Posterior Polymorphous Corneal Dystrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Posterior Polymorphous Corneal Dystrophy (PPMD, PPCD), also known as Schlichting dystrophy, is an autosomal dominant disorder of the corneal endothelium and Descemet’s membrane, producing a wide variability in clinical presentation. PPMD is a subtype of congenital hereditary corneal dystrophies, which often manifest as bilateral, non-inflammatory corneal opacities that may result in corneal edema of the stroma and degradation directly affecting vision.[1] Congenital hereditary endothelial dystrophies have recently been classified into two types; CHED Type 1, now recognized as a variant of PPMD, is the autosomal dominant disorder that presents after the first year of life and is on the same locus on chromosome 20 as PPMD.[2][3] Whereas, CHED Type 2, now simply CHED, is an autosomal recessive disease and presents immediately after birth. Although the exact prevalence of PPMD is not known, studies of the prevalence of corneal dystrophies in the U.S. estimate that 60% of congenital corneal dystrophies have endothelial involvement.[4] PPMD usually presents in early childhood or adolescence as clouding of the stroma that may cause blurred vision. Ocular diseases correlated with PPMD include secondary glaucoma and keratoconus.[5][6][7] PPMDs have also been associated with extraocular diseases, such as abdominal hernias and Alport Syndrome.[8][9] Other infantile corneal dystrophies include congenital hereditary endothelial dystrophies, congenital hereditary stromal dystrophies, and posterior amorphous corneal dystrophies.[10]
Disease Entity
Molecular mechanisms and genetics
PPMD is inherited in an autosomal dominant manner, and it has been mapped to four different loci on chromosomes 1, 8, 10, and 20, thus showing locus heterogeneity.[11][12] The PPCD1 locus (20p11.2–q11.2) encodes OVOL2, a zinc-finger transcription factor that directly represses expression of ZEB1.[13] ZEB1, another zinc-finger transcription factor, is expressed at the PPCD3 loci (10p11.2). Together these genes control epithelial-to-mesenchymal transition (EMT) and its converse process, mesenchymal-to-epithelial transition (MET); both are important for embryological development of the cornea.[14] Interestingly, recent developments have identified the transcription factor GHRL2 expressed at the PPCD4 locus (8q22.3–q24.12). GRHL2 has also been shown to directly repress transcription of ZEB1 and thus play a role in EMT.[12] The collagen type VIII alpha 2 gene (COL8A2) is found at the PPCD2 locus (1p34.3–p32.3) and has been proposed as a causative agent in the development of PPCD2; however, insufficient evidence in the literature has supported this notion.[11]
Pathophysiology
Due to abnormal developmental differentiation, the endothelial layer transforms into cells similar to stratified, squamous epithelium, thus causing an abnormal basement membrane and thickening of Descemet’s membrane.[1][15] The epithelium-like endothelial cells in a PPMD lesion are usually pleomorphic, multi-layered, exhibit positive staining for cytokeratin and have multiple microvilli on their surfaces.[16] These abnormal epithelialized cells are keratinized, migrate randomly, secrete defective basement membrane cells, cause peripheral synechiae, and ineffectively remove fluid from the corneal stroma, increasing the risk of edema and glaucoma. The thickened Descemet’s membrane and possible resulting stromal edema may result in blurred vision and corneal clouding. Additionally, the resulting thickened cornea may result in a false reading of applanation tonometry.[15]
Diagnosis
Differential Diagnosis [1][17]
- Iridocorneal endothelial syndrome (ICE)
- Rupture of the Descemet’s membrane
- Primary congenital glaucoma
- Peter’s anomaly
- Axenfield-Rieger syndrome
- Fuch’s endothelial dystrophy
- Posterior amorphous corneal dystrophy
- Pre-Descemet’s corneal dystrophy
Clinical Presentation
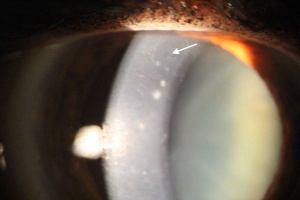
Diagnosis of PPMD is mainly clinical, requiring slit-lamp microscopy and in some cases, specular microscopy to determine the changes in Descemet’s membrane.[18][19] On slit lamp examination, direct and retro-illumination can be used to visualize lesions. Usually bilateral, but sometimes asymmetric, diffuse corneal opacification on physical examination along with corneal edema and thickening are indicative of PPMD.[18][19][20] Other corneal findings include endothelial vesicular changes, "snail-track" band lesions, opacities, and possibly a peripheral ring.[15][19][20] Patients usually present with decreased visual acuity.[15] The multi-layered collagen may present as nodules on the posterior side of the Descemet’s membrane. The vesicular changes usually have blue-grey halos and are usually present near the endothelium or Descemet’s membrane of the posterior surface.[1] Band-like lesions usually are linear and parallel.[15][21][22] If a patient presents with a thickened or enlarged cornea with suspected elevated intraocular pressure and corneal edema, PPMD should be considered. A thorough family history is also helpful in determining the etiology.
