All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Primary congenital glaucoma (PCG) is a rare disease due to genetically-determined abnormalities in the trabecular meshwork and anterior chamber angle resulting in elevated intraocular pressure (IOP), without other ocular or systemic developmental anomalies. Other terms have been used previously to describe this entity, including trabeculodysgenesis, goniodysgenesis and primary infantile glaucoma, however the term PCG replaces these in the 2013 International Classification of Childhood Glaucoma.[2] There are three variants based on the age of presentation as follows:
1) newborn onset (0-1 month)
2) infantile onset (>1-24 months)
3) late onset or late-recognized (>24 months)
4) spontaneously arrested cases (very rare, classic findings of eye stretching including Haab striae with normal IOP; must follow as glaucoma suspects nonetheless)
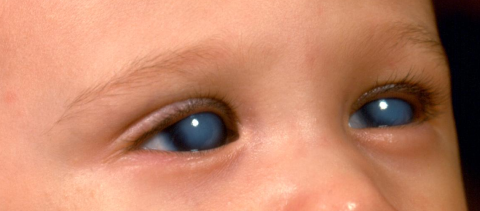
PCG commonly presents between the ages of 3-9 months, but the most severe form is the newborn onset. Elevated IOP is associated with the classic “triad” of symptoms (photophobia, epiphora and blepharospasm), which occurs due to rapid expansion of the child’s eye causing buphthalmos (Greek = “ox eye”), corneal enlargement, horizontal or oblique breaks in Descemet membrane (Haab striae) and subsequent corneal edema and opacification. If Haab striae and buphthalmos are seen without elevated IOP, optic nerve cupping or corneal edema, then the patient has spontaneously arrested PCG. [2]
The prognosis for children with PCG is quite variable, with some achieving good vision, while others go blind. While PCG accounts for less than 0.01% of all patients with eye diseases, it has been blamed for 5% of childhood blindness worldwide.[3] Vision loss is secondary to corneal scarring or optic nerve damage, and often amblyopia in asymmetric or unilateral cases. Surgical management is the primary treatment modality. If IOP is controlled, vision in the better eye ultimately can be 20/60 or better.[4][5]
Disease Overview and Definition
PCG is the most common form of primary childhood glaucoma, and is one, of two primary childhood glaucomas, that presents in children younger than 4 years of age (the other primary childhood glaucoma being juvenile open angle glaucoma). PCG and other childhood glaucomas presenting in infancy may present with one or more of the classic triad of epiphora, photophobia and blepharospasm; however if ocular or systemic abnormalities, either congenital or acquired, are also present, then PCG can be ruled out, and secondary childhood glaucomas need to be considered (i.e. Axenfeld-Rieger syndrome, aniridia, Peters anomaly, persistent fetal vasculature, oculodermal melanocytosis, posterior polymorphous dystrophy, microphthalmos, microcornea, ectopia lentis, Sturge-Weber syndrome, Lowe syndrome, neurofibromatosis, trauma, inflammation, steroid-induced, tumors, retinopathy of prematurity, glaucoma following cataract surgery).
Due to the elasticity of the eye in young children, the 2013 International Classification System for Childhood Glaucoma defined childhood glaucoma as irreversible or reversible damage to the whole eye (not just the optic nerve as glaucoma is defined for adults). Thus, additional important clinical signs in PCG, besides elevated IOP and optic nerve cupping, are corneal enlargement and clouding, Haab striae, and buphthalmos. Not all signs are always present, however, and other parts of the eye also stretch with elevated IOP. Diagnosis of PCG can be delayed if corneas remain clear, despite being enlarged, and bilateral PCG can be missed if signs and symptoms are mild in one eye. Irreversible vision loss results if elevated IOP is untreated or uncontrolled in PCG. Optic nerve damage occurs, and focal corneal edema overlying Haab striae, which can be single or multiple, can lead to permanent corneal scarring and opacification. This corneal scarring can obscure the visual axis or cause astigmatism with or without refractive amblyopia. Amblyopia may also develop due to optic nerve damage, anisometropia, strabismus or a combination.
Demographics
Incidence of PCG varies by geographic location; rates can be as high as 1 in 1250 in some Eastern Europeans or as low as 1 in 20000 in Western countries. The rate is 5-10 times higher in children from consanguineous relationships.[6] It is bilateral in 65-80% of cases.[7] [3]Male to female ratio is 3 to 2 in the United States and Europe. [8]In Japan, a study compared PCG patients with and without CYP1B1 mutations and found a ratio of male to female incidence of 6:5 in patients with CYP1B1 mutations, and 19:2 in those without CYP1B1 mutations.[9]
Etiology
Most PCG cases are sporadic (with no family history), however, about 10-40% are familial, with an autosomal recessive inheritance pattern and penetrance varying from 40-100%. [2] Autosomal dominant inheritance has also been reported. [10]Five loci have been identified by linkage analyses: GLC3A (located on choromosome 2p22-p21), GLC3B (1p36.2-p36.1), GLC3C (14q24.3), GLC3D (14q24.2-q24.3, not overlapping with GLC3C), and GLC3E (9p21).[3][11]
Thus far, a gene associated with PCG has been identified in three of these five loci. Further details are below.
The GLC3A loci contains the cyctochrome P4501B1 (CYP1B1) gene, which was the first reported PCG-causing gene. It codes for an enzyme that metabolizes compounds vital for the developing eye, such as fatty acids and vitamins[6], and is expressed in fetal and adult neuroepithelium and ciliary body. [2][12]Severe trabecular meshwork atrophy is seen in mouse models deficient of CYP1B1. [13]In zebrafish, CYP1B1 has been found to indirectly affect neural crest migration to the anterior segment and angle by playing a role in ocular fissure closure. [14] While the exact mechanism by which CYP1B1 mutations causes PCG is unknown, we know that levels of a protein product of this gene are inadequate for appropriate embryogenic ocular development, resulting in goniodysgenesis. A twin study demonstrated that CYP1B1 gene activity may be implicated in a common pathway PCG, juvenile open-angle glaucoma, and primary open angle glaucoma. Recent studies propose that the mutation may also interfere with the ability of retinal ganglion cells to respond to the stress generated by high IOP and the resultant increase in reactive oxygen species.[15][16]CYP1B1 mutations are associated with 15-20% of PCG cases in Japan and the United States, 75-100% of cases in Saudi Arabia, and all cases in Slovakia Roma. [17][18]
The GLC3D locus contains latent transforming growth factor beta binding protein 2 (LTBP2). LTBP2 is expressed in trabecular meshwork and ciliary processes however its role in the eye is unknown.[17] In nonocular tissues, it is involved in tissue repair and cell adhesion. [18][19] LTBP2 null mutations have been reported in consanguineous Iranian and Pakistani families, and Slovakian Roma with PCG. Homozygosity for a variant of an LTBP2 mutation was associated with worse outcomes even while undergoing more surgical interventions.[3][10]
GLC3E contains the tunica interna endothelial cell kinase (TEK, also known as TIE2) gene. The angiopoietin/TEK (ANGPT/TEK) signaling pathway is required in Schlemm canal development, and includes 3 ligands (ANGPT1, ANGPT2, and ANGPT4) and 2 receptors (TEK and TIE). TEK-knockout mice can have no Schlemm canal and TEK-hemizygous mice have a severely underdeveloped Schlemm canal.[20] Additionally, ANGPT1 mutations have been identified in a few PCG patients who do not have other known PCG-causing genes, revealing another member of the ANGPT/TEK signaling pathway that may be a cause of PCG.[21] A recent study conducted in China re-demonstrated TEK mutations as causative in PCG. In patients with the loss of function p.C264F variant located in the ectodomain of TIE2, it is thought that disruption of disulfide bond formation leads to misfolding and subsequent instability of protein products. The p.L504P variant in the same ectodomain appears to replace a leucine with a proline at a critical location in a beta-pleated sheet, preventing proper intra-strand hydrogen bonding.[22]
Lastly, though not associated with the above loci, but originally associated with juvenile open angle glaucoma and primary open angle glaucoma, the myocilin/trabecular meshwork-induced glucocorticoid response protein (MYOC) gene on chromosome 1q24 may also explain a small proportion of PCG cases, up to 5.5%.[3][11][23]
PCG patients may have one or more genes affected, and the previously discussed genes may regulate or interact with each other.[24] CYP1B1 may play a role as a modifier gene for MYOC expression, [25] and a digenic mode of inheritance has been considered for CYP1B1 and MYOC,[26] and CYP1B1 and TEK.[27]
Currently, the chance of identifying a genetic cause is 40% when genetic testing is done.[3]
Risk Factors
The only known risk factors are genetic - consanguinity and affected siblings. Parents of PCG patients should be aware that the chance of a second child with PCG is a small but real risk that usually is no more than 3%. If two children have the disease, then the risk of subsequent children increases to as high as 25%, with the assumption of autosomal recessive inheritance.[8] In 2018, Yu-Wai-Man et al compiled the clinical utility gene card for PCG which describes situations for which gene testing may be useful. [3] Carriers of the CYP1B1 gene mutation and double null CYP1B1 alleles are, on average, more likely to have higher IOP and require more surgeries. [6]
General Pathology
Many theories have been developed, yet the exact nature of anterior chamber development and the pathophysiology of PCG has yet to be well-understood. It is generally agreed that elevated IOP occurs due to poor aqueous outflow secondary to abnormal development of the anterior chamber angle.
