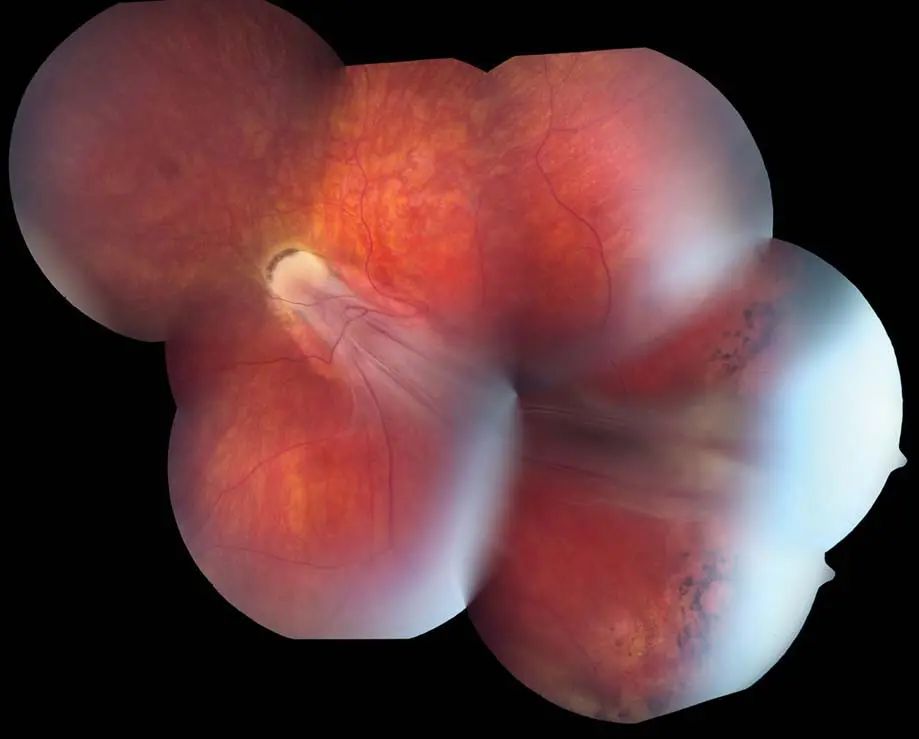
Retinopathy of Prematurity
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Retinopathy of prematurity (ROP), initially described as "retrolental fibroplasia" by Theodore Terry in 1942, was the leading cause of blindness in children in the United States (US).[2] To date, 3 "epidemics" of blindness due to ROP have been described.[3][4] The first epidemic occurred in the 1940s and 1950s in industrialized countries, primarily due to unmonitored supplemental oxygen. Regulation and monitoring of high oxygen at birth caused ROP to virtually disappear, but as a result of advances in neonatal care, premature infants survived at earlier gestational ages and lower birth weights, and ROP re-emerged to be a serious problem, leading to the second epidemic that began in the 1970s in industrialized countries. Then in the mid-1990s, the third epidemic began in low- and middle-income countries (initially in Eastern Europe and Latin America, spreading to East and South Asia and then to sub-Saharan Africa) due to both high rates of preterm birth and varying levels of neonatal care in these countries. Some countries or regions within countries lack the technology and resources to optimize their care, and often ROP is seen in larger and older infants exposed to unregulated oxygen (similar to what had occurred in the US decades earlier).[5] In the US and other high-income countries, ROP affects extremely premature infants and involves incomplete vascularization of the retina as well as oxygen-induced damage, though the latter is believed to play less of a role now.[6] Therefore, the manifestation and timing of ROP differs greatly throughout the world.
Despite advances in oxygen management in high income countries, however, recent data from the IRIS registry shows that ROP remains the leading cause of vision loss in the United States.[7]
Etiology
In utero, the fetus is in a hypoxic state. When infants are born prematurely, the relative oxygen level is sometimes increased even when oxygenation is at ambient levels. High supplemental oxygen can be damaging to capillaries.
The cause of ROP is premature birth and additional factors that cause a mismatch between normal retinal vascularization and the oxygen need of the developing retina. There is also consideration of maternal factors affecting nutrition and inflammation/infection.
Risk Factors
Key Risk Factors (in high-income countries)
- Low birthweight (<1500 grams*)
- Gestational age of <30 weeks*
- High, unregulated oxygen at birth and/or fluctuations in oxygenation
- Poor postnatal growth
*May need to be modified for lower-income countries.
Other Proposed Risk Factors
- Respiratory distress syndrome, duration of mechanical ventilation, and steroid use for bronchopulmonary dyplasia; considered risk factors for the development of ROP[8][9]
- Bronchopulmonary dyplasia; considered a risk factor for the progression of ROP[10]
- CNS injuries: intraventricular hemorrhage, periventricular leukomalacia; considered risk factors for the progression of ROP[11][12]
- Low plasma immunoglobulin factor 1 (IGF-1) levels: IGF-1 is thought to mediate vascular endothelial growth factor (VEGF); hence, low plasma IGF-1 levels may be a potential risk factor for the development of ROP[11]
- Hyperglycemia[13]
- Sepsis, White race, blood transfusion, and multiple births[9]
- Proteinuria due to shared embryogenesis between retinal vascularity and renal function[11]
General Pathology
In histologic studies from the 1970s of infants with retrolental fibroplasia/ROP, the earliest lesions seen in the acute phase were arteriovenous shunts. Neovascularization that may penetrate the vitreous, microvascular changes (including tufting), and attenuation of capillaries around the arteries and veins were also noted.[14] However, it remains unknown if shunts are early warning signs of more severe disease in cases of currently classified treatment-warranted (type 1).