Treatment and Prognosis
Management
Most cases of PPMD are asymptomatic, and these cases generally do not require treatment.[10][23] Intraocular pressure should be continuously monitored in patients with PPMD and controlled with medications that decrease aqueous humor production (B-blockers, alpha-adrenergic agonists, and carbonic anhydrase inhibitors).[23] For patients with bilateral, corneal opacities, penetrating keratoplasties has been shown to help.[15][23] Recently developed, Descemet’s Membrane Endothelial Keratoplasty may be considered in patients with normal corneal stroma and epithelium.[15][23] However, surgery should be a final option after all medical interventions have been attempted.
Prognosis
The severity of PPMD determines the prognosis of the condition.[15] Most cases are asymptomatic, and the experience for these patients can be either a nonprogressive disease or a slow, progressive disease. Mild symptoms also have a good prognosis and generally do not require surgical intervention.[15] However, more severe cases of the disease can lead to corneal edema, visual disturbances, and increased intraocular pressure. These cases often require surgery. High intraocular pressure was associated with decreased visual acuity post-surgery.[23]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4(1):7. doi:10.1186/1750-1172-4-7
- ↑ Toma NMG, Ebenezer ND, Inglehearn CF, Plant C, Ficker LA, Bhattacharya SS. Linkage of congenital hereditary endothelial dystrophy to chromosome 20. Hum Mol Genet. 1995;4(12):2395-2398. doi:10.1093/hmg/4.12.2395
- ↑ Nischal KK. Genetics of Congenital Corneal Opacification—Impact on Diagnosis and Treatment. Cornea. 2015;34:S24-S34. doi:10.1097/ICO.0000000000000552
- ↑ Musch DC, Niziol LM, Stein JD, Kamyar RM, Sugar A. Prevalence of Corneal Dystrophies in the United States: Estimates from Claims Data. Investig Opthalmology Vis Sci. 2011;52(9):6959. doi:10.1167/iovs.11-7771
- ↑ Cibis GW, Krachmer JA, Phelps CD, Weingeist TA. The clinical spectrum of posterior polymorphous dystrophy. Arch Ophthalmol (Chicago, Ill 1960). 1977;95(9):1529-1537. http://www.ncbi.nlm.nih.gov/pubmed/302697. Accessed June 5, 2019.
- ↑ Blair SD, Seabrooks D, Shields WJ, Pillai S, Cavanagh HD. Bilateral progressive essential iris atrophy and keratoconus with coincident features of posterior polymorphous dystrophy: a case report and proposed pathogenesis. Cornea. 1992;11(3):255-261. http://www.ncbi.nlm.nih.gov/pubmed/1587135. Accessed June 5, 2019.
- ↑ Bechara SJ, Grossniklaus HE, Waring GO, Wells JA. Keratoconus associated with posterior polymorphous dystrophy. Am J Ophthalmol. 1991;112(6):729-731. http://www.ncbi.nlm.nih.gov/pubmed/1957913. Accessed June 5, 2019.
- ↑ Aldave AJ, Yellore VS, Yu F, et al. Posterior polymorphous corneal dystrophy is associated withTCF8 gene mutations and abdominal hernia. Am J Med Genet Part A. 2007;143A(21):2549-2556. doi:10.1002/ajmg.a.31978
- ↑ Teekhasaenee C, Nimmanit S, Wutthiphan S, et al. Posterior polymorphous dystrophy and Alport syndrome. Ophthalmology. 1991;98(8):1207-1215. http://www.ncbi.nlm.nih.gov/pubmed/1923357. Accessed June 5, 2019.