The American Academy of Ophthalmology's Pathology Atlas contains a virtual microscopy image of Congenital Glaucoma (not PCG, given presence of cataract).
Pathophysiology
In 1955 and 1966, an imperforate membrane covering the anterior chamber angle was described by Barkan and Worst (called Barkan membrane). [28][29]This structure has not been confirmed histologically, and after numerous investigations, Barkan membrane is no longer thought to exist, and aqueous outflow obstruction is thought to occur at the trabecular meshwork itself.[8][30] Furthermore, clinicopathologic studies with light microscopy, and scanning and transmission electron microscopy, have shown abnormalities throughout the outflow pathway. A high ciliary muscle insertion into the trabecular meshwork is often seen. Often times, increased amounts of collagen in trabecular beam cores have been demonstrated, with preserved trabecular spaces. And other times, elastic-type fibers, fibrillary collagen and granular material or ground substance have been seen in the trabecular spaces and the inner wall of Schlemm canal. The most severe cases were associated with no visualization of the Schlemm canal and collector channels. [31][32][33][34]
Previously, it was thought that abnormal cleavage and mesodermal tissue atrophy in anterior chamber angle development was the root cause of PCG. Currently, the ruling hypothesis by Anderson begins in the third trimester when normal angle development involves posterior sliding of the ciliary body and peripheral iris (uveal tissues), away from the cornea and sclera. Anderson proposed that excessive or premature accumulation of collagenous beams within the trabecular meshwork inhibit posterior sliding of the ciliary body and peripheral iris, leading to an anterior insertion of the iris root and ciliary muscle, which can obstruct the trabecular meshwork, and narrow or completely compress the Schlemm canal.[35][36] More recently, ultrasound biomicroscopy and anterior segment optical coherence tomography have been used to determine angle abnormalities in PCG patients.[30]
Researchers have been correlating neural crest cell (NCC) migration and differentiation with angle development and the role of CYP1B1 in ocular fissure closure, which ultimately affects NCC migration. [37][38][34]
Primary Prevention
There is no known way to prevent PCG. Early detection and treatment are essential to maximize visual potential. In the future, prenatal genetic screening may emerge as a preventative measure. It can be offered to parents in at-risk populations, such as those with family history or in consanguineous relationships in areas with higher PCG prevalence (Slovakia, Saudi Arabia, China, etc.). Parents with unborn children who test positively for mutations in CYP1B1 on genetic screening can be alerted about the potential need for urgent surgical management soon after birth. [6]
Symptoms
Patients with PCG experience one or more of the "clinical triad" of symptoms including epiphora, photophobia and blepharospasm. This triad of symptoms is classically associated with PCG due to the corneal edema that results from the elevated IOP, and causes irritation. Reduced vision can also occur from corneal edema/opacification or progressive myopia and/or astigmatism. Amblyopia can further worsen vision.
Signs
The main clinical signs of PCG include elevated IOP >21 mmHg, corneal edema and/or enlargement of the eye with buphthalmos, and Haab striae. The IOP at presentation is usually between 30-40 mmHg, though it can be outside this range. [39] IOP in the low-20s mmHg is acceptable if the optic nerve is healthy and the patient’s eye growth is within normal limits, but may not be if there are other more severe signs of PCG.
With IOP in the 30-40s mmHg, the cornea becomes cloudy due to diffuse and/or focal edema. As in adult eyes, the endothelial cell layer cannot pump fluid out of the cornea in an eye with elevated IOP. In young children however, there is the additional insult of corneal stretching from the high IOP causing not only enlargement of the cornea, but Descemet breaks, leading to “striae,” which are areas of bare stroma bordered by two separated edges of Descemet membrane that become ridges due to deposition of hyaline.[8] These are called Haab striae and are associated with acute overlying focal corneal edema when the IOP is high. They occur in about 25% of PCG eyes presenting at birth, and more than 60% of PCG eyes identified at 6 months of age.[40] There may be single or multiple, and are oriented horizontally or obliquely. After normalization of IOP, corneal edema may clear; however, Haab striae remain and may be associated with corneal scarring. The poorly controlled cases of PCG may end up with dense stromal opacification even after IOP is controlled.
A newborn’s cornea is typically 9.5-10.5 mm in diameter and increases to 10.0-11.5 mm by age 1.[41] Any diameter above 12.0 mm before 1 year of age suggests an abnormality, especially if there is asymmetry between the two eyes. If the diameter is greater than 13 mm at any age, glaucoma suspicion should be high. Along with corneal stretching in the setting of elevated IOP, there is stretching of the scleral wall and all tissues within the eye leading to buphthalmos. Corneal enlargement stops around age 3 years, while sclera can continue to stretch up to age 10 years of age.[8]
Other signs related to the eye distension include abnormally deep anterior chamber, myopia (mainly due to elongation and enlargement of the eye), astigmatism (from Haab striae and corneal stretching), anisometropia (almost always present in unilateral PCG), and optic nerve cupping.
The optic nerve cupping in very young children may be seen solely due to optic canal stretching and posterior bowing of the lamina cribrosa without a decrease in the neuroretinal rim.[42][43] When the IOP is normalized, there can be notable reversal of cupping. While cupping may resolve, retinal nerve fiber layer damage, if present, is permanent. In older children and those with advanced glaucoma, cupping occurs due to neuroretinal rim tissue loss, especially at the vertical disk poles.[8]
Any asymmetry between eyes in the aforementioned signs should raise suspicion of glaucoma. Lastly, amblyopia, either deprivation or both, may also be present with the other signs mentioned above.
Diagnosis
The diagnosis of PCG can often be made clinically, even without an accurate measurement of IOP. The hallmark of the disease, however, is an elevated IOP and ocular stretching in the absence of other ocular and systemic conditions that can cause glaucoma, such as Axenfeld-Reiger syndrome, aniridia, or surgical removal of cataract in infancy (i.e. glaucoma following cataract surgery).
History
PCG patients often present to the physician’s office due to abnormal appearance of the eyes such as a cloudiness or a blue tint to the eyes, or patient behavior such as eye rubbing or shying away from light. While there may be tearing, there is no ocular discharge and usually no eye redness. The patients are otherwise healthy. A positive family history is helpful but often is not present since most cases are sporadic.
Physical examination
The physical exam follows the standard ophthalmology exam for infants and young children, with special note of signs related to ocular expansion due to the distensibility of the infant eye. The vision assessment should specifically look for asymmetry in unilateral cases and the presence of nystagmus and/or reduced fixation response in bilateral cases.
The anterior segment exam should specifically look at the corneal diameter. Measurement can be done in clinic by holding a ruler in front of the patient’s eyes, however it may be much more accurate under anesthesia if the patient is not cooperative in clinic. (Details in “diagnostic testing.”)
The cornea should also be examined for clarity and the presence of one or multiple Haab striae (horizontal or oblique breaks in Descemet membrane, described above). These may be obvious or subtle, and sometimes can best be seen after dilation using retroillumination. Diffuse corneal edema and/or focal corneal edema or opacification may obscure Haab striae. The anterior segment exam should also look for anterior chamber depth (deep in PCG), and structural abnormalities of the cornea, iris, and lens (no frank abnormalities are seen in PCG, with the exception of mild iris changes in newborn-onset PCG).