Pathophysiology
Retinopathy of prematurity occurs in premature infants who are born before the retinal vessels complete their normal growth, and occurs in 2 phases:
- Delays in physiologic retinal vascular development that leads to damages in newly developed capillaries. Occurs in the setting of oxygen stresses and other stresses (described below)
- Aberrant neovascularization that grows into the vitreous instead of the retina
Normal retinovascular development in humans is believed to occur initially through vasculogenesis, or de novo formation of vessels from precursor endothelial cells, before and at about 14–16 weeks of gestation, vascularizing the posterior pole through 22 weeks of gestation. Following vasculogenesis, angiogenesis occurs via budding from existing vessels to extend the retinal vessels into the periphery and the other plexi. The vascularization of the deeper plexi is associated with Müller cells in human. In mice, astrocytes may also play a role in sensing physiologic hypoxia and upregulating VEGF. Ensuing migrating endothelial cells are attracted by a gradient of VEGF toward the ora serrata.[15]
In a representative animal model of ROP that recapitulated stresses to premature infants,[16] regulation of signaling through the VEGF receptor 2 (VEGFR2) specifically restored the orientation of dividing endothelial cells to allow them to grow in an ordered fashion toward the ora serrata.[17] This discovery showed that inhibition of an overactivated angiogenic pathway through VEGFR2 in endothelial cells caused abnormal vascularization into the vitreous and interfered with normal retinal vascular development. Regulation of the VEGFR2 pathway not only inhibited intravitreal and extraretinal neovascularization but also facilitated angiogenesis into the peripheral retina.[18][19] This process is different from the pathophysiology of many adult retinovascular diseases. [20] Clinical studies have attempted to regulate VEGFR2 signaling in endothelial cells using intravitreal neutralizing antibodies to VEGF, because these can be delivered safely in the premature infant eye with intravitreal injections. However, the intravitreal delivery of an antibody or fusion protein that binds the ligand (VEGF) does not allow for specific regulation of VEGFR2 in endothelial cells, since VEGF receptors on glia and neural cells are also affected. An additional study in a representative model showed that intravitreal neutralizing antibody to VEGFA led to retinal capillary dropout after oxygen stresses, followed by reactivation of neovascularization into the vitreous.[21] This is similar to what happens in some infant eyes.[22] In addition, reduced expression of VEGFA in the experimental model caused thinning of the retinal layers, whereas reduction in only some of the forms of VEGF did not lead to retinal thinning.[23] This research led to the idea to pursue studies identifying an appropriate dose of intravitreal anti-VEGF medications that would be effective and safe.[24] Recently, a clinical study compared infants with type 1 ROP, who received bilateral intravitreal bevacizumab (IVB) 0.25 mg, to an untreated control group of infants with less severe RP matched by sex, degree of prematurity, and postmenstrual age (PMA). Vascularization into the peripheral avascular retina was measured on retinal images taken with the same contact camera. The treated group all responded with ROP regression and had greater extension of retinal vascularization peripherally than the less severe, untreated group.[25] This study provides evidence supporting the basic research and suggests that inhibition of vitreous VEGF may allow linear intraretinal blood vessel development to extend to the ora serrata and reduce neovascularization into the vitreous. Long-term and additional studies are needed, including for safety.
In ROP, premature birth delays the normal process of retinal vascularization. Other factors, such as oxygen-induced vascular injury, also play a role. Risk factors for ROP can include high oxygen at birth, fluctuations in oxygenation, poor postnatal growth, poor nutrition, and possible oxidative stress. The role of oxygen in the causation of ROP is complex. Some studies have shown that keeping the oxygen saturation at a lower level from birth can reduce the rate of advanced ROP, but other studies have found increased mortality rates following lower oxygen saturation.[26]
Primary Prevention
Screenings of infants at risk with appropriate timing of exams and follow up is essential to identify infants in need of treatment.[9] It is important to recognize that screening recommendations may vary by location. For example, in some countries in Asia, ROP can occur in babies of older gestational age or larger birth weight.[27]
The text and table below summarize the current recommendations for ROP screening in the US.[28]
- Low birthweight (≤1500 g)
- Gestational age ≤30 weeks
- Birthweight between 1500 and 2000 g or gestational age >30 weeks, but believed by their pediatrician or neonatologist to be at risk for ROP (e.g., history of hypotension requiring inotropic support; received supplemental oxygen for more than a few days or without oxygen saturation monitoring)
Recommendations published by a multisociety consortium in 2018 state that infants should be screened "by an ophthalmologist who is experienced in the examination of preterm infants for ROP using a binocular indirect ophthalmoscope."[28]
| Gestational Age at Birth | Postmenstrual Age (weeks) | Chronologic Age (weeks) |
|---|---|---|
| 22 weeks | 31 | 9, but consider earlier screening per clinical judgment |
| 23 weeks | 31 | 8, but consider earlier screening per clinical judgment |
| 24 weeks | 31 | 7 |
| 25 weeks | 31 | 6 |
| 26 weeks | 31 | 5 |
| 27 weeks | 31 | 4 |
| 28 weeks | 32 | 4 |
| 29 weeks | 33 | 4 |
| 30 weeks | 34 | 4 |
| >30 weeks with high risk factors | - | 4 |
Diagnosis
The International Committee for Classification of Retinopathy of Prematurity (ICROP) developed a diagnostic classification of ROP in 1984.[29] It has since has been further refined, most recently in 2021 with the International Classification of Retinopathy of Prematurity, 3rd edition (ICROP3).[30][31][22] These diagnostic classifications define ROP by location (zone), severity (stage) and vascular characteristics in the posterior pole (normal, pre-plus, or plus disease).[31]
Location (Zone)
To define the location, 3 concentric zones were established based on the most posterior zone, as the retina may be vascularized to different extents in different regions of the retina (i.e., nasal vs temporal vs superior vs inferior). Since retinal vascular development proceeds from the optic nerve to the ora serrata, the zones are centered on the optic disc rather than the macula.
Zone I: The area defined by a circle centered on the optic nerve, the radius of which extends from the center of the optic disc to twice the distance from the center of the optic disc to the center of the macula.
Zone II: The area extending centrifugally from the edge of zone I to a circle with a radius equal to the distance from the center of the optic disc to the nasal ora serrata.
- Posterior Zone II: A region of 2 disc diameters peripheral to the zone I border. This was an addition in ICROP3 to allow for nuance in characterizing more posterior disease, which is often more aggressive.
Zone III: The residual temporal crescent of retina anterior to zone II. By convention, zones II and III are considered to be mutually exclusive.
ICROP3 also added the notion of a “notch” in describing the location of ROP, which is an incursion by the ROP lesion of 1 to 2 clock hours from one zone into another.”[22] If present, it should be documented by the most posterior zone with the qualifier "secondary to notch." For example, if most of the vascularization is in zone II with a notch that extends into zone I, the location would be “zone I secondary to notch.”
Disease Severity (Stage)
Prior to the development of ROP in the premature infant, vascularization of the retina is "incomplete."
More than one stage may be present in the same eye. However, staging for the eye as a whole is determined by the most severe stage present.
Stage 1: Demarcation Line: This line is thin and flat (in the retina plane) and separates the avascular retina anteriorly from the vascularized retina posteriorly.
Stage 2: Ridge: The ridge arises from the demarcation line and has height and width, which extends above the plane of the retina. The ridge may change from white to pink and vessels may leave the plane of the retina posterior to the ridge to enter it. Small, isolated tufts of neovascular tissue lying on the surface of the retina, commonly called "popcorn," may be seen posterior to this ridge structure but do not constitute the degree of fibrovascular growth that is a necessary condition for stage 3.
