Aniridia
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
OMIM Entries for Aniridia:
- 106210: ANIRIDIA; AN
- 194072: WILMS TUMOR, ANIRIDIA, GENITOURINARY ANOMALIES, AND MENTAL RETARDATION SYNDROME; WAGR
- 206700: ANIRIDIA, CEREBELLAR ATAXIA, AND MENTAL RETARDATION (GILLESPIE SYNDROME)
- 607108: PAIRED BOX GENE 6; PAX6
Disease
Aniridia (ANIRIDIA II, AN 2) is a rare genetic disorder in which there is a variable degree of hypoplasia or absence of iris associated with other ocular features, some present from birth and some arising progressively over time.[1] [2]
The major diagnostic feature is congenital partial or complete hypoplasia of the iris; foveal hypoplasia with reduced visual acuity is almost always present and associates with early onset nystagmus. Other frequently associated ocular abnormalities, generally with later onset, include cataract, glaucoma and corneal opacification and vascularization secondary to limbal stem cell deficiency.[3]
In the vast majority of cases aniridia occurs isolated without systemic involvement due to dominantly inherited mutations or deletions of the paired box gene-6 (PAX6). It can also occur, in a minority of cases as part of the WAGR (Wilms tumor-aniridia-genital anomalies-retardation) contiguous gene syndrome in which the adjacent PAX6 and Wilms tumor (WT1) genes are both deleted.
Epidemiology
Aniridia is seen in approximately 1.8/100 000 live births.[4]The incidence ranges from 1:40 000 to 1:100.000. No significant racial or gender predilection has been described.[5]
Classification
Three phenotypes are recognized:
- Isolated aniridia without systemic involvement
- WAGR Syndrome
- Gilliespie Syndrome
The autosomal dominant (AD) mode of inheritance accounts for two-thirds of cases and as no systemic implications. Penetrance is complete but expressivity is variable.[1]It is caused by mutation in the PAX6 gene on chromosome 11p13 or deletion of the regulatory regions that control its expression.
The sporadic form accounts for about one third of patients.[6]It occurs due to de novo deletions on chromosome 11p13 involving the PAX6 gene. Larger deletions affecting the adjacent WT1 (Wilms tumor) gene are the underlying cause of the WAGR syndrome (Wilms tumor, Aniridia, Genitourinary anomalies, and mental Retardation).[7]
Twenty five to thirty percent of patients with sporadic aniridia develop Wilms’ tumor. According to Gronskov et al patients with sporadic aniridia had a relative risk of 67 (CI, 8.1-241) of developing Wilms tumor and if there is contiguous gene deletion of PAX6 and WT1 patients have up to 50% risk of developing this tumor. [3][4][8]
Autosomal recessive aniridia accounts for approximately 2% of all cases. It is associated with cerebellar ataxia and intellectual disability (Gillespie syndrome).[8] Nelson et al considered that the specific iris abnormality characterized by circumpupillary aplasia leading to a fixed dilated pupil is pathognomonic for Gillespie syndrome. In fact, it may help distinguish from other forms of aniridia and a presumptive diagnosis of Gillespie syndrome can be made during the first months of life. Owing to its rarity, the inheritance pattern and molecular basis of this syndrome are still unclear. However, at least one form may be caused by a heterozygous PAX6 mutation.
Pathogenesis
Different theories have been proposed to explain aniridia. The formation of the anterior segment involves a complex interaction of surface ectoderm, neuroectoderm, and neural crest. Some researchers consider aniridia a subtype of coloboma while others propose mesodermal and ectodermal theories.
In the ectodermal theory aniridia is caused by failure in the optic vesicle rim development between the 12th and 14th weeks of gestation.[5] Supporting this theory is the association of aniridia with other ectodermal defects: anomalies in the retina, absence of the fovea, and absence of iris musculature.