IOP can be reliably measured in clinic with various devices (described below) if the child is calm, sleeping or feeding. Distraction with toys or movies may help during measurement. If the child is crying and uncooperative, then the IOP measurement will likely be artificially elevated and exam under anesthesia may be required.
Gonioscopy can be performed in the clinic on older children, and must be done under anesthesia for younger patients. Further description is in “diagnostic testing.”
After dilation, cycloplegic retinoscopy should be performed to look for myopia, astigmatism, and any corneal irregularity. The fundus exam should include a careful exam of the optic nerve to look for asymmetric or large cupping and for any structural abnormalities. There may be reversal of cupping after reduction in IOP in infants with glaucoma. [44]
Axial length measurement (described in "diagnostic testing") is also a key part of the physical exam in PCG.
The sequence and method the exam is done in clinic may be modified to optimize obtaining IOP measurements when the child is calm. For example, initial anterior segment exam may be done with a penlight or direct ophthalmoscope instead of a portable slit lamp. IOP could be the first part of the exam if the child is sleeping.
Clinical diagnosis
The clinical diagnosis of PCG can be difficult, especially when a child does not cooperate with IOP measurement. If a reliable IOP measurement is elevated in the setting of other classic signs of ocular stretching (above), then the diagnosis of glaucoma is made, and if no other ocular or systemic developmental anomalies are seen, then PCG is the diagnosis. The presence of Haab striae suggests congenital glaucoma, and if seen without ocular developmental anomalies or systemic syndromes, then PCG is the diagnosis. If IOP is normal with Haab striae, then one may have a case of spontaneously arrested PCG, which still needs to be followed over time for elevated IOP.
Diagnostic procedures
The main diagnostic test for PCG is the measurement of the IOP, which should be done prior to instilling dilating drops. In a cooperative infant or young child, this measurement can be obtained in the clinic setting with a Perkins applanation tonometer, Tono-pen (a portable Mackay-Marg-type tonometer) and/or Icare rebound tonometer. In older patients, standard Goldmann applanation tonometry can be performed. A pneumotonometer may be useful to confirm IOP during examination under anesthesia or in clinic if available, and may be less influenced by corneal abnormalities. A Schiötz indentation tonometry is not recommended in these patients due to under- or overestimation of IOP in childhood glaucoma.[45][46] For the uncooperative patient, an examination under anesthesia should be performed.
Of note, the Icare rebound tonometer has decreased the need for examinations under anesthesia as it does not require a topical anesthetic.[47] Two models available in the United States (Icare TAO1i and Icare ic100) require the patient to be upright, while the newest model, recently approved in the US (Icare ic200), allows measurement in a supine patient. The IOP measured by Icare in cooperative, awake children with known or suspected glauacoma, has been shown to be within 3 mmHg of IOP obtained by Goldmann applanation tonometry in 63% and is higher than measured by Goldmann applanation tonometry in 75% of children.[48] By contrast, Icare tonometry may under-measure the IOP compared to Tono-pen readings in the setting of corneal edema.[49]
Because anesthetic agents variably alter the IOP, with most lowering IOP, measurements should be obtained as soon as possible after induction of anesthesia and before intubation. If the IOP is actually elevated, it often remains greater than 20 mmHg under anesthesia, which suggests glaucoma. The normal IOP is lower in infants and young children than adults. A newborn has an average IOP of 10-12 mm Hg, increasing to 14 mm Hg by age 7 or 8 years of age. An asymmetric measurement or an elevated IOP measurement in the presence of other clinical signs helps make the diagnosis of glaucoma.
Corneal diameter measurement is another key diagnostic procedure for PCG. Some providers check horizontal diameters only, while some check horizontal and vertical diameters. If there is pannus or scarring obscuring the limbus, the measurement may not be accurate. In the office a millimeter ruler can be placed above the eyes and if the child is not cooperative, a close-up digital photograph can be taken with the ruler in place, and a measurement can be made from the photo. This is most amenable to horizontal corneal diameter measurement. While under anesthesia, calipers with the tips placed at the limbus 180 degrees apart are used across the widest diameter, and then measured with a graduated ruler to check the measurement. Ideally, the measurement can be estimated to the nearest 0.25 mm.[2]
Examination for Haab striae is done with an oblique slit beam with a portable slit lamp if the patient is younger or under anesthesia, or on a regular slit lamp in the clinic if the patient is older. Retroillumination can also be used to identify Haab striae. . In older patients with treated PCG, corneal endothelial protuberances and hyperproliferation of the Descemet membrane/pre-Descemet’s layer complex have been demonstrated with anterior segment OCT (ASOCT). These may demarcate areas in which the edges of the Descemet membrane have re-approximated during the healing process.[50]
If a view through the cornea allows it, gonioscopy is done in clinic if tolerated, ideally a Sussman (or similar) indirect gonioscopy lens as it fits easily between a young child’s small palpebral fissure. Using a gonioscopy lens without a handle may be easier as it allows the examiner to hold open the eyelids while placing the lens. More commonly, for initial diagnosis of PCG, gonioscopy is performed under under anesthesia with a Koeppe or similar direct gonioscopy lens and portable slit lamp. There are different sized Koeppe lenses to fit different corneal diameters. The Koeppe lens is best handled with a glove to avoid fingerprint smudges. The Koeppe lens cup is filled with balanced salt solution and placed quickly on the eye or placed on the eye and tilted with one edge abutting the sclera while filling the space between the lens and eye with solution. Then a binocular microscope such as the portable slit lamp is angled towards the angle of interest and the lens can be shifted slightly toward the angle to optimize the view.
Gonioscopy in these cases helps guide surgical planning in cases of PCG, and may also identify other angle abnormalities which might identify other secondary glaucoma types, for example Axenfeld-Rieger anomaly (many irido-corneal attachments with anteriorly placed Schwalbe line). Infants with PCG usually do not have a visible scleral spur because the peripheral iris inserts into the trabecular meshwork (in contrast to normal infants whose peripheral iris and ciliary body have recessed to the scleral spur or posterior to it). There may also be scalloped edges of the peripheral iris and pale peripheral iris stroma in front of the angle causing a “morning mist” appearance. If there are peripheral anterior synechiae, posterior embryotoxon, or other abnormalities, then the diagnosis is unlikely PCG. Gonioscopy photographs can be taken by instilling the eye with coupling gel and angling the camera lens (i.e. RetCam) obliquely toward the angle of interest and adjusting the focus until the angle comes into clear view.
Axial length is measured with A-scan ultrasonography, ideally using the immersion and not contact method, either in clinic or under anesthesia. It is best done under anesthesia, during baseline examination to determine if the axial length is greater than normal for the patient’s age, and repeated approximately every 3-4 months to assess if the growth rate is greater than average. Of note, measuring axial length itself is not an indication for examination under anesthesia if a patient is otherwise doing well, and can be performed at intervals when examination under anesthesia is needed for clinical management. Sampaolesi and Kiskis provided linear regressions from data of normal children. Sampaolesi used immersion A-scans and found the normal axial length for a one-month-old lies between 17.25 mm (5th percentile) and 20.25 mm (95th percentile). Sampaolesi also recommended that axial length be measured after dilation with cycloplegic drops.[2][51]
Optic nerve evaluation is performed with either indirect or direct ophthalmoloscopy with attention to the cup-to-disc ratio. In the setting of a small pupil, a magnified view of the nerve can be obtained by using a direct ophthalmoscope through a Koeppe gonioscopy lens on the eye. Fundus photography is also recommended for comparisons between serial examinations. B-scan ultrasonography is recommended if the cornea does not allow fundus examination to rule out posterior disease. Severe optic nerve cupping may sometimes be noted on the posterior B-scan.
Pachymetry is used to measure central corneal thickness. The central cornea may be thicker due to corneal edema, and has also shown to be thinner in PCG patients without corneal edema, likely due to stretching of their tissues. [52] Other small studies have shown either no significant difference in central corneal thickness between normal eyes and eyes treated for PCG, or the central corneal thickness was thicker in eyes treated for PCG than in normal eyes.[53] [54] Corneal hysteresis and corneal resistance factor have been found to be lower in eyes with PCG compared to normal eyes.[53][54]
Perimetry can be attempted starting around age 7-8 years of age if the patient does not have nystagmus, cognitive impairment or severe vision loss. Quicker testing algorithms such as SITA-FAST may allow children to perform more reliably.[55] Goldman perimetry can be very helpful in young children.