Stage 3: Extraretinal Fibrovascular Proliferation: Intravitreal neovascularization, or that which extends from the ridge into the vitreous. This extraretinal proliferating tissue is continuous with the posterior aspect of the ridge, causing a ragged appearance as the proliferation becomes more extensive. Seemingly flat-appearing extraretinal neovascularization can occur in eyes with zone I or posterior zone II disease, in the absence of an obvious ridge or demarcation line.
Stage 4: Partial Retinal Detachment: In the initial ICROP classification, this was the final stage and was known as the cicatricial phase.[29] It was later divided into stage 4A (extrafoveal partial retinal detachments) and stage 4B (foveal partial retinal detachments). Stage 4 retinal detachments are generally concave and most are circumferentially oriented. Retinal detachments usually begin at the point of fibrovascular attachment to the vascularized retina, and the extent of detachment depends on the amount of neovascularization present.[30] They can be exudative or tractional.
Stage 5: Total Retinal Detachment: Retinal detachments are generally tractional and usually funnel-shaped. The configuration of the funnel itself is used for subdivision of this stage, depending on if the anterior and posterior portions are open or narrowed.[30] ICROP3 recommends subcategorizing stage 5 into 3 configurations: stage 5A, when the optic disc is visible by ophthalmoscopy; stage 5B, when the optic disc is not visible secondary to retrolental fibrovascular tissue or closed-funnel detachment; and stage 5C:, which contain stage 5B findings accompanied by anterior segment abnormalities (e.g., anterior lens displacement, marked anterior chamber shallowing, iridocapsular adhesions, capsule-endothelial adhesions with central corneal opacification).[22] Ultrasonography (B-scan) can be useful for the classification of stage 5B and 5C ROP, but it is not necessary.
Aggressive ROP (A-ROP)
Aggressive ROP (A-ROP) is a new category initially presented in ICROP3. A-ROP includes aggressive posterior ROP, which was first recognized in the international classification in 2005 to indicate a rapidly progressing, posterior form of ROP that can bypass the typical progression of stages ("rush disease".[31] A-ROP includes peripheral features such as vascular loops and areas of avascular retina, sometimes without obvious demarcation lines or ridges. Fundus fluorescein angiography may delineate the vascular changes more clearly in this disease.[32]
With ICROP3, the term aggressive ROP (A-ROP) replaced aggressive-posterior ROP "because of increasing recognition that aggressive disease may occur in larger preterm infants and beyond the posterior retina, particularly in regions of the world with limited resources."[22] The key diagnostic features of A-ROP are, according to ICROP3, "the tempo of disease and appearance of vascular abnormalities, but not location of disease. Eyes with A-ROP often demonstrate a form of stage 3 disease that may appear as deceptively featureless networks of so-called flat neovascularization, though the extra retinal neovascularization of classic stage 3 ROP also can be seen.[22]
Extent
The extent of disease is recorded as hours of the clock or as 30° sectors. As the observer looks at each eye, the 3-o’clock position is to the right and nasal in the right eye and temporal in the left eye, and the 9-o’clock position is to the left and temporal in the right eye and nasal in the left eye.[29] Extent is useful in stages 4 and 5 ROP, but in general is no longer used in the diagnosis of treatment-warranted (type 1) ROP.
Vascular Characteristics in the Posterior Pole/Zone I (normal, pre-plus or plus disease)
Plus Disease Spectrum
In ICROP3, the recommendation is to evaluate the vessels within zone I.[22] ICROP3 emphasizes that the terms below should be thought of as "a continuous spectrum of retinal vascular changes," [22] In this spectrum of abnormal dilatation and tortuosity, plus disease is the most severe form.
Pre-Plus Disease
Pre-plus disease has been described as vascular abnormalities of the posterior pole that are insufficient for the diagnosis of plus disease, but that demonstrate more arterial tortuosity and more venous dilatation than normal.[31] In ICROP3, these vascular abnormalities can either demonstrate more arterial tortuosity or more venous dilatation than normal.[22]
Plus Disease
In the original ICROP classification, plus disease was characterized by additional signs of increased venous dilatation and arteriolar tortuosity of the posterior retinal vessels, which can increase in severity to include iris vascular engorgement, poor pupillary dilatation, and vitreous haze.[29] Thus, all patients with suspected ROP should be seen, including those with poor dilation of pupils after topical mydriatics, to rule out plus disease and more importantly A-ROP.[22]
The new recognition of plus disease being on a spectrum reduces the rigidity of the use of standard photos, as advocated in previous clinical trials. The ICROP3 criteria requires at least 2 quadrants with vascular dilatation and tortuosity.[33]
Later Phases of ROP (regression and reactivation)
Regression
A term introduced in ICROP3, regression refers to disease involution and resolution. Regression may be complete or incomplete, and may include persistence of retinal abnormalities.[22] Signs of vascular regression include decreased plus disease, increased vascularization into the peripheral avascular retina, involution of the tunica vasculosa lentos, better pupillary dilation, greater media clarity, and resolution of intraretinal hemorrhages. Regression is characterized by thinning and whitening of neovascular tissue.
Reactivation
ICROP3 also introduced the term reactivation, which refers to recurrence of acute-phase features but not necessarily recurrence of type 1 ROP.[22] Reactivation may occur after incomplete or complete regression of the original ROP and is seen more frequently after anti-VEGF treatment than after spontaneous regression. Reactivated disease may not progress through the normal sequence of stages of acute-phase disease. Vascular reactivation includes the recurrence of pre-plus or plus disease. Extraretinal new vessels can occur and may be relatively delicate compared with those of acute ROP. Hemorrhages can occur around fronds of extraretinal vessels. Alternatively, extraretinal vessels may appear as a fibrovascular ridge, which can progress to fibrosis, contraction, and tractional detachment. These forms of progressive stage 4 ROP can involve fibrosis at the original ridge that regressed and also have some features similar to those seen after laser treatment.[34][35] Documentation of reactivation should specify the presence and location(s) of new ROP features, noted by zone and stage using the modifier "reactivated." If multiple ridges are present, "reactivated" is applied to the more anterior ridge, which is typically more active.