On the basis of mesodermal theory is the association of some aniridias with hypoplastic discs. In these cases, aniridia is due to inadequate migration or proliferation of mesenchymal elements during the second month of gestation. However this theory fails to explain the common association between neuroectodermal anomalies and aniridia (i.e., fovea hypoplasia).[5] [9]
Beauchamp et al have also described a third theory for iris maldevelopment, where aniridia is explained in part on the basis of excessive remodeling and cell death.[9]
Histopathology
Although iris hypoplasia is the most characteristic histophathological feature and it can be quite severe, small portions of iris tissue can always be found. The anterior border layer of the iris stump is usually moderately cellular, consisting of closely packed melanocytes that create a flat iris surface having few crypts. Iris vasculature is occasionally prominent and relatively large vessels can be seen directing towards the normal avascular anterior border layer. The ciliar body is also hypoplasic, but in a lesser degree. The anterior chamber angle can be normal or congenital angle abnormalities can be present. Congenital angle abnormalities have been divided into two categories: incomplete cleavage and anomalous. In incomplete cleavage the angle recess, whose position relative to the ciliary body and scleral spur is normal, is filled with loose mesenchymal tissue. In a series of gonioscopic examination in aniridic patients with and without glaucoma, Grant and Walton found that initially the stroma of the iris extends forward onto the trabecular meshwork forming synechiae-like attachments, followed by a more homogeneous sheet, resulting in eventual angle closure. Less frequently, they found that the angle remained open until adolescence and then was closed by the anterior rotation of the entire iris leaflet. Corneal findings in cases of advanced glaucoma include a thick fibrovascular pannus, calcific and non-calcific degenerations of the Bowman’s layer, and deep stromal vascularization. Cataract formation is present in most aniridic eyes. Nuclear, cortical, posterior subcapsular, and anterior subcapsular cataracts may occur with various degrees of severity.
Molecular and Genetic Basis of Aniridia
Isolated aniridia is caused by heterozygous mutations in the paired box gene-6 (PAX6; 607108) on chromosome 11p13 or deletion of a regulatory region controlling its expression. Pax genes are a family of developmental genes that encode nuclear transcription factors.[10] [11] PAX6 was cloned in 1991, and to date, no other genetic loci have been implicated in aniridia.[12] Most PAX6 mutations cause haploinsufficiency probably resulting from a mechanism of nonsense-mediated decay.[13]
Structure and function of the PAX6 gene
PAX6 is a highly conserved gene in evolution, which codes for a transcriptional regulator protein. This protein plays a key role in ocular, neural tube, olfactory bud, and pancreatic tissue development.[12] It is expressed early in normal eye morphogenesis. It regulates cellular proliferation, differentiation, migration and adhesion. Its targets include PAX6 itself and genes encoding other developmental regulators, cell adhesion molecules and structural proteins, such as lens crystallins and corneal keratins.[1] PAX6 protein expression continues in the adult retina, lens, and cornea.
The PAX6 gene, spanning 22 kb of genomic DNA, contains 14 exons and encodes 422 aminoacids. PAX6 contains 2 DNA-binding domains, the paired domain and the paired-type homeodomain, both with DNA-binding capability, separated by a lysine-rich linker region. A C-terminal proline, serine, and threonine-rich (PST) domain act as a transcriptional activator.[3]
Five non-pathogenic normal allelic variants of PAX6 gene are known.
Pathologic allelic variants
Three hundred PAX6 mutations have been identified; 286 are associated with congenital eye malformations; 257 of them cause aniridia and the reminder 29 cause related ocular phenotypes such as Peters anomaly, foveal hypoplasia, and optic nerve anomalies:[1] [13]
- The Aniridia phenotype may be the result of:
- Nonsense mutations (39%)
- Splice mutations (13%)
- Frameshift deletions and insertions (25%)
- Inframe insertions and deletions (6%)
- Missense mutations (12%)
- Run-on mutations (5%)
- Most lead to loss of protein function.
- In the non-aniridia eye disorders, 69% are missense mutations.
In the Aniridic phenotype, 94% of all intragenic point mutations lead to the introduction of a premature termination codon (PTC), or to C-terminal extensions (CTE), or aminoacid substitutions (missense mutations).