- ↑ Jump up to: 10.0 10.1 Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7. doi:10.1186/1750-1172-4-7
- ↑ Jump up to: 11.0 11.1 Aldave AJ, Han J, Frausto RF. Genetics of the corneal endothelial dystrophies: an evidence-based review. Clin Genet. 2013;84(2):109-119. doi:10.1111/cge.12191
- ↑ Jump up to: 12.0 12.1 Liskova P, Dudakova L, Evans CJ, et al. Ectopic GRHL2 Expression Due to Non-coding Mutations Promotes Cell State Transition and Causes Posterior Polymorphous Corneal Dystrophy 4. Am J Hum Genet. 2018;102(3):447-459. doi:10.1016/j.ajhg.2018.02.002
- ↑ Davidson AE, Liskova P, Evans CJ, et al. Autosomal-Dominant Corneal Endothelial Dystrophies CHED1 and PPCD1 Are Allelic Disorders Caused by Non-coding Mutations in the Promoter of OVOL2. Am J Hum Genet. 2016;98(1):75-89. doi:10.1016/j.ajhg.2015.11.018
- ↑ Hong T, Watanabe K, Ta CH, Villarreal-Ponce A, Nie Q, Dai X. An Ovol2-Zeb1 Mutual Inhibitory Circuit Governs Bidirectional and Multi-step Transition between Epithelial and Mesenchymal States. Stumpf MPH, ed. PLoS Comput Biol. 2015;11(11):e1004569. doi:10.1371/journal.pcbi.1004569
- ↑ Jump up to: 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 Cibis G, Gulani AC. Posterior Polymorphous Corneal Dystrophy. StatPearls Publishing; 2019. http://www.ncbi.nlm.nih.gov/pubmed/28613630. Accessed June 5, 2019.
- ↑ Goshe JM, Li JY, Terry MA. Successful Descemet’s stripping automated endothelial keratoplasty for congenital hereditary endothelial dystrophy in a pediatric patient. Int Ophthalmol. 2012;32(1):61-66. doi:10.1007/s10792-011-9511-3
- ↑ Farid M, Rhee MK, Akpek EK, et al. Corneal Edema and Opacification Preferred Practice Pattern®. 2018. doi:10.1016/j.ophtha.2018.10.022
- ↑ Jump up to: 18.0 18.1 Krachmer JH. Posterior polymorphous corneal dystrophy: a disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans Am Ophthalmol Soc. 1985;83:413-475. http://www.ncbi.nlm.nih.gov/pubmed/3914130. Accessed June 5, 2019.
- ↑ Jump up to: 19.0 19.1 19.2 Henriquez AS, Kenyon KR, Dohlman CH, et al. Morphologic characteristics of posterior polymorphous dystrophy. A study of nine corneas and review of the literature. Surv Ophthalmol. 29(2):139-147. http://www.ncbi.nlm.nih.gov/pubmed/6334374. Accessed June 5, 2019.
- ↑ Jump up to: 20.0 20.1 Ahn YJ, Choi S Il, Yum HR, Shin SY, Park SH. Clinical Features in Children with Posterior Polymorphous Corneal Dystrophy. Optom Vis Sci. 2017;94(4):476-481. doi:10.1097/OPX.0000000000001039
- ↑ Cibis GW, Krachmer JA, Phelps CD, Weingeist TA. The Clinical Spectrum of Posterior Polymorphous Dystrophy. Arch Ophthalmol. 1977;95(9):1529-1537. doi:10.1001/archopht.1977.04450090051002
- ↑ Lefebvre V, Sowka JW, Frauens BJ. The clinical spectrum between posterior polymorphous dystrophy and iridocorneal endothelial syndromes. Optom - J Am Optom Assoc. 2009;80(8):431-436. doi:10.1016/J.OPTM.2009.02.009
- ↑ Jump up to: 23.0 23.1 23.2 23.3 23.4 Dahrouj M, Vislisel JM, Raecker M, Maltry AC GK. Posterior Polymorphous Corneal Dystrophy (PPMD). https://webeye.ophth.uiowa.edu/eyeforum/cases/208-PPMD.htm. Published 2015. Accessed June 5, 2019.