Persistent Avascular Retina
ICROP3 described persistent avascular retina (PAR) as cases of incomplete vascularization of the peripheral avascular retina. It is described by both its location (e.g., posterior zone II) and extent (e.g., nasal).[22] It can occur spontaneously or after vascularization into the peripheral avascular retina, a feature more recognized now with the increased use of anti-VEGF therapy. PAR may be a risk factor for delayed reactivation after anti-VEGF therapy, and cases of reactivation leading to retinal detachment have been reported in toddlers.[36][37] Retinal neovascularization and vitreous hemorrhage have even been reported 10 years after IVB treatment (without laser ablation therapy).[38] Laser ablation of PAR removes the opportunity for vascularization of the peripheral avascular retina and potential visual field expansion, although delaying laser until older PMAs may minimize this effect.
Diagnostic Procedures
Following pupillary dilation using eye drops, the retina is examined with an indirect ophthalmoscope. The peripheral portions of the retina are pushed into view using scleral depression. After examination, either separate sterile equipment or appropriate cleaning protocols should be used to avoid possible cross-contamination by infectious agents.[39] When using dilation drops, be aware of possible adverse effects to the cardiorespiratory and gastrointestinal system of the infant and use the lowest doses needed to minimize side effects.
Artificial intelligence (AI) is increasingly being studied for potential roles in ROP screening.[11] Smartphone-based screening methods are also being explored as a low-cost alternative for lower-income settings.[40]
Differential Diagnosis
- Familial exudative vitreoretinopathy: A genetic disorder that appears similar to ROP but occurs in full-term infants. It may also present early within the first week of life.[41] For about 50% of patients, genetic counseling and testing of family members can aid in identifying gene variants
- Persistent fetal vasculature: A cause of tractional retinal detachment which may be difficult to differentiate from ROP but is typically unilateral and is not correlated with prematurity
- Incontinentia pigmenti: A genetic syndrome associated with dermatologic, central nervous system (CNS), and dental abnormalities. When noted, retinal abnormalities often include avascularity, neovascularization, and exudative and tractional retinal detachments.
- Coats disease: A sporadic X-linked condition which may lead to total exudative retinal detachment. Often unilateral and found in males.[42]
- Cutis marmorata telangiectatica congenita: A rare capillary malformation with skin and CNS manifestations. When present, ocular findings include peripheral retinal neovascularization and glaucoma, but the most common anomaly is body asymmetry[43][44][45]
- Norrie disease: A rare X-linked recessive disorder with fibrovascular changes. It appears similar to ROP but is also associated with progressive hearing loss. Ocular findings, which include microophthalmia, are typically bilateral and symmetric. The disease appears at birth and progresses throughout infancy.
Management
Follow-Up Intervals
Preterm infants meeting screening criteria should have retinal exams performed by ophthalmologists with adequate training in ROP management. There is increasing use of obtaining retinal images by trained personnel that are then reviewed by ophthalmologists.[46] The initial exam should be based on the infant’s age (see Table). Follow-up recommendations were updated in 2019 by the American Academy of Pediatrics and depend on the location and stage of ROP.[28]
The timings of follow-up examinations are based on retinal exam findings as classified by ICROP3.[28] [31]
- Recommended follow-up visit within 1 week:
- Zone I: immature vascularization, stage 1 or 2 ROP
- Posterior zone II: immature vascularization
- Suspected presence of A-ROP
- Recommended follow-up visit in 1–2 weeks:
- Zone I: unequivocally regressing ROP
- Posterior zone II: immature vascularization
- Zone II: stage 2 ROP
- Recommended follow-up visit in 2 weeks:
- Zone II: immature vascularization, stage 1 ROP, or unequivocally regressing ROP
- Recommended follow-up visit in 2–3 weeks:
- Zone II: regressing ROP
- Zone III: stage 1 or 2 ROP
Termination of acute retinal screening examinations is based on age and retinal findings. Examinations can be stopped in these cases:
- Retina is fully vascularized
- Zone III retinal vascularization without previous ROP in zones I or II (may need a confirmatory exam if PMA <35 weeks)
- PMA = 45 weeks and no type 1 ROP (i.e. ,"pre-threshold disease", defined as stage 3 ROP in zone II or any ROP in zone I, or worse ROP)
- If previously treated with anti-VEGF injections, follow until at least PMA =65 weeks (FYI: infant will need close follow-up during the time of highest risk for disease reactivation [PMA = 45–55 weeks])[28]
- ROP has fully regressed (ensure there is no abnormal vascular tissue present that can reactivate and progress)
After acute retinal screening examinations are terminated, preterm infants should be seen within 4–6 months after discharge from the neonatal intensive care unit for vision development. Preterm infants are at increased risk for developing strabismus, amblyopia, high refractive error, cataract, and glaucoma.
Treatment
The first surgical treatment for ROP accepted to be safe and effective was cryotherapy to the avascular retina, as designated by the CRYO- ROP study in 1986. This reduced unfavorable structural outcomes in eyes with threshold ROP.[8] Threshold ROP was defined as 5 contiguous or 8 cumulative clock hours of stage 3 ROP in zone I or zone II with plus disease.[47]
Subsequently, argon and diode lasers were used to treat the avascular retina of threshold ROP. Lasers were an improvement over cryotherapy since they were more portable, better tolerated, and less damaging than cryotherapy.[48]
Currently, ROP treatment guidelines are based on the Early Treatment of Retinopathy of Prematurity (ETROP) Study.[49]
Laser Photocoagulation
Laser photocoagulation treatment is currently recommended for the following (defined as "type 1" ROP):
- Zone I: any-stage ROP with plus disease or stage 3 ROP without plus disease
- Zone I: stage 3 ROP without plus disease
- Zone II: stage 2 or 3 ROP with plus disease
Eyes meeting these criteria should be treated as soon as possible, ideally within 72 hours. However, increasingly eyes with zone I, type 1 ROP are being treated with anti-VEGF agents. The number of clock hours of disease is no longer a determining factor for treatment.
Anti-VEGF Agents
Anti-VEGF treatment has shown promise for the treatment of stage 3 ROP with plus disease in zone I (not zone II).[50] Recent clinical trials have been performed to test de-escalating doses of bevacizumab[24] or ranibizumab[51] for type 1 ROP. Treatment efficacy was seen with lower bevacizumab doses and with ranibizumab 0.2 mg.[24][51] Treatment with aflibercept in the FIREFLEYE study has also been found to be beneficial[52] but was found to be noninferior to laser in clinical trials.[53]
Post-treatment Monitoring
Follow-up is recommended in 3–7 days following either laser photocoagulation or anti-VEGF injections to monitor for reductions in retinal dilation, tortuosity, and/or stage 3 ROP.[28] Reduction in features can be seen within a week. Following anti-VEGF injections, close monitoring is needed in case endophthalmitis and other complications, including damage to the retina or lens, occur. Eyes must be watched carefully for ROP regression and reactivation. Very late recurrences of proliferative ROP have been reported following anti-VEGF therapy.