PTC (null) mutations comprise nonsense mutations, frame-shifting insertions and deletions and most splice mutations. CTE mutations cause the open reading frame to continue into the 3’ untranslated region. Missense mutations produce a reduced-function protein, resulting in the variant ocular phenotypes or (if protein function is greatly reduced) in aniridia.
Compound heterozygous PAX6 mutations
Glaseret al and Solomon et al described three cases of children inheriting two different PAX6 mutations, one from each affected parent. Two of these infants died soon after birth with anophthalmia and brain abnormalities. The third child survived with severe microphthalmia and microcephaly. [14] [15]
Chromosomal rearrangements in isolated aniridia and WAGR syndrome
Chromosomal deletions affecting all or part of the PAX6 region, or translocations and inversions that disrupt the transcription unit or control elements can also cause isolated aniridia, typically classical aniridia. A small proportion of sporadic aniridia cases are caused by contiguous deletion of both PAX6 and the nearby WT1. The deletion provides the first of two ‘hits’ needed to inactivate both WT1 alleles. Absence of one WT1 allele in the germline leads to a high risk (~45%) of Wilms tumor.
Other genes and Aniridia-like phenotypes
FOXC1 and PITX2 mutations usually cause Axenfeld-Rieger syndrome (MIM 602482 and 180500). PITX3 mutations most often cause anterior segment dysgenesis and cataracts.
Clinical Overview
Natural History
Aniridia is a rare, sight-threatening disorder that affects the cornea, iris, intraocular pressure, lens, fovea, and optic nerve. Individuals with aniridia characteristically have a variable degree of iris hypoplasia and foveal hypoplasia, which leads to nystagmus and impaired visual acuity (usually 20/100 - 20/200 BCVA). Other features include corneal changes, glaucoma, cataract, lens subluxation, strabismus, optic nerve coloboma and hypoplasia. Progressive sight-threatening complications include cataracts, glaucoma, and corneal opacification. The phenotype may vary between and within families; however, affected individuals usually show little inter-ocular difference.
Most cases are diagnosed at birth with an obvious iris/pupillary abnormality or in infancy with nystagmus (usually apparent by six weeks of age). Photophobia may also be present. Slit-lamp examination commonly discloses small anterior polar cataracts, sometimes with persistent pupillary membrane strands attached.
Iris
The term aniridia is a misnomer, since a small portion of iris tissue is almost always found on gonioscopic or ultrasound biomicroscopy. Variations range from almost total absence to only mild hypoplasia of the iris. In the less severe cases, a round, normal-appearing pupil may be found. The pupil size may be normal, but there may be loss of the iris surface architecture or the presence of iris transillumination.[1][3][16] Other iris changes include partial iris defects (resembling a coloboma) or eccentric/misshapen pupils and iris ectropion.[5]
Lens
Congenital lens opacities (especially anterior polar) are common. Occasionally there is persistent vascularization of the anterior lens capsule (tunica vasculosa lentis) or remnants of pupillary membrane.[1]The lens opacities are rarely dense enough to require lens extraction in infancy, but visually significant lens opacities eventually develop in 50%-85% of patients, usually during the first two decades of life. [1][3]Lens subluxation or dislocation occur but are infrequent and often occur in the setting of glaucoma and buphthalmos. Scheider et al, Houston et al found that the anterior capsule of aniridic cataracts is very fragile. Typically lens subluxates superiorly.
Intraocular Pressure
Ocular hypertension and glaucoma are common. Congenital glaucoma with or without buphthalmos can happen in infants with aniridia; although it usually develops in later childhood or adulthood. However, children with aniridia should receive regular eye exams to evaluate and monitor for high pressure through out childhood. In the former, the reported incidence is 6% to 75% according to Nelson et al.[5] Glaucoma develops due to angle abnormalities which obstruct the outflow of the aqueous humor through Schlemm’s canal. Margo et al studied the histopathology of seven enucleated eyes from children with aniridia and glaucoma. They reported an abnormal angle development. Grant and Walton analyzed a series of gonioscopic examinations in aniridic patients with glaucoma versus aniridic patients without glaucoma and found that initially the stroma of the iris extends forward onto the trabecular meshwork forming synechiae-like attachments, followed by a more homogeneous sheet, eventually resulting in angle closure. Less frequently, they found that the angle remained open until adolescence and then was closed by the anterior rotation of the entire iris leaflet.