Standard tabletop optical coherence tomography (OCT) can be considered once a child can be examined at the regular slit lamp to evaluate the retinal nerve fiber layer and ganglion cell layer. It may be helpful especially if the child cannot perform perimetry. While devices currently do not carry normative data for children, studies have collected data on normal children. [56][57][58][59] Handheld and mounted spectral-domain OCT devices are emerging technologies that can be used during examination under anesthesia.[60][61]
Laboratory test
Ophthalmology providers may work with genetics team members to send for genetic testing if available.
Differential diagnosis
The differential diagnosis depends on the presenting symptom(s) and is considered when key features of PCG, such as elevated IOP and optic nerve cupping, are absent.
For epiphora, the differential diagnosis includes nasolacrimal duct obstruction, conjunctivitis, corneal abrasion, keratitis, and uveitis. Photophobia and blepharospasm are unlikely with nasolacrimal duct obstruction and conjunctivitis, however, may be seen with corneal abrasion/injury, keratitis, or uveitis.
For corneal clouding or opacification, the differential diagnosis includes birth trauma generally involving forceps causing Descemet tears that are vertical or oblique (unlike Haab striae which are usually more horizontal or curvilinear), corneal dystrophies (such as congenital hereditary endothelial dystrophy or posterior polymorphous dystrophy), congenital or developmental abnormalities (such as sclerocornea, Peters anomaly), keratitis (from intrauterine infection or inflammation such as herpetic infection, congenital syphilis, and maternal rubella, and Riley-Day syndrome), storage diseases, inborn errors of metabolism (such as mucopolysaccharidoses, mucolipidoses, cystinosis and oculocerebrorenal (Lowe) syndrome), and choristomas.
For corneal enlargement, the differential diagnosis includes high axial myopia and megalocornea. Megalocornea is an inherited disorder in which infants have clear corneas with diameters > 14 mm, deep anterior chambers, and iridodonesis. There have been reports within one family of megalocornea coexistent with congenital glaucoma.[62]
For optic nerve cupping, the differential diagnosis includes physiologic cupping, optic nerve coloboma, optic nerve atrophy, optic nerve hypoplasia, and an optic nerve malformation.
Note many of the above diagnoses may coexist with congenital glaucoma, however it would not be considered primary congenital glaucoma, but under the category of secondary childhood glaucoma.
Management
The management of PCG is directed toward lowering and controlling the IOP and treating the secondary complications such as refractive change and amblyopia that develop during the course of the disease.
General treatment
The mainstay of treatment is angle surgery, either goniotomy or trabeculotomy, to lower IOP by improving aqueous outflow. If angle surgery is not successful, trabeculectomy enhanced with mitomycin C or glaucoma implant surgery with a Molteno, Baerveldt, or Ahmed implant can be performed. In refractory cases, cycloablation can be performed using an Nd:YAG laser, diode laser, or cryotherapy, with diode laser being the most widely used device. Medical therapy, either topically or orally, is typically used as a temporizing measure prior to surgery and to help decrease corneal clouding to facilitate goniotomy, and to supplement IOP control after surgery. In managing secondary congenital glaucoma, medical therapy is the first-line treatment. [63]
Medical therapy
Medical therapy for PCG is typically used as an adjunct to surgery. Most medications in the United States have not been approved for children, however many studies have been performed that inform practitioners on their safety and efficacy in children. Timolol is the first choice in pediatric glaucoma. In cases with insufficient reduction of the intraocular pressure (IOP), the combination of timolol once a day and dorzolamide twice a day brings about a good control of the IOP. Both medications are effective and well tolerated. The alpha2-agonists have more and potentially serious adverse effects in children and are contraindicated for children younger than 2 years of age. Latanoprost tends to be less effective in lowering IOP in children than in adults.[64]
Beta-blockers (beta-adrenergic antagonists): Topical beta-blockers play a large role in PCG treatment and include timolol (non-selective beta-1 and beta-2 blocker, concentrations of 0.1% available in some countries, 0.25% and 0.5% solutions, and 0.25% and 0.5% gel-forming solution), and betaxolol (selective beta-1-blocker, concentrations of 0.25% and 0.5% solutions). Given potentially high plasma levels of the medication from topical instillation in small children, the lowest concentration available should be initiated first. The solution drops are approved for BID dosing though may be just as effective dosed once in the morning. The gel-forming solutions are approved for once daily dosing. Beta-blockers typically reduce IOP by 20-30%. Side effects are mainly systemic and include respiratory distress, caused by apnea or bronchospasm (which may present as coughing instead of wheezing), and bradycardia. Beta-blockers should be avoided in patients with bradycardia, second- or third-degree atrioventricular block, and active asthma or “reactive airways.” Betaxolol may be less likely to cause pulmonary distress (e.g. asthma attacks) and cardiac side effects.[65]
Carbonic anhydrase inhibitors: Oral carbonic anhydrase inhibitors include acetazolamide (Diamox, dose 10-20 mg/kg/day divided into 3 or 4 doses) and methazolamide (Neptazane, dose < 2 mg/kg/day, divided into 2 doses[2][66]). Acetazolamide can be prepared in a flavored syrup (have the pharmacist crush the tablets and suspend the powder in syrup) with a concentration of 50 mg/ml for ease of use. Children can also take the tablet crushed in applesauce or something similar. It reduces the IOP about 20-35%. Side effects occur in >40% of patients and include lethargy, decreased appetite, weight loss, gastrointestinal discomfort, diarrhea and metabolic acidosis. Topical carbonic anhydrase inhibitors include dorzolamide 2% (Trusopt) and brinzolamide 1% (Azopt) drops twice a day (BID) or three times a day (TID). These medications may produce less reduction in IOP (about 25%) than oral carbonic anhydrase inhibitors, but also appear to have fewer systemic side effects. Rarely, side effects can occur, particularly in premature infants, such as metabolic acidosis.[67] Topical carbonic anhydrase inhibitors ideally should be avoided or used as a later option in the setting of compromised corneas, especially of a corneal transplant.[2]
Combination beta-blocker/carbonic anhydrase inhibitor: Timolol 0.5%-dorzolamide 2% (Cosopt) drop BID has been shown to be effective in reducing IOP in children requiring more than one topical medication. It is approved for BID dosing, but cautious use in young children is warranted due to the higher concentration of timolol.
Adrenergic agonists (avoid or use with caution in children younger than age 6 years or weight less than 20 kg): Apraclonidine 0.5% (Iopidine) and brimonidine (Alphagan, Alphagan P, 0.1%, 0.15%, 0.2%) are alpha-2 selective agonists and are dosed BID to TID. Their effectiveness has not been studied specifically for PCG. The side effects in children limit their use. Due to being highly lipophilic, brimonidine passes through the blood-brain barrier potentially causing severe sleepiness, respiratory depression, apnea and coma, especially in neonates and infants, thus it is strictly contraindicated in patients 2 years old or younger. It may also cause bradycardia, hypotension, hypotonia, and hypothermia. Apraclonidine is more hydrophilic which reduces its blood-brain barrier penetration and thus has fewer central nervous system side effects than brimonidine. It must still be used with caution and is best used for short- or intermediate-term IOP lowering. Tachyphylaxis and ocular allergy limit its effectiveness long-term.
Combination beta-blocker/alpha-2 adrenergic agonists: Timolol 0.5%-brimonidine 0.2% (Combigan) must not be prescribed to children if there is a contraindication to the individual components.
Prostaglandin analogs: Latanoprost 0.005% (Xalatan), travoprost 0.004% (Travatan), bimatoprost 0.01% (Lumigan), and tafluprost (Zioptan, preservative-free) are dosed nightly. Latanoprost reduces the IOP in PCG 15-20%.[68] While the FDA has not approved prostaglandin analogs in children, Europe has approved latanoprost for children. Side effects mainly include lash growth, conjunctival injection, and less commonly iris pigmentation alteration, allergy, uveitis and periocular hyperpigmentation. Side effects seem more prominent with use of travoprost and bimatoprost and less with latanoprost. Long-term side effects are still unknown in children. Prostaglandin-related periorbitopathy has been described in children.[69] This class of medication is relatively contraindicated when active inflammation or uveitis is present.
Combination beta-blocker/prostaglandin analog: Available in countries outside the United States.