Despite treatment, some eyes will progress. In the CRYO-ROP study, approximately 30% of eyes progressed to posterior pole macular fold or retinal detachment.[33] In these situations, vitreoretinal surgery may be needed. At the reported 15-year outcome from the CRYO-ROP study, new retinal folds, detachments, or obscuring the posterior pole occurred in 4.5% of eyes given treatment and 7.7% of eyes in the control group; thus, it was recommended that eyes that experience threshold ROP have long-term, regular follow-up.[54]
Additional monitoring is necessary for the fibrovascular progression of ROP sometimes seen after anti-VEGF injections or laser treatments.[55] Progressive stage 4 ROP after laser is predicted by clock-hour extent of changes at the ridge, vitreous condensation, and persistent or new plus disease. Similar findings may be present after anti-VEGF treatment, but these changes can occur at the original ridge and around the optic nerve.[34] [22] Stage 4 and 5 ROP may require vitreous surgery by a pediatric retina-trained surgeon. Surgery is performed with the intent of preserving the natural lens whenever possible and to address the vitreoretinal adhesions that create the complex tractional detachments.[56][57] All attempts are made to avoid creating breaks during vitrectomy. Small studies have compared scleral buckling and vitrectomy for stage 4 ROP, and lens-sparing vitrectomy was found to have better outcomes.[58] However, there are times when scleral buckling can be considered, especially in cases of rhegmatogenous retinal detachment.[55]
Complications
The most feared complication of ROP is retinal detachment or macular folds, which can lead to severe vision deficits and blindness. There are a number of other complications related to this disease that can affect visual development. Myopia is a common finding in premature infants with or without ROP, and research is ongoing to determine if myopia is reduced after anti-VEGF injections and laser photocoagulation. Infants with regressed ROP also have an increased incidence of strabismus, amblyopia, and anisometropia. However, progressive stage 4 or 5 ROP can be treated to preserve vision and the eye, as preservation of vision is helpful for the child's development.[59]
Even in the absence of macular folds or structural abnormalities, exudative detachment may occur after laser photocoagulation for type 1 ROP, leading to macular scarring and decreased visual outcomes.[60]
Extreme prematurity is a risk for ROP and for reduced neurocognitive function. Some large studies have found an association with reduced neurocognitive function following administration of certain types of anti-VEGF agents for ROP.[61] [62] However, these large-scale studies have selection bias. Smaller studies have not reported adverse effects on neurocognitive function following anti-VEGF treatment for ROP. While one meta-analysis found that anti-VEGF treatment was not associated with severe cognitive impairment,[63] another did note an increased risk of moderate cognitive impairment.[64] Long-term follow-up is needed from key clinical trials: BEAT-ROP, ROP3 and 4, RAINBOW, FIREFLEYE and BUTTERFLEYE. Although data from BEAT-ROP were limited to a small sample size,[65] neurodevelopmental data from FIREFLEYE and RAINBOW are encouraging.[66] [67]
Prognosis
If ROP progression leads to untreatable retinal detachment, the outcome is poor. The CRYO-ROP study showed that at the 15-year follow-up visit, treatment reduced the risk of unfavorable outcome from 52% to 30%.[54] The same study showed improved outcomes in the treated group for visual acuity at the 3-year, 10-year, and 15-year follow-up visits.[54] Better outcomes are being reported with anti-VEGF agents, though additional studies are needed.
Key Clinical Trials
Cryotherapy for ROP (CRYO-ROP)
CRYO-ROP was the first major treatment trial for ROP and enrolled patients from January 1986 through November 1987.[54]
- Enrollment Criteria: Birthweight <1251 g
- Interventions: Cryotherapy to peripheral avascular retina in severe (threshold) ROP
- Outcomes: At 15 years, fewer infants had visual acuity of 20/200 or worse with cryotherapy than with observation (44.7% vs 64.3%, respectively; p<0.001), and fewer treated eyes had unfavorable structural outcomes (30% vs 51.9%; p<0.001)
- Clinical Implications: Established benefit of surgical intervention for threshold ROP. These criteria were later extended to laser treatment
Early Treatment for Retinopathy of Prematurity (ETROP)
After establishing the benefit of cryotherapy for threshold ROP, ETROP enrolled patients from October 1999 through September 2002 to study laser treatment for pre-threshold eyes.[49][68]
- Enrollment Criteria: Birthweight <1251 g
- Interventions: Laser therapy to peripheral avascular retina in type 1 and type 2 ROP
- Outcomes: At 6 years, fewer type 1 eyes treated with laser therapy had visual acuity of 20/200 or worse than type 2 eyes (25.1% vs 32.1%, respectively; p=0.02). No differences were seen in visual acuity (23.6% vs 19.4%; p=0.37), and no significant side effects were noted[68]
- Clinical Implications: Established benefit of laser treatment in high-risk pre-threshold type 1 ROP
Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP)
BEAT-ROP was the first major study of anti-VEGF agents in ROP and enrolled patients from March 2008 through August 2010.[50]
- Enrollment Criteria: Birthweight <1500 g; GA < 0 weeks; stage 3+ zone I or zone II posterior ROP
- Interventions: Bilateral IVB (0.625 mg in 0.025 mL of solution) vs bilateral laser therapy
- Outcomes: Lower recurrence rate of stage 3 ROP with IVB than with laser therapy (4.3% vs 21.9%, respectively; p = 0.002). This lower recurrence rate for was seen in infants with zone I ROP, but not in infants with zone II posterior disease
- Clinical Implications: Established benefit of IVB monotherapy over laser therapy for stage 3+ ROP with zone I disease. Bevacizumab had a similar benefit to laser for posterior zone II disease, although conventional laser therapy permanently destroys the peripheral retina
Pediatric Eye Disease Investigator Group (PEDIG) ROP1
Following the positive results of BEAT-ROP, PEDIG ROP1 studied the efficacy and systemic safety of de-escalating doses of bevacizumab. This study enrolled patients from April 2017 through May 2019.[24] [69][70][71]
- Enrollment Criteria: Premature infants with type 1 ROP in at least 1 eye