Cornea
Aniridia-associated keratopathy (AAK) is a late and progressive manifestation. It is a significant threat to vision and is thought to have an incidence of 20%.[2] AAK is caused mainly by limbal stem-cell deficiency but also by a combination of other factors such as an abnormal differentiated epithelium, abnormal cell adhesion, impaired healing response and infiltration of conjunctival cells. The trigger event for corneal deterioration may be surgical intervention with excessive manipulation of the limbus, or after the application of topical antimetabolites in order to treat the aniridia-associated glaucoma. Inadequate tear production is common, exacerbating the ocular surface disease. The first signs of AAK appear in the first decade of life, with thickening and vascularization of the peripheral cornea, which gradually advances into the central cornea, ending in pancorneal vascularization, opacification and keratinisation. Central corneal thickness is often increased.
AAK may be caused by a deficiency in matrix metalloproteinase 9 (MMP-9), which is also regulated by PAX6. In PAX6 mutation animal models, MMP-9 deficiency results in the accumulation of fibrin and the infiltration of inflammatory cells. There is also a significant increase in stromal cell apoptosis, which disrupts the orderly arrangement of the collagen fibers of the cornea, results in subsequent loss of transparency.
Optic Nerve
Optic nerve hypoplasia occurs in approximately 10% of cases; optic nerve colobomas are also seen occasionally.[3]
Retina
Foveal hypoplasia is usually present. This can occur either independently or as a part of panocular involvement. Patients present with a reduced foveal reflex, macular hypopigmentation and crossing of the usual foveal vascular zone by retinal vessels. Pendular horizontal nystagmus is usually present by 6 weeks of age. ERG testing discloses variable retinal dysfunction from nearly normal to severely abnormal. Rods and cones are equally affected. The etiology of this retinal dysfunction remains unclear. Perhaps it may be due to foveal aplasia or hypoplasia, secondary to PAX6 mutation or phototoxicity as a result of iris maldevelopment.
Refractive error and strabismus
Both are frequent in aniridic patients. According to Nelson et al esotropia is the most frequent deviation encountered. High refractive errors are not uncommon.
Ptosis
Up to 10% pf patients may have bilateral, usually symmetrical ptosis.[3]
Systemic Features
Central Nervous System
Patients with isolated aniridia may show reduced olfaction (probably the most common functional deficit) and cognition , behavioral problems, or developmental delay. MRI studies have demonstrated abnormalities of the anterior comissure, anterior cingulated cortex, cerebellum, temporal and occipital lobes, corpus callosum, pineal gland and olfactory bulb. [1][3]
Hearing
Individuals with aniridia may show central auditory processing difficulties (from abnormal interhemispheric transfer), which may cause hearing difficulties.
WAGR syndrome
Children with sporadic aniridia must be investigated to determine if it is caused by a chromosomal rearrangement affecting both the PAX6 and WT1 genes (Wilms tumour, pediatric nephroblatoma predisposition gene). However, chances of Wilms tumor is very very rare in familial aniridia. Classical WAGR syndrome includes Wilms tumor with Aniridia, Genitourinary abnormalities and mental Retardation, but the phenotype is highly variable. The term ‘WAGR syndrome’ is used even in the absence of all four classical features.
- Wilms tumor risk for individuals with a cytogenetically visible deletion of 11p13 or a submicroscopic deletion that involves PAX6 and WT1 is up to 50%. Patients with WAGR, Wilms tumors are more likely to be bilateral, present earlier and to have more favorable tumor histology with better prognosis than those with isolated Wilms tumor.
- Aniridia is the most consistent symptom and is typically severe. Although cases without classical aniridia have been described.
- Genitourinary abnormalities include cryptorchidism (in 60% of males), uterine abnormalities, hypospadias, ambiguous genitalia, streak ovaries, urethral strictures, ureteric abnormalities, and gonadoblastoma.