Miotic agents: These do not play much of a role in PCG likely due to their immature angle anatomy and high ciliary muscle insertion. They include echothiophate, phospholine iodide (irreversible cholinesterase and pseudocholinesterase inhibitors) and pilocarpine (direct parasympathomimetic). Miotic are useful perioperatively for angle surgery. Pilocarpine (0.5-6%, most common 1-2%) is dosed once to four times a day, usually 2-3 times a day after angle surgery. Side effects include miosis, decreased heart rate, apnea, sweating, and hypersalivation, and theoretically may induce cataract and retinal detachment.
Modified prostaglandin analogs and rho-associated protein kinase inhibitors: Latanoprostene bunod (Vyzulta) and netarsudil (Rhopressa) have not been studied in patients younger than 18 years of age.
Providers can start with a either a carbonic anhydrase inhibitor, beta-blocker or prostaglandin analog, or a combination, and progressively add another medication class, keeping in mind medications are generally a temporizing measure prior to surgery. If prescribed before initial surgery, medications should not be used without fairly frequent follow-up, and ideally surgery performed within 2 weeks of PCG diagnosis. Early discussion preparing family and caregivers for surgery is necessary. Medications should be continued until surgery, and may help maximize corneal clearing by reducing the IOP. After surgery, medications may still be needed as an adjunct and family and caregivers should be made aware of this. Compliance may be an issue when the medication regimen becomes complex and should be addressed. PCG requires lifelong serial measurements of IOP, corneal diameter, axial length, refractive error, and optic nerve cupping. If an adequate assessment is not possible in the outpatient clinic, an examination under anesthesia should be performed.
Surgery
There are four major surgical options for PCG; however once the diagnosis of PCG is established, angle surgery is the first procedure of choice to incise/open the trabecular meshwork with the hope of allowing aqueous flow from the anterior chamber directly into Schlemm canal. It is generally agreed that angle surgery is most successful in infantile-onset PCG, and less so in newborn or late-recognized PCG. Goniotomy is preferred by some surgeons when the cornea is clear enough to permit visualization of anterior segment structures (although some prefer trabeculotomy regardless of the corneal clarity, see below). An incision is made across the trabecular meshwork under direct gonioscopic visualization using a goniotomy knife (Swan knife, needle-knife, disposable 25-gauge needle on a syringe) and surgical goniotomy lens (i.e. Barkan or other goniotomy lens). Traditionally, it is first performed nasally, however modifications can be made to complete it temporally as well at one surgical session. If a surgeon is comfortable with devices and modified techniques such as using the Kahook dual blade, Trabectome, gonioscopy-assisted transluminal trabeculotomy with suture or lighted microcatheter, or Omni, these devices can be used safely to perform a goniotomy in children, however it is not recommended to use these devices in a pediatric eye prior to extensive experience in an adult eye. There is no data to suggest these modified techniques do better than traditional goniotomy or trabeculotomy. Complications include hyphema, anterior chamber shallowing, peripheral anterior synechiae, and rarely, iridodialysis, cyclodialysis, cataract, scleral perforation, epithelial ingrowth, and retinal detachment.[70][2]
When the cornea is not clear enough to permit visualization of the angle, or if preferred due to technical factors or surgeon experience or preference, trabeculotomy ab externo (“trabeculotomy”) is the procedure of choice. Access to Schlemm canal is obtained externally via a partial scleral flap to allow cannulation of Schlemm canal. The older technique opened ~90 degrees of Schlemm canal with a curved rigid pronged probe called a trabeculotome, which can then be rotated gently into the anterior chamber to incise through the trabecular meshwork. A trabeculotome curved in the opposite direction can then be used to cannulate another 90 degrees of Schlemm canal and complete 180 degrees of trabeculotomy. Alternatively, and preferred by many angle surgeons at this time, a 6-0 polypropylene (Prolene) suture or an illuminated microcatheter can be threaded into the entire Schlemm canal and pulled across the anterior chamber to complete a 360-degree trabeculotomy. Complications include hyphema, unintentional filtering blebs, choroidal detachment, cyclodialysis, iridodialysis, lens injury, and infection.
Traditional goniotomy and trabeculotomy ab externo (incising 2 quadrants) have success rates ranging for goniotomy 30-65% and for trabeculotomy 40-80%, with success reported as low as 10% to as high as 94%.[71][2]
Combined trabeculotomy and trabeculectomy (CTT) can be performed if Schlemm canal could not be cannulated or prior trabeculotomy failed, in which a trabeculectomy is added to the trabeculotomy by removing a block of tissue in the scleral flap bed followed by a surgical iridectomy as done in regular trabeculectomy. Mitomycin C (MMC) may be used with care. CTT can be an initial surgical procedure, especially in Indian and Middle Eastern patients.[72][73]
Filtering surgery is considered when one or more angle surgeries have failed and includes traditional trabeculectomy with or without MMC, and glaucoma drainage device implantation. Trabeculectomy may be best done using techniques of the Moorfields Safer Surgery System, including fornix-based conjunctival flaps, small radial cuts, MMC under the sclera flap and subconjunctival tissue with wider spread to enhance posterior aqueous flow and reduce bleb-related complications. Use of an anterior chamber maintainer in all cases and releasable sutures are also recommended.[74][75][76] EX-PRESS mini glaucoma shunts are not used commonly in PCG as safety and efficacy have not been established long-term in young children. Severe complications of trabeculectomy include vitreous loss, ectasia, scleral collapse, retinal detachment, and phthisis. The child is also at lifelong risk of complications and infection including bleb leak, wound rupture, blebitis, and endophthalmitis.
Reported success rates for trabeculectomy performed for PCG range between 50-87%.[2] The risk of failure is 5.6 times higher in patients age 1 year or less.[77] The higher risk of failure in advanced PCG young patients is due to buphthalmos, lack of scleral rigidity, and highly active healing and scarring.
When trabeculectomy fails or is not a desirable option, then the other filtering option with glaucoma drainage device (GDD) surgery or cyclodestructive procedures are the next surgical choices. All models of GDDs (Molteno, Baerveldt, and Ahmed valve) can be used in PCG patients, and GDDs can be implanted safely in neonates with attention to eye and implant parameters. Generally, it is advisable to use a fornix incision with conjunctival and Tenon capsule incision 8 mm posterior to the limbus, and double-layer closure with running 8-0 polyglactin (Vicryl) suture on a vascular needle. The flexible implants (Baerveldt and Ahmed) can be trimmed posteriorly so as to prevent plate-optic nerve touch. The amount to trim can be calculated with the online Freedman-Margeta GDD calculator (http://people.duke.edu/~freed003/GDDCalculator/).[78] Some surgeons place the first tube inferior nasal to preserve conjunctiva superiorly for possible trabeculectomy when the patient is older, however others prefer superior temporal placement of the first tube, for better efficacy, and are able to perform successful trabeculectomy superior nasal at a future time. Complications from GDDs are many and include those of trabeculectomy plus cornea-tube touch, tube erosion through the conjunctiva or cornea, implant migration, and cataract. Infection rates are low.[79] The Ahmed valve may additionally fail due to fibrovascular ingrowth into the valve chamber.[80]
Success rates vary widely for PCG and childhood glaucoma. For the Molteno, the range is 56-95%, with slightly higher success with the double-plate implant compared to the one-plate implant.[81][82] The Baerveldt success rates range from 80-95% at 12 months, decreasing to < 50% by 60 months.[83][84] The Ahmed glaucoma valve has about a 55% success rate at 5 years.[85][86]
Cyclodestructive procedures are useful tools in managing refractory PCG after all other options have been tried, to reduce aqueous production. Results are unpredictable and complications exist. Laser cyclophotocoagulation (CPC) has largely replaced cyclocryotherapy, and diode laser is preferred to Nd:YAG laser due to decreased adverse events such as sympathetic ophthalmia. Transscleral and endoscopic application of laser are both options, with endoscopic preferred if the eye anatomy allows. Transscleral Micropulse-CPC may have less severe complications than traditional transscleral CPC and be as effective in children,[87] though further research is needed. The limbal anatomy may be distorted and blind application of transscleral CPC may be better guided with ultrasound biomicroscopy.[88] A general rule of thumb for all cyclodestructive procedures is to maintain 1-2 clock hours of untouched ciliary processes, even after repeated sessions, thus careful documentation of treated areas is recommended. Rare complications include hypotony, retinal detachment, visual loss, and phthisis.