- Interventions: Low-dose IVB (0.25 mg, 0.125 mg, 0.063 mg, and 0.031 mg) or very low-dose IVB (0.016 mg, 0.008 mg, 0.004 mg, and 0.002 mg), with a higher dose in the fellow eye (if both eyes had type 1 ROP)
- Outcomes: At 12 months, both the study eyes and fellow eyes received additional treatment at similar rates (55% and 56%, respectively). Rates of poor structural outcomes were low (3% poor retinal outcomes, 5% anterior segment abnormalities). No differences were seen in rates of reactivation between doses, but a trend toward shorter time to reactivation was seen in the study eyes that received very low-dose IVB vs low-dose IVB (mean 76.4 days vs 85.7 days, respectively)[70]
- Clinical Implications: Although the study was not sufficiently powered to determine the optimal dosing of IVB, doses of bevacizumab as low as 0.004 mg (<1% of BEAT-ROP dose) may be effective at preventing poor structural outcomes, with the caveat that need for additional treatment may be needed
Pediatric Eye Disease Investigator Group (PEDIG) ROP2Y
This was a 2-year follow-up study of PEDIG ROP1.[54]
- Enrollment Criteria: See PEDIG ROP1
- Interventions: See PEDIG ROP1
- Outcomes: At the 2-year follow-up visit, no correlation was seen between the total dose of IVB and neurodevelopmental outcomes, as assessed by Bayley scores (−0.19 for cognitive [95% CI, −0.44 to 0.11], −0.19 for motor [95% CI, −0.45 to 0.11], and −0.14 for language [95% CI, −0.41 to 0.16]). High myopia rates (16%;) were similar to those reported in earlier studies
- Clinical Implications: Continued to demonstrate that long-term safety and efficacy may be seen with low-dose IVB
RAINBOW
This treatment trial tested ranibizumab, another anti-VEGF agent, and enrolled patients from June 2016 to January 2018.[72][51]
- Enrollment Criteria: Birthweight <1500 g
- Interventions: Intravitreal ranibizumab (0.1 mg or 0.2 mg) vs laser therapy
- Outcomes: No significant differences in rates of structural abnormalities between ranibizumab and laser therapy, up to 2 years. At 2 years, structural abnormalities were present in 2% of patients in the ranibizumab 0.2-mg group, 2% of patients in the 0.1-mg group, and 9% of patients in the laser therapy group. High myopia was less frequent after 0.2 mg ranibizumab (5%) than after laser therapy (20%; odds ratio 0.19; p=0.012).[72][51]
- Clinical Implications: Demonstrated effectiveness of ranibizumab, although the study was not designed to determine superiority relative to other anti-VEGF agents.
FIREFLEYE
This study examined aflibercept, the next anti-VEGF agent to be studied for ROP treatment, and enrolled patients between September 2019 and August 2020.[53]
- Enrollment Criteria: Birthweight <1500 g or GA <32 weeks; weight >800 gr at time of treatment
- Interventions: Intravitreal aflibercept 0.4 mg vs laser therapy
- Outcomes: Treatment success after 24 weeks with intravitreal aflibercept (85.5%) was similar to that seen with laser therapy (82.1%) but did not meet the 5% noninferiority criteria. Serious adverse events were seen in 13.3% (ocular) and 24.0% (systemic) of infants given intravitreal aflibercept, consistent with the known safety profile, relative to 7.9% (ocular) and 36.8% (systemic) of infants given laser treatment[53]
- Clinical Implications: Further data are required to understand the long-term efficacy of intravitreal aflibercept for ROP. However, these data, showing similar efficacy of aflibercept to laser therapy, in combination with results from BUTTERFLEYE (see below), provided enough evidence for the US Food and Drug Administration to approve aflibercept for use in ROP
BUTTERFLEYE
Aflibercept was further studied in this trial, which enrolled participants between October 2019 and August 2022 in the US. A manuscript describing full results is yet to be published, but trial details can be found in its official clinical trial registration at ClinicalTrials.gov.
- Enrollment Criteria: Birthweight <1500 g or GA <32 weeks; weight >1000 g at time of treatment
- Interventions: Intravitreal aflibercept 0.4 mg vs laser therapy
- Outcomes: Although publication of results is still pending, presented data showed results consistent with those reported for FIREFLEYE; i.e., ~80% of infants achieved absence of both active ROP and unfavorable structural outcomes at 52 weeks, without additional unexpected ocular adverse events
- Clinical Implications: Further data are required, but aflibercept appears to have efficacy similar to that of laser treatment
Ongoing Studies
PEDIG ROP 3 and 4 are currently underway. PEDIG ROP 3 is comparing low-dose IVB (0.063 mg) and laser therapy for type 1 ROP and PEDIG ROP 4 is evaluating IVB 0.063 mg and 0.25 mg for type 1 ROP. RAINBOW has a 5-year extension study whose results are still pending, and FIREFLEYE and BUTTERFLEYE are still following patients for long-term efficacy data.
Other treatments such as blood transfusions (BORN study),[73] arachidonic and docosahexaenoic acid supplementation (Mega Donna Mega study),[74] and the combination of less-dense laser with bevacizumab are also under investigation.[75]
Additional Resources
- American Association for Pediatric Ophthalmology and Strabismus. Retinopathy of prematurity. https://aapos.org/glossary/retinopathy-of-prematurity. Accessed April 11, 2025.
- Turbert D. What is retinopathy of prematurity? American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/what-is-retinopathy-prematurity. Published September 30, 2024. Accessed April 11, 2025.
References
- ↑ Retinopathy of Prematurity. American Academy of Ophthalmology. https://www.aao.org/education/image/retinopathy-of-prematurity-20 Accessed July 6, 2023.
- ↑ Terry TL. Retrolental fibroplasia. J Pediatr. 1946;29(6):770-773.
- ↑ Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77-82.
- ↑ Gilbert C, Malik ANJ, Nahar N, et al. Epidemiology of ROP update – Africa is the new frontier. Semin Perinatol. 2019;43(6):317-322.
- ↑ Darlow BA, Gilbert C. Retinopathy of prematurity – A world update. Semin Perinatol. 2019;43(6):315-316.
- ↑ Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367(26):2515-2526.
- ↑ Lim HW, Pershing S, Moshfeghi DM, Heo H, Haque ME, Lambert SR; IRIS® Registry Analytic Center Consortium. Causes of Childhood Blindness in the United States Using the IRIS® Registry (Intelligent Research in Sight). Ophthalmology. 2023 Sep;130(9):907-913. doi: 10.1016/j.ophtha.2023.04.004. Epub 2023 Apr 8. PMID: 37037315; PMCID: PMC10524509.
- ↑ 8.0 8.1 Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106(4):471-479.