- Intellectual disability and behavioral abnormalities in WAGR syndrome are highly variable and both may not be present in some patients:
- Seventy percent of individuals with WAGR syndrome have intellectual disability (defined as IQ <74);
- Behavioral abnormalities include attention deficit hyperactivity disorder (ADHD), autism spectrum disorders (anxiety, depression, and obsessive compulsive disorder).
- Neurologic abnormalities occur in up to one-third of individuals with WAGR syndrome. Findings include epilepsy, hypertonia or hypotonia, microcephaly, enlarged ventricles and corpus callosum agenesis.
- Development kidney abnormalities, focal segmental glomerulosclerosis and end-stage renal disease (ESRD) are more common than Wilms tumor. The rate of ESRD is 36% with unilateral Wilms tumor and 90% with bilateral Wilms tumor. Approximately 25% of individuals with WAGR syndrome have proteinuria ranging from minimal to overt nephrotic syndrome.
- A variety of dysmorphic features and metabolic abnormalities, including obesity, craniofacial dysmorphism, hemihypertrophy, growth retardation, scoliosis, and kyphosis.
Gillespie Syndrome
It is a rare variant form of aniridia associated with cerebellar ataxia, ptosis (congenital hypotonia of the eye muscles), and intellectual disability. The molecular basis of classical Gillespie syndrome remains unidentified. Owing to its rarity the inheritance pattern is unclear. PAX6 intragenic mutations have been reported in two individuals with phenotypes overlapping this syndrome, however these cases probably have atypical aniridia.
Genotype-Phenotype Correlations
PTC (null) mutations tend to produce uniform ocular changes of the anterior segment and cause classical aniridia with iris aplasia and progressive sight-threatening ocular changes.[1][17]
CTE mutations (run on mutations) appear to cause a severe phenotype in the panocular aniridia spectrum.
PAX6 missense mutations result in the variant ocular phenotypes or (if protein function is greatly reduced) in aniridia. In these cases, the severity of functional loss is difficult to predict because it depends largely on the affected aminoacid and its degree of phylogenetic conservation. Variant phenotypes include foveal hypoplasia, autosomal dominant keratitis, developmental abnormalities of the optic nerve, and Peters’ anomaly, sometimes associated with neurodevelopmental abnormalities.
Differential Diagnosis
The differential diagnosis includes:
- Anterior Segment development abnormalities: Rieger and Peters anomalies, iris coloboma and albinism (oculocutaneous and ocular);
- Other causes of infantile nystagmus and reduced vision without iris anomalies: retinal dystrophy, congenital cataract, optic nerve hypoplasia and congenital infection;
- Causes of iris absence or hypoplasia in adults: traumatic aniridia, prior ocular surgery and iridocornealendothelial (ICE) syndromes.
Rieger anomaly is characterized by iris stromal hypoplasia, ectropion uveae, corectopia, full-thickness iris defects, and in about 50% of cases childhood onset glaucoma. It may be distinguished from aniridia by the presence of posterior embryotoxon with attached iris strands, relatively good visual acuity, and the absence of nystagmus or foveal abnormality.
Peters’ anomaly presents with central corneal opacity of variable degree and an underlying defect involving the posterior stroma, Descemet’s membrane and endothelium with or without iridocorneal or lenticulocorneal adhesions. Peter’s anomaly may be associated with other ocular anomalies, such as chorioretinal colobomas, iris coloboma, aniridia, persistent fetal vasculature, microphthalmia, and optic nerve hytpoplasia.
Iris coloboma is a developmental defect that results in a focal absence of the iris and a keyhole-shaped pupil; the remaining iris is normal. Contrary to aniridia, the majority of isolated iris colobomas are not associated with reduced visual acuity or nystagmus.
Oculocutaneous albinism (OCA) and ocular albinism typically present in early infancy with nystagmus, diffuse iris transillumination (but structurally complete), hypopigmented fundus, and, in the case of OCA, skin and hair hypopigmentation, which distinguish these disorders from aniridia.