Success for transscleral CPC ranges from 30-79% with retreatment in ~70% of patients, and has been comparable to implanting a second GDD in children. [89][90][91][92] Endoscopic cyclophotocoagulation (ECP) has been reported to be 64% successful at 1 year, and 16% by 5 years, with sequential ECP bringing the rate up to 81% at 1 year, and 34% at 5 years.[93][94]
Surgical follow up
In the short term, these patients require frequent follow up to follow response to treatment and monitor for hypotony, infection, and excessive inflammation. For young patients, or patients with less than 2 years of IOP control, follow-up is recommended at least every 3-4 months. Regular life-long follow-up is needed (at least every 6 months) because even if long-term IOP control from a surgical intervention is achieved, asymptomatic relapse can occur at any time and will need to be managed with medications or further surgery. Additionally, vision-threatening complications may occur at any time, especially after filtering surgeries.
Of note, GDD patency can be assessed with B-scan ultrasonography in the clinic.[95]
Complications
Complications for surgeries are described in the “Surgery” section.
Untreated IOP or delayed treatment in an infant eye may lead to severe complications and significant visual impairment in addition to permanent optic nerve damage and glaucomatous visual field defects. High IOP causes corneal edema and corneal stretching with development of Haab striae. With prolonged corneal edema, both diffuse and focal overlying Haab striae, the cornea can become permanently opacified. Buphthalmos with axial elongation, and Haab striae cause abnormally high refractive errors including myopia and astigmatism, that can impair vision both by blurring vision and causing refractive amblyopia, which can be exacerbated by anisometropia in unilateral cases. In severe buphthalmos, with continued stretching, the lens could dislocate, and risk of retinal complications increases (i.e. lacquer cracks and retinal detachments). Overcoming these complications can be difficult in severe cases. Corneal transplantation for corneal opacification is avoided if possible due to high risk of failure and complications in young children.
Prognosis
The prognosis for untreated PCG is almost always buphthalmos and blindness, except in the rare case of spontaneously arrested PCG. When PCG is being managed, the prognosis seems to depend on the age of onset, which depends on the severity of the angle anomaly. Eyes with more severe angle abnormalities tend to present earlier and to have worse prognosis compared with infantile-onset cases.[2] Interestingly, angle surgery is most successful in patients presenting between 2 months and 1 year of age (infantile-onset PCG) with 90% success, and less in newborn or late-recognized PCG with <50% success. In general, IOP control is obtained in greater than 80% of patients.[96] Visual prognosis depends on the degree of corneal scarring, anisometropia, refractive or deprivation amblyopia, and optic nerve damage. With maximal glaucoma and amblyopia therapy, one study found median VA of 20/60.[5][4] Strict attention to maximizing IOP control, correction of refractive errors and treatment of amblyopia is crucial. Early referral to visual assistance programs offered through the county or school system can be beneficial.
Consideration must also be given to the burden on caregivers, whose actions likely will affect the patient’s prognosis. Recent studies have highlighted that one-third of PCG caregivers could have moderate to severe depression, and quality of life is poorer for the caregiver if the patient is older and has had the disease longer.[97]
Additional Resources
- AAO, Basic and Clinical Science Course, Section 6: Pediatric Ophthalmology and Strabismus, 2019-2020, p 277-284.
- Ho, C L, Walton, D S. PCG: 2004 Update. JPOS 2004; 41:271-288.
- Weinreb R.N., Grajewski A.L., Papadopoulos M., Grigg J.,Freedman S.F. Childhood glaucoma: the 9th consensus report of the World Glaucoma Association. Netherlands: Kugler Publications, 2013.
- Patient Information: Glaucoma for Children by the American Association of Pediatric Ophthalmology and Strabismus
- American Academy of Ophthalmology. Congenital glaucoma, cloudy corneas. https://www.aao.org/image/congenital-glaucoma-cloudy-corneas-2 Accessed July 01, 2019.
References
- ↑ American Academy of Ophthalmology. Congenital glaucoma, cloudy corneas. https://www.aao.org/image/congenital-glaucoma-cloudy-corneas-2 Accessed July 01, 2019.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Weinreb R.N., Grajewski A.L., Papadopoulos M., Grigg J.,Freedman S.F. Childhood glaucoma: the 9th consensus report of the World Glaucoma Association. Netherlands: Kugler Publications, 2013.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Yu-Wai-Man C, Arno G, Brookes J, Garcia-Feijoo J, Khaw PT, Moosajee M. Primary congenital glaucoma including next-generation sequencing-based approaches: clinical utility gene card. Eur J Hum Genet. 2018;26(11):1713-8.
- ↑ 4.0 4.1 Neustein RF, Bruce BB, Beck AD. Primary Congenital Glaucoma Versus Glaucoma Following Congenital Cataract Surgery: Comparative Clinical Features and Long-term Outcomes. Am J Ophthalmol. 2016;170:214-22.
- ↑ 5.0 5.1 Biglan AW, Hiles DA. The visual results following infantile glaucoma surgery. J Pediatr Ophthalmol Strabismus. 1979;16(6):377-81.
- ↑ 6.0 6.1 6.2 6.3 Shah M, Bouhenni R, Benmerzouga I. Geographical Variability in CYP1B1 Mutations in Primary Congenital Glaucoma. J Clin Med. 2022 Apr 6;11(7):2048. doi: 10.3390/jcm11072048. PMID: 35407656; PMCID: PMC8999900.
- ↑ Duke-Elder S. Congenital Deformities. St Louis: CV Mosby; 1969. 548-65 p.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 DeLuise VP, Anderson DR. Primary infantile glaucoma (Congenital glaucoma). Surv Ophthalmol. 1983;28:1-18
- ↑ Ohtake Y, Tanino T, Suzuki Y, Miyata H, Taomoto M, Azuma N, et al. Phenotype of cytochrome P4501B1 gene (CYP1B1) mutations in Japanese patients with primary congenital glaucoma. Br J Ophthalmol. 2003;87(3):302-4.
- ↑ 10.0 10.1 Lewis CJ, Hedberg-Buenz A, DeLuca AP, Stone EM, Alward WLM, Fingert JH. Primary congenital and developmental glaucomas. Hum Mol Genet. 2017;26(R1):R28-R36.
- ↑ 11.0 11.1 Online Mendelian Inheritance in Man, OMIM (TM). An Online Catalog of Human Genes and Genetic Disorders Updated 20 January 2012 [Internet]. 2011.
- ↑ Bejjani BA, Xu L, Armstrong D, Lupski JR, Reneker LW. Expression patterns of cytochrome P4501B1 (Cyp1b1) in FVB/N mouse eyes. Exp Eye Res. 2002;75(3):249-57.
- ↑ Teixeira LB, Zhao Y, Dubielzig RR, Sorenson CM, Sheibani N. Ultrastructural abnormalities of the trabecular meshwork extracellular matrix in Cyp1b1-deficient mice. Vet Pathol. 2015;52(2):397-403.
- ↑ Williams AL, Eason J, Chawla B, Bohnsack BL. Cyp1b1 Regulates Ocular Fissure Closure Through a Retinoic Acid-Independent Pathway. Invest Ophthalmol Vis Sci. 2017;58(2):1084-97.
- ↑ Suri F, Yazdani S, Narooie-Nejhad M, Zargar SJ, Paylakhi SH, Zeinali S, Pakravan M, Elahi E. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 2009 Nov;116(11):2101-9. doi: 10.1016/j.ophtha.2009.04.045. Epub 2009 Sep 10. PMID: 19744731.
- ↑ Alghamdi A, Aldossary W, Albahkali S, Alotaibi B, Alrfaei BM. The loss of microglia activities facilitates glaucoma progression in association with CYP1B1 gene mutation (p.Gly61Glu). PLoS One. 2020 Nov 10;15(11):e0241902. doi: 10.1371/journal.pone.0241902. PMID: 33170892; PMCID: PMC7654781.
- ↑ 17.0 17.1 Schlotzer-Schrehardt U, Zenkel M, Kuchle M, Sakai LY, Naumann GO. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73(6):765-80.
- ↑ 18.0 18.1 Hyytiainen M, Keski-Oja J. Latent TGF-beta binding protein LTBP-2 decreases fibroblast adhesion to fibronectin. J Cell Biol. 2003;163(6):1363-74.