- ↑ 9.0 9.1 9.2 Charles JB, Ganthier R Jr, Appiah AA. Incidence and characteristics of ROP in a low-income inner-city population. Ophthalmology. 1991; 98(1):14-17.
- ↑ Chang JW. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS ONE. 2019;14(7):e0219934.
- ↑ 11.0 11.1 11.2 11.3 Bujoreanu Bezman L, Tiutiuca C, Totolici G, et al. Latest trends in retinopathy of prematurity: research on risk factors, diagnostic methods and therapies. Int J Gen Med. 2023;16:937-949.
- ↑ Chang E, Josan AS, Purohit R, et al. A network meta-analysis of retreatment rates following bevacizumab, ranibizumab, aflibercept, and laser for retinopathy of prematurity. Ophthalmology. 2022;129(12):1389-1401.
- ↑ Almeida AC, Silva GA, Santini G, et al. Correlation between hyperglycemia and glycated albumin with retinopathy of prematurity. Sci Rep. 2021;11(1):22321.
- ↑ Kushner BJ, Essner D, Cohen IJ, et al. Retrolental fibroplasia: pathologic correlation. Arch Ophthalmol. 1977;95(1):29-38.
- ↑ Lutty GA, McLeod DS. Development of the hyaloid, choroidal and retinal vasculatures in the fetal human eye. Prog Retin Eye Res. 2018;62:58-76.
- ↑ York JR, Landers S, Kirby RS, et al. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24(2):82-87.
- ↑ Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007;109(4):1345-1352.
- ↑ Geisen P, Peterson LJ, Martiniuk D, et al. Neutralizing antibody to VEGF reduces intravitreous neovascularization and may not interfere with ongoing intraretinal vascularization in a rat model of retinopathy of prematurity. Mol Vis. 2008;14:345-357.
- ↑ Simmons AB, Bretz CA, Wang H, et al. Gene therapy knockdown of VEGFR2 in retinal endothelial cells to treat retinopathy. Angiogenesis. 2018;21(4):751-764.
- ↑ Hartnett ME. Retinopathy of prematurity: evolving treatment with anti-vascular endothelial growth factor. Am J Ophthalmol. 2020;218:208-213.
- ↑ McCloskey M, Wang H, Jiang Y, et al. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013;54(3):2020-2026.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 22.13 Chiang MF, Quinn GE, Fielder AR, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;128(10):e51-e68.
- ↑ Becker S, Wang H, Simmons AB, et al. Targeted knockdown of overexpressed VEGFA or VEGF164 in Müller cells maintains retinal function by triggering different signaling mechanisms. Sci Rep. 2018;8(1):2003.
- ↑ 24.0 24.1 24.2 24.3 Wallace DK, Dean TW, Hartnett ME, et al. A dosing study of bevacizumab for retinopathy of prematurity: late recurrences and additional treatments. Ophthalmology. 2018;125(12):1961-1966.
- ↑ Sauer L, Chandler M, Hartnett ME. Extending peripheral retinal vascularization in retinopathy of prematurity (ROP) through regulation of VEGF signaling. Am J Ophthalmol. 2024;260:190-199
- ↑ Chow LC, Wright KW, Sola A; for the CSMC Oxygen Administration Study Group. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111(2):339-345.
- ↑ Vedantham V. Retinopathy of prematurity screening in the Indian population: it's time to set our own guidelines!. Indian J Ophthalmol. 2007;55(5):329-330.
- ↑ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 Fierson WM, Chiang MF, Good W, et al; for the American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142(6):e20183061.
- ↑ 29.0 29.1 29.2 29.3 Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130-1134.
- ↑ 30.0 30.1 30.2 ICROP Committee for Classification of Late Stages ROP. An international classification of retinopathy of prematurity, II: the classification of retinal detachment. Arch Ophthalmol. 1987;105:906-912
- ↑ 31.0 31.1 31.2 31.3 31.4 International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991-999.
- ↑ Temkar S, Azad SV, Chawla R, et al. Ultra-widefield fundus fluorescein angiography in pediatric retinal vascular diseases. Indian J Ophthalmol. 2019;67(6):788-794.
- ↑ 33.0 33.1 Cryotherapy for Retinopathy of Prematurity Cooperative Group. The natural ocular outcome of premature birth and retinopathy. Arch Ophthalmol. 1994;112:903-912.
- ↑ 34.0 34.1 Hartnett ME, McColm JR. Retinal features predictive of progressive stage 4 retinopathy of prematurity. Retina. 2004;24(2):237-241.
- ↑ Yonekawa Y, Thomas BJ, Thanos A, et al. The cutting edge of retinopathy of prematurity care: expanding the boundaries of diagnosis and treatment. Retina. 2017;37(12):2208-2225.
- ↑ Ittiara S, Blair MP, Shapiro MJ, et al. Exudative retinopathy and detachment: a late reactivation of retinopathy of prematurity after intravitreal bevacizumab. JAAPOS. 2013;17(3):323-325.
- ↑ Snyder LL, Garcia-Gonzalez JM, Shapiro MJ, et al. Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2016;47(3):280-283.
- ↑ Taylor K, Ghergherehchi L, Rao P, et al. Very late-onset reactivation of retinopathy of prematurity post anti-VEGF bevacizumab treatment for type 1 ROP: a case report. JAAPOS. 2021;25(3):180-184.
- ↑ Sammons JS, Graf EH, Townsend S, et al. Outbreak of adenovirus in a neonatal intensive care unit: critical importance of equipment cleaning during inpatient ophthalmologic examinations. Ophthalmology. 2019;126(1):137-143.
- ↑ Wintergerst MWM, Petrak M, Li JQ, et al. Non-contact smartphone-based fundus imaging compared to conventional fundus imaging: a low-cost alternative for retinopathy of prematurity screening and documentation. Sci Rep. 2019;9(1):19711.
- ↑ Chawla R, Bypareddy R, Chandra P, et al. Familial exudative vitreoretinopathy: presentation in the first week of life. J Pediatr Ophthalmol Strabismus. 2015;52(5):317-318.
- ↑ Grosso A, Pellegrini M, Cereda MG, et al. Pearls and pitfalls in diagnosis and management of coats disease. Retina. 2015;35(4):614-623.
- ↑ Bui TNPT, Corap A, Bygum A. Cutis marmorata telangiectatica congenita: a literature review. Orphanet J Rare Dis. 2019;14(1):283.
- ↑ Sassalos TM, Fields TS, Levine R, et al. Retinal neovascularization from a patient with cutis marmorata telangiectatica congenita. Retin Cases Brief Rep. 2021;15(1):77-80.