Other causes of nystagmus and poor vision in infancy (e.g. retinal dystrophy, congenital cataracts, optic nerve hypoplasia) lack the iris changes seen in aniridia.
Traumatic aniridia, prior ocular surgery, iridocornealendothelial (ICE) syndromes, are causes of partial or complete absence of iris in adults. Medical history, age of onset and history of trauma or surgery and lack of other ocular features of aniridia help in the correct diagnosis.
Diagnosis
Clinical Diagnosis
Aniridia is diagnosed by clinical examination:
- Slit lamp examination is important to detect iris and papillary abnormalities; corneal opacification and vascularization, cataract, and glaucoma may also be detected.
- Slit lamp fundoscopy and/or indirect ophthalmoscope can demonstrate foveal hypoplasia and any associated optic disc malformations.
- Optical coherence tomography (OCT) may be used to document foveal hypoplasia in atypical cases, although it may be difficult to perform in the presence of nystagmus and in young children.
- High-frequency ultrasound biomicroscopy (UBM) is useful in infants with corneal opacity or severe corneal edema, as it can demonstrate complete or partial iris hypoplasia.
Genetic Diagnosis
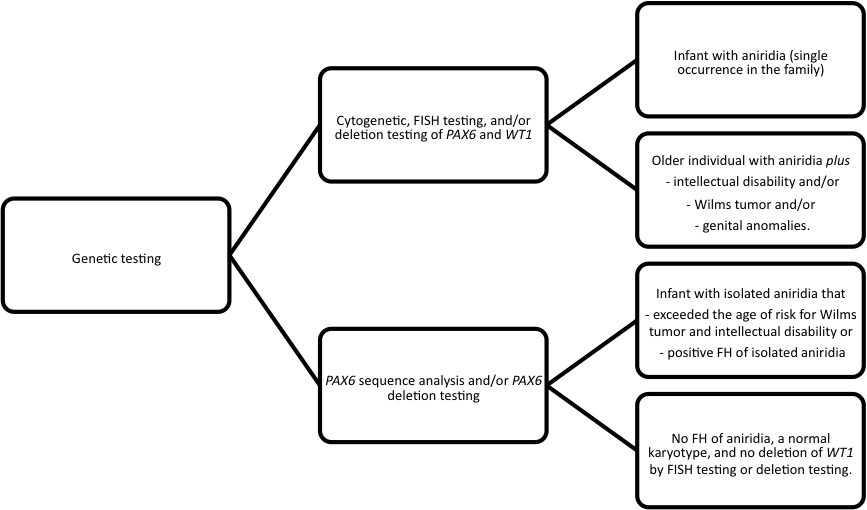
When evaluating an infant with aniridia it is essential to assess family history (FH), but even in the absence of obvious family history, ophthalmic examination of the parents for PAX6 spectrum anomalies should be performed.[3] If there is an affected parent it is unlikely that the child has a deletion extending to WT1, although rare cases have been reported. [3]
Tests
Table 1: Genetic Tests for Aniridia by Phenotype and Family history[3]
Testing Strategy
Management
Clinical Workup
The severity of the ocular condition helps predict future visual function and may be assessed by determining:
- Best corrected visual acuity (BCVA);
- Degree of iris hypoplasia;
- Presence of foveal and optic nerve hypoplasia.
Evaluation of corneal involvement, cataract and glaucoma is also important. These later-onset manifestations play an important role in further reduction of visual function.
Treatment and Surveillance
Patients should have regular eye examinations. Correction of refractive errors and treatment of amblyopia are simple and essential measures. Optical low vision aids for those with significant visual impairment and help with schooling and social support should be provided. Tinted or photocromic lenses can be used to reduce light sensitivity associated with the large papillary aperture.