- ↑ Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280(9):7409-12.
- ↑ Souma T, Tompson SW, Thomson BR, Siggs OM, Kizhatil K, Yamaguchi S, et al. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest. 2016;126(7):2575-87.
- ↑ Thomson BR, Souma T, Tompson SW, Onay T, Kizhatil K, Siggs OM, et al. Angiopoietin-1 is required for Schlemm's canal development in mice and humans. J Clin Invest. 2017;127(12):4421-36.
- ↑ Qiao Y, Chen Y, Tan C, Sun X, Chen X, Chen J. Screening and Functional Analysis of TEK Mutations in Chinese Children With Primary Congenital Glaucoma. Front Genet. 2021 Dec 10;12:764509. doi: 10.3389/fgene.2021.764509. PMID: 34956319; PMCID: PMC8703195.
- ↑ Kaur K, Reddy AB, Mukhopadhyay A, Mandal AK, Hasnain SE, Ray K, et al. Myocilin gene implicated in primary congenital glaucoma. Clin Genet. 2005;67(4):335-40.
- ↑ Banerjee A, Chakraborty S, Chakraborty A, Chakrabarti S, Ray K. Functional and Structural Analyses of CYP1B1 Variants Linked to Congenital and Adult-Onset Glaucoma to Investigate the Molecular Basis of These Diseases. PLoS One. 2016;11(5):e0156252.
- ↑ Mookherjee S, Acharya M, Banerjee D, Bhattacharjee A, Ray K. Molecular basis for involvement of CYP1B1 in MYOC upregulation and its potential implication in glaucoma pathogenesis. PLoS One. 2012;7(9):e45077.
- ↑ Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, et al. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70(2):448-60.
- ↑ Kabra M, Zhang W, Rathi S, Mandal AK, Senthil S, Pyatla G, et al. Angiopoietin receptor TEK interacts with CYP1B1 in primary congenital glaucoma. Hum Genet. 2017;136(8):941-9.
- ↑ Worst JGF. The pathogenesis of congential glaucoma. An embryological and goniosurgical study. Springfield, Ill: Charles C. Thomas; 1966.
- ↑ Barkan O. Pathogenesis of congenital glaucoma: gonioscopic and anatomic observation of the angle of the anterior chamber in the normal eye and in congenital glaucoma. Am J Ophthalmol. 1955;40:1-11.
- ↑ 30.0 30.1 Pilat AV, Proudlock FA, Shah S, Sheth V, Purohit R, Abbot J, et al. Assessment of the anterior segment of patients with primary congenital glaucoma using handheld optical coherence tomography. Eye (Lond). 2019;33(8):1232-9.
- ↑ Sampaolesi R, Argento C. Scanning electron microscopy of the trabecular meshwork in normal and glucomatous eyes. Invest Ophthalmol Vis Sci. 1977;16(4):302-14.
- ↑ Maul E, Strozzi L, Munoz C, Reyes C. The outflow pathway in congenital glaucoma. Am J Ophthalmol. 1980;89(5):667-73.
- ↑ Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in congenital glaucoma. Am J Ophthalmol. 1981;92(4):508-25.
- ↑ 34.0 34.1 Garcia-Anton MT, Salazar JJ, de Hoz R, Rojas B, Ramirez AI, Trivino A, et al. Goniodysgenesis variability and activity of CYP1B1 genotypes in primary congenital glaucoma. PLoS One. 2017;12(4):e0176386.
- ↑ Maumenee AE. Further observations on the pathogenesis of congenital glaucoma. Trans Am Ophthalmol Soc. 1962;60:140-6.
- ↑ Anderson DR. The development of the trabecular meshwork and its abnormality in primary infantile glaucoma. Trans Am Ophthalmol Soc. 1981;79:458-85.
- ↑ Kupfer C, Kaiser-Kupfer MI. Observations on the development of the anterior chamber angle with reference to the pathogenesis of congenital glaucomas. Am J Ophthalmol. 1979;88(3 Pt 1):424-6.
- ↑ Williams AL, Eason J, Chawla B, Bohnsack BL. Cyp1b1 Regulates Ocular Fissure Closure Through a Retinoic Acid-Independent Pathway. Invest Ophthalmol Vis Sci. 2017;58(2):1084-97.
- ↑ Walton DS. Diagnosis and treatment of glaucoma in childhood. In: Epstein DL, editor. Chandler and Grant's Glaucoma. 3rd ed. Phila: Lea & Febinger; 1986.
- ↑ Morin JD, Bryars JH. Causes of loss of vision in congenital glaucoma. Arch Ophthalmol. 1980;98(9):1575-6.
- ↑ Kiskis AA, Markowitz SN, Morin JD. Corneal diameter and axial length in congenital glaucoma. Can J Opththalmol. 1985;20:93.
- ↑ Kessing SV, Gregersen E. The distended disc in early stages of congenital glaucoma. Acta Ophthalmol (Copenh). 1977;55(3):431-5.
- ↑ Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol. 1977;84(3):358-70.
- ↑ Ely AL, El-Dairi MA, Freedman SF. Cupping reversal in pediatric glaucoma--evaluation of the retinal nerve fiber layer and visual field. Am J Ophthalmol. 2014;158(5):905-15.
- ↑ Fayed MA, Chen TC. Pediatric intraocular pressure measurements: Tonometers, central corneal thickness, and anesthesia. Surv Ophthalmol. 2019.
- ↑ Eisenberg DL, Sherman BG, McKeown CA, Schuman JS. Tonometry in adults and children. A manometric evaluation of pneumatonometry, applanation, and TonoPen in vitro and in vivo. Ophthalmology. 1998;105(7):1173-81.
- ↑ Grigorian F, Grigorian AP, Olitsky SE. The use of the iCare tonometer reduced the need for anesthesia to measure intraocular pressure in children. J Aapos. 2012;16(6):508-10.
- ↑ Flemmons MS, Hsiao YC, Dzau J, Asrani S, Jones S, Freedman SF. Icare rebound tonometry in children with known and suspected glaucoma. J Aapos. 2011;15(2):153-7.
- ↑ McKee EC, Ely AL, Duncan JE, Dosunmu EO, Freedman SF. A comparison of Icare PRO and Tono-Pen XL tonometers in anesthetized children. J aapos. 2015;19(4):332-7.
- ↑ Gupta S, Mahalingam K, Singh A, Selvan H, Somarajan BI, Gupta V. Posterior corneal morphological changes in primary congenital glaucoma. Indian J Ophthalmol. 2022 Jul;70(7):2571-2577. doi: 10.4103/ijo.IJO_317_22. PMID: 35791159.
- ↑ Sampaolesi R, Caruso R. Ocular Echometry in the Diagnosis of Congenital Glaucoma. Arch Ophthalmol. 1982;100(4):574–577.
- ↑ Henriques MJ, Vessani RM, Reis FA, de Almeida GV, Betinjane AJ, Susanna R, Jr. Corneal thickness in congenital glaucoma. J Glaucoma. 2004;13(3):185-8.
- ↑ 53.0 53.1 Doozandeh A, Yazdani S, Ansari S, Pakravan M, Motevasseli T, Hosseini B, Yasseri M. Corneal profile in primary congenital glaucoma. Acta Ophthalmol. 2017;95(7):e575-e581.
- ↑ 54.0 54.1 Zareei A, Razeghinejad MR, Salouti R. Corneal Biomechanical Properties and Thickness in Primary Congenital Glaucoma and Normal Eyes: A Comparative Study. Med Hypothesis Discov Innov Ophthalmol. 2018;7(2):68-72.
- ↑ Donahue SP, Porter A. SITA visual field testing in children. J AAPOS. 2001;5:114.
- ↑ Salchow DJ, Oleynikov YS, Chiang MF, Kennedy-Salchow SE, Langton K, Tsai JC, et al. Retinal nerve fiber layer thickness in normal children measured with optical coherence tomography. Ophthalmology. 2006;113(5):786-91.
- ↑ Ahn HC, Son HW, Kim JS, Lee JH. Quantitative analysis of retinal nerve fiber layer thickness of normal children and adolescents. Korean J Ophthalmol. 2005;19(3):195-200.
- ↑ Hess DB, Asrani SG, Bhide MG, Enyedi LB, Stinnett SS, Freedman SF. Macular and retinal nerve fiber layer analysis of normal and glaucomatous eyes in children using optical coherence tomography. Am J Ophthalmol. 2005;139(3):509-17.