- ↑ Taleb EA, Nagpal MP, Mehrotra NS, et al. Retinal findings in a case of presumed cutis marmorata telangiectatica congenita. Retin Cases Brief Rep. 2018;12(4):322-325.
- ↑ Quinn GE, Ying GS, Repka MX, et al. Timely implementation of a retinopathy of prematurity telemedicine system. JAAPOS. 2016;20(5):425-430.e1.
- ↑ Agarwal K, Jalali S. Classification of retinopathy of prematurity: from then till now. Community Eye Health. 2018;31(101):S4-S7.
- ↑ Hunter DG, Repka MX. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology. 1993;100(2):238-244.
- ↑ 49.0 49.1 Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-1694.
- ↑ 50.0 50.1 Mintz-Hittner HA, Kennedy KA, Chuang AZ; for the BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603-615.
- ↑ 51.0 51.1 51.2 51.3 Stahl A, Lepore D, Fielder A, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394(10208):1551-1559.
- ↑ Eftekhari Milani A, Bagheri M, Niyousha MR, et al. Comparison of clinical outcomes of intravitreal bevacizumab and aflibercept in type 1 prethreshold retinopathy of prematurity in posterior zone II. J Curr Ophthalmol. 2022;34(1):87-92.
- ↑ 53.0 53.1 53.2 Stahl A, Sukgen EA, Wu WC, et al; for the FIREFLEYE Study Group. Effect of intravitreal aflibercept vs laser photocoagulation on treatment success of retinopathy of prematurity: the FIREFLEYE randomized clinical trial. JAMA. 2022;328(4):348-359.
- ↑ 54.0 54.1 54.2 54.3 54.4 Palmer EA, Hardy RJ, Dobson V, et al; for the Cryotherapy for Retinopathy of Prematurity Cooperative Group. 15-year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123(3):311-318.
- ↑ 55.0 55.1 Hansen ED, Hartnett ME. A review of treatment for retinopathy of prematurity. Expert Rev Ophthalmol. 2019;14(2):73-87.
- ↑ Capone A Jr, Trese MT. Evolution of stage 4 retinopathy of prematurity. In: Hartnett ME, Drack A, Capone A Jr, et al, eds. Pediatric Retina. 3rd ed. Wolters Kluwer; 2021:832-837.
- ↑ Capone A Jr, Trese MT, Hartnett ME. Treatment of stages 4 and 5 retinopathy of prematurity. In: Hartnett ME, Drack A, Capone A Jr, et al, eds. Pediatric Retina. 3rd ed. Wolters Kluwer; 2021:838-846.
- ↑ Hartnett ME, Maguluri S, Thompson HW, et al. Comparison of retinal outcomes after scleral buckle or lens-sparing vitrectomy for stage 4 retinopathy of prematurity. Retina. 2004 Oct;24(5):753-757.
- ↑ Hartnett ME, Rodier DW, McColm JR, et al. Long-term vision results measured with Teller Acuity Cards and a new Light Perception/Projection Scale after management of late stages of retinopathy of prematurity. Arch Ophthalmol. 2003;121(7):991-996.
- ↑ Zhang DL, Shapiro MJ, Schechet SA, et al. Macular sequelae following exudative retinal detachment after laser photocoagulation for retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2020;51(12):698-705.
- ↑ Morin J, Luu TM, Superstein R, et al. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016;137(4):e20153218.
- ↑ Natarajan G, Shankaran S, Nolen TL, et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics. 2019;144(2):e20183537.
- ↑ Tsai CY, Yeh PT, Tsao PN, et al. Neurodevelopmental outcomes after bevacizumab treatment for retinopathy of prematurity: a meta-analysis. Ophthalmology. 2021;128(6):877-888.
- ↑ Diggikar S, Gurumoorthy P, Trif P, et al. Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis. Front Pediatr. 2023;11:1055813.
- ↑ Kennedy KA, Mintz-Hittner HA; for the BEAT-ROP Cooperative Group. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. JAAPOS. 2018;22(1):61-65.e1.
- ↑ Stahl A, Nakanishi H, Lepore D, for the; FIREFLEYE Next Study Group. Intravitreal aflibercept vs laser therapy for retinopathy of prematurity: two-year efficacy and safety outcomes in the nonrandomized controlled trial FIREFLEYE Next. JAMA Netw Open. 2024;7(4):e248383.
- ↑ Marlow N, Stahl A, Lepore D, et al; for the RAINBOW Investigators Group. 2-year outcomes of ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW extension study): prospective follow-up of an open label, randomised controlled trial. Lancet Child Adolesc Health. 2021;5(10):698-707.
- ↑ 68.0 68.1 The Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the Early Treatment for Retinopathy of Prematurity study. Arch Ophthalmol. 2010;128(6):663-671.
- ↑ Wallace DK, Kraker RT, Freedman SF, et al. Short-term outcomes after very low-dose intravitreous bevacizumab for retinopathy of prematurity. JAMA Ophthalmol. 2020;138(6):698-701.
- ↑ 70.0 70.1 Freedman SF, Hercinovic A, Wallace DK, et al. Low- and very low-dose bevacizumab for retinopathy of prematurity: reactivations, additional treatments, and 12-month outcomes. Ophthalmology. 2022;129(10):1120-1128.
- ↑ Wallace DK, Hercinovic A, Freedman SF, et al. Ocular and developmental outcomes of a dosing study of bevacizumab for retinopathy of prematurity. JAAPOS. 2023;27(1):10.e1-10.e8.
- ↑ 72.0 72.1 Fleck BW, Reynolds JD, Zhu Q, et al. Time course of retinopathy of prematurity regression and reactivation after treatment with ranibizumab or laser in the RAINBOW trial. Ophthalmol Retina. 2022;6(7):628-637.
- ↑ Teofili L, Papacci P, Orlando N, et al. BORN study: a multicenter randomized trial investigating cord blood red blood cell transfusions to reduce the severity of retinopathy of prematurity in extremely low gestational age neonates. Trials. 2022;23(1):1010.
- ↑ Pivodic A, Johansson H, Smith LE, et al. Evaluation of the Retinopathy of Prematurity Activity Scale (ROP-ActS) in a randomised controlled trial aiming for prevention of severe ROP: a substudy of the Mega Donna Mega trial. BMJ Open Ophthalmol. 2022;7(1):e000923.
- ↑ Namvar E, Bolkheir A, Emadi Z, et al. Outcomes of near confluent laser versus combined less dense laser and bevacizumab treatment of prethreshold ROP Type 1 Zone 2: a randomized controlled trial. BMC Ophthalmol. 2022;22(1):454.