Intraocular pressure and Glaucoma
All patients with aniridia should undergo annual glaucoma screening throughout life with measurement of intraocular pressure, examination of the angle for evidence of closure, optic disc examination and visual field testing when possible. Measurement of central corneal thickness is also important, as aniridic patients have corneas up to 100 μm thicker than average and this influence intraocular pressure readings.[2] Treatment of glaucoma associated with aniridia is difficult and if present in infancy is even more difficult to treat. Medical treatment is usually the initial approach although it usually proves to be insufficient. Trabeculectomy with or without antimetabolites is among the potential surgical options and success varies ranging from 0% to 83%.[1][3] Drainage tube surgery (with or without antimetabolites) or cyclodiode laser treatment may be necessary in refractory cases. Filtration surgery success rate ranges from 66% to 100%. [3]
Cornea
Management of mild aniridic keratopathy (AAK) includes preservative-free lubricants. In moderate AAK serum drops and amniotic membranes may be temporary useful to enhance the survival and expansion of surviving limbal stem cells. In severe cases, a limbal cell transplant is recommended. Penetrating keratoplasty (PK) may be considered; however, PK alone has a poor prognosis, probably due to the primary limbal stem cell insufficiency. Homologous Lamellar limbokeratoplasty appears to be very effective: its success rate is increased by the use of systemic immunosuppressants. [18]
Lens
Cataract extraction is indicated in those patients with severe lens opacities. Mild to moderate lens opacity may not require surgery, as it should be remembered that in aniridia visual improvement after surgery is limited by foveal hypoplasia. Children rarely require lens surgery. Cataract surgery in aniridic patients has an increased risk of intraoperative complications due to the poor zonular stability. It also influences the type of IOL implanted. Black diaphragm aniridic IOLs are used to reduce glare or light sensitivity and are associated with improvement in visual acuity, but may be associated with a slightly higher rate of surgical complications. [19]
Aniridic Fibrosis syndrome
Patients with aniridia and multiple ocular procedures should be monitored for this syndrome and at the first sign surgical intervention is recommended.
Wilms Tumor
Children with WAGR deletions require renal ultrasound examinations every three months and follow-up by a pediatric oncologist until 8 years of age.
Hearing
Detailed audiological examination is recommended as children with aniridia may have abnormal hearing.
Genetic counseling
Mode of Inheritance
Both isolated aniridia and WAGR syndrome are inherited in an AD manner.
Risk to Family Members
Isolated aniridia
In isolated aniridia, most patients have an affected parent, however a proband with isolated aniridia and no FH may have a de novo gene mutation or gene deletion. The risk for the sibs of the proband depends on the genetic status of the parents. If a parent has isolated aniridia or an identifiable PAX6 mutation, the risk is 50%. When the parents don’t have any clinically signs, the risk to the sibs appears to be low.
The child of a proband with isolated aniridia has a 50% chance of inheriting the PAX6 mutation and developing aniridia.
WAGR Syndrome
If WAGR syndrome is caused by contiguous deletion of PAX6 and WT1 that is detected only by FISH or deletion testing usually occurs de novo. Rarely an asymptomatic parent may be mosaic for such a deletion; thus, it is appropriate to offer FISH testing or deletion testing to both parents.
In probands with WAGR syndrome caused by a cytogenetically visible deletion, it is appropriate to offer cytogenetic testing to both parents to determine if either parent has a balanced chromosome rearrangement.
The risk for the sibs is increased if a parent has a balanced chromosome rearrangement and depends on the nature of the chromosome rearrangement. The risk is equivalent to the general population if the proband has a de novo contiguous gene deletion and neither parent has evidence of mosaicism for the deletion.
Prenatal Testing
Prenatal testing is indicated in pregnancies with risk of isolated aniridia, WAGR syndrome caused by a cytogenetic deletion and by a cryptic deletion. Preimplantation genetic diagnosis (PGD) is an option if the disease-causing PAX6 mutation has been identified or a chromosome rearrangement detectable by chromosome analysis or FISH has been demonstrated.
