Axenfeld Rieger Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
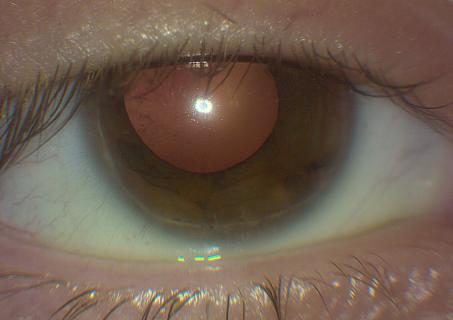
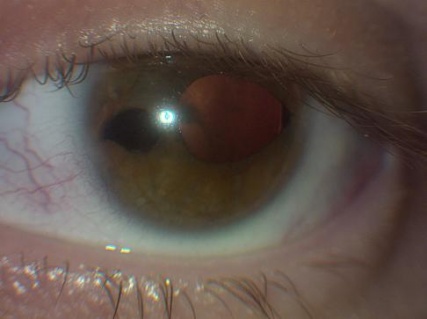
Axenfeld-Rieger syndrome (ARS) refers to an autosomal dominant genetic condition characterized by anterior segment dysgenesis and systemic abnormalities. In 1920, Axenfeld characterized the anomaly which bears his name when he described posterior embryotoxon and iris strands adherent to the anteriorly displaced Schwalbe’s line[1] (Figure 1). Posterior embryotoxon is a clinical and histologic term referring to displacement of Schwalbe’s line anterior to the limbus in