- ↑ Rao A, Sahoo B, Kumar M, Varshney G, Kumar R. Retinal nerve fiber layer thickness in children <18 years by spectral-domain optical coherence tomography. Semin Ophthalmol. 2013;28(2):97-102.
- ↑ Rotruck JC, House RJ, Freedman SF, Kelly MP, Enyedi LB, Prakalapakorn SG, et al. Optical Coherence Tomography normative peripapillary retinal nerve fiber layer and macular data in children ages 0-5 years. Am J Ophthalmol. 2019.
- ↑ Hsu ST, Chen X, Ngo HT, House RJ, Kelly MP, Enyedi LB, et al. Imaging Infant Retinal Vasculature with OCT Angiography. Ophthalmol Retina. 2019;3(1):95-6.
- ↑ Pearce WG. Autosomal dominant megalocornea with congenital glaucoma: evidence for germ-line mosaicism. Can J Ophthalmol. 1991;26(1):21-6.
- ↑ Greco, A. et al., 2022, 'Childhood Glaucoma and Medical Treatment: An Up to Date', in G. L. Giudice (ed.), Vision Correction and Eye Surgery, IntechOpen, London. 10.5772/intechopen.100579.
- ↑ Coppens G, Stalmans I, Zeyen T, Casteels I. The safety and efficacy of glaucoma medication in the pediatric population. J Pediatr Ophthalmol Strabismus. 2009 Jan-Feb;46(1):12-8. doi: 10.3928/01913913-20090101-05. PMID: 19213271.
- ↑ Buckley MM, Goa KL, Clissold SP. Ocular Betaxolol. A Review of Its Pharmacological Properties, and Therapeutic Efficacy in Glaucoma and Ocular Hypertension. Drugs. 1990;40:75-90.
- ↑ Maren TH, Haywood JR, Chapman SK, Zimmerman TJ. The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci. 1977;16(8):730-742.
- ↑ Capino AC, Dannaway DC, Miller JL. Metabolic Acidosis with Ophthalmic Dorzolamide in a Neonate. J Pediatr Pharmacol Ther. 2016;21(3):256-9.
- ↑ Maeda-Chubachi T, Chi-Burris K, Simons BD, Freedman SF, Khaw PT, Wirostko B, et al. Comparison of latanoprost and timolol in pediatric glaucoma: a phase 3, 12-week, randomized, double-masked multicenter study. Ophthalmology. 2011;118(10):2014-21.
- ↑ Kim JS, Blizzard S, Woodward JA, Leyngold IM, Liss J, Freedman SF. Prostaglandin-Associated Periorbitopathy in Children and Young Adults with Glaucoma. Ophthalmol Glaucoma. In Press. Available online 3 April 2020.
- ↑ El Sayed Y, Esmael A, Mettias N, El Sanabary Z, Gawdat G. Factors influencing the outcome of goniotomy and trabeculotomy in primary congenital glaucoma. Br J Ophthalmol. [Online ahead of print]
- ↑ Huang H, Bao WJ, Yamamoto T, Kawase K, Sawada A. Postoperative outcome of three different procedures for childhood glaucoma. Clin Ophthalmol. 2019;13:1-7.
- ↑ Mandal AK, Gothwal VK, Nutheti R. Surgical outcome of primary developmental glaucoma: a single surgeon's long-term experience from a tertiary eye care centre in India. Eye. 2007;21(6):764-74.
- ↑ Khalil DH, Abdelhakim MA. Primary trabeculotomy compared to combined trabeculectomy-trabeculotomy in congenital glaucoma: 3-year study. Acta Ophthalmol. 2016;94(7):e550-e4.
- ↑ Jayaram H, Scawn R, Pooley F, Chiang M, Bunce C, Strouthidis NG, et al. Long-Term Outcomes of Trabeculectomy Augmented with Mitomycin C Undertaken within the First 2 Years of Life. Ophthalmology. 2015;122(11):2216-22.
- ↑ Khaw PT, Chiang M, Shah P, Sii F, Lockwood A, Khalili A. Enhanced trabeculectomy: the Moorfields Safer Surgery System. Dev Ophthalmol. 2012;50:1-28.
- ↑ Low S, Hamada S, Nischal KK. Antimetabolite and releasable suture augmented filtration surgery in refractory pediatric glaucomas. J Aapos. 2008;12(2):166-72.
- ↑ Beck AD, Wilson WR, Lynch MG, Lynn MJ, Noe R. Trabeculectomy with adjunctive Mitomycin c in pediatric glaucoma. Am J Ophthalmol. 1998;126:648-57.
- ↑ Margeta MA, Kuo AN, Proia AD, Freedman SF. Staying away from the optic nerve: a formula for modifying glaucoma drainage device surgery in pediatric and other small eyes. J aapos. 2017;21(1):39-43.e1.
- ↑ Ishida K, Mandal AK, Netland PA. Glaucoma Drainage Implants in Pediatric Patients. Ophthalmol Clin North Am. 2005;18:431-42.
- ↑ Tung I, Marcus I, Thiamthat W, Freedman SF. Second glaucoma drainage devices in refractory pediatric glaucoma: failure by fibrovascular ingrowth. Am J Ophthalmol. 2014;158(1):113-7.
- ↑ Cunliffe IA, Molteno AC. Long-term follow-up of Molteno drains used in the treatment of glaucoma presenting in childhood. Eye. 1998;12(Pt 3a):379-85.
- ↑ Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with Mitomycin-C for children in the first two years of life. Am J Ophthalmol. 2003;136(6):994-1000.
- ↑ Tai AX, Song JC. Surgical outcomes of Baerveldt implants in pediatric glaucoma patients. J AAPOS. 2014;18(6):550-3.
- ↑ Mandalos A, Tailor R, Parmar T, Sung V. The Long-term Outcomes of Glaucoma Drainage Device in Pediatric Glaucoma. J Glaucoma. 2016;25(3):e189-95.
- ↑ Razeghinejad MR, Kaffashan S, Nowroozzadeh MH. Results of Ahmed glaucoma valve implantation in primary congenital glaucoma. J aapos. 2014;18(6):590-5.
- ↑ Pakravan M, Esfandiari H, Yazdani S, Doozandeh A, Dastborhan Z, Gerami E, et al. Clinical outcomes of Ahmed glaucoma valve implantation in pediatric glaucoma. Eur J Ophthalmol. 2019;29(1):44-51.
- ↑ Abdelrahman AM, El Sayed YM. Micropulse Versus Continuous Wave Transscleral Cyclophotocoagulation in Refractory Pediatric Glaucoma. J Glaucoma. 2018;27(10):900-5.
- ↑ Way AL, Nischal KK. High-frequency ultrasound-guided transscleral diode laser cyclophotocoagulation. Br J Ophthalmol. 2014;98(7):992-4.
- ↑ Bock CJ, Freedman SF, Buckley EG, Shields MB. Transscleral diode laser cyclophotocoagulation for refractory pediatric glaucomas. J Pediatr Ophthalmol Strabismus. 1997;34(4):235-9.
- ↑ Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109(2):316-23.
- ↑ Autrata R, Rehurek J. Long-term results of transscleral cyclophotocoagulation in refractory pediatric glaucoma patients. Ophthalmologica. 2003;217(6):393-400.
- ↑ Sood S, Beck AD. Cyclophotocoagulation versus sequential tube shunt as a secondary intervention following primary tube shunt failure in pediatric glaucoma. J Aapos. 2009;13(4):379-83.
- ↑ Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J Aapos. 2001;5(4):221-9.
- ↑ Glaser TS, Mulvihill MS, Freedman SF. Endoscopic cyclophotocoagulation (ECP) for childhood glaucoma: a large single-center cohort experience. J AAPOS. 2019;23(2):84 e1- e7.
- ↑ Baig NB, Lin AA, Freedman SF. Ultrasound evaluation of glaucoma drainage devices in children. J AAPOS. 2015;19(3):281-4.
- ↑ Shaffer RN. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis). Trans Am Ophthalmol Soc. 1982;80:321-5.
- ↑ Kantipuly A, Pillai MR, Shroff S, Khatiwala R, Raman GV, Krishnadas SR, et al. Caregiver Burden in Primary Congenital Glaucoma. Am J Ophthalmol. 2019.