Additional Resources
- Aniridia Foundation International (AFI)
- Aniridia Network International
- International WAGR Syndrome Association
- eyeGENE® - National Ophthalmic Disease Genotyping Network Registry
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Melanie Hingorani, Isabel Hanson and Veronica van Heyningen, Aniridia, European Journal of Human Genetics. (2012) 20, 1011–1017; doi:10.1038/ ejhg.2012.100; published online 13 June 2012;
- ↑ Jump up to: 2.0 2.1 2.2 Lee H, Khan R, O’Keefe M: Aniridia: current pathology and management. Acta Ophthalmol. 2008; 86: 708–715;
- ↑ Jump up to: 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 Melanie Hingorani, Anthony Moore, Aniridia, Includes: Isolated Aniridia, Wilms Tumor-Aniridia-Genital Anomalies-Retardation (WAGR) Syndrome, Bookshelf ID: NBK1360, 2008;
- ↑ Jump up to: 4.0 4.1 Berlin HS, Ritch R. The treatment of glaucoma secondary to aniridia. Mt Sinai J Med. 1981;48:11;
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 Nelson LB, Spaeth GL, Nowinski TS, et al. Aniridia. A review. Surv Ophthalmol.1984; 28:621–642;
- ↑ Lim HT, Seo EJ, Kin GH et al, Comparison between Aniridia with and without PAX 6 mutations, clinical and molecular analysis of 14 Korean patients, Ophthalmology. 2012; 119:1258-1264;
- ↑ Ton, C. C. T., Hirvonen, H., Miwa, H., et al Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991; 67: 1059-1074, 1991;
- ↑ Jump up to: 8.0 8.1 Gronskov K, Olsen JH, Sand A, et al.T. Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet. 2001;109:11–8. [PubMed: 11479730]
- ↑ Jump up to: 9.0 9.1 Beauchamp GR, Meisler DM, An alternative hypothesis for iris maldevelopment (aniridia). J Pediatr Ophthalmol Strabismus. 1986 Nov-Dec;23(6):281-3;
- ↑ Ton CC, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell.1991;67:1059–74;
- ↑ Dahl E, Koseki H, Balling R., Pax genes and organogenesis, Bioessays. 1997 Sep;19(9):755-65;
- ↑ Jump up to: 12.0 12.1 Prosser, J., van Heyningen, V. PAX6 mutations reviewed. Hum. Mutat. 11: 93-108, 1998. [PubMed: 9482572, related citations] [Full Text: John Wiley & Sons, Inc.];
- ↑ Jump up to: 13.0 13.1 Sérgio Rodrigues Gonçalves, Correlações Genótipo-Fenótipo numa população de doentes com aniridia, Trabalho final do 6º ano com vista à atribuição do grau de mestre no âmbito do ciclo de estudos de mestrado integrado em medicina, Faculdade de Medicina da Universidade de Coimbra, Março 2009
- ↑ Glaser T, Jepeal L, Edwards JG et al, PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet 1994; 7:463-471;
- ↑ Solomon BD, Pineda-Alvarez DE, Balog JZ et al. Compound heterozygosity for mutations in PAX6 in a patient with complex brain anomaly, neonatal diabetes mellitus, and microophthalmia. Am J Med Genet A 2009; 149A:2543-2546;
- ↑ Edward L. Raab, Azay A. Aaby, Jeffrey N. Bloom et al. Pediatric Ophthalmology and Strabismus, . San Francisco: American Academy of Ophthalmology; 2011
- ↑ Simpson TI, Price DJ. Pax6; a pleiotropic player in development. Bioessays. 2002; 24:1041-1051.
- ↑ Holland EJ, Djalilian AR et al. Management of aniridic keratopathy with keratolimbal allograft: A limbal stemm cell transplantation technique. Ophthalmology. 2003;110, 125-130;
- ↑ Reinhard T, Engelhardt S, Sundmacher R. Black diaphragm aniridia intraocular lens for congenital aniridia: long-term follow-up. J Cataract Refract Surg. 2000; 26(3):375-81;
- Mintz-Hittner HA. Aniridia. In: Ritch R, Shields MP, Krupin T, eds. The glaucomas, St Louis: CV Mosby; 1996;
- Tzoulaki I, White IMS, Hanson IM. PAX6 mutations: genotype-phenotype correlations. BMC Genetics. 2005;6:27–39. [PMC free article: PMC1156885] [PubMed: 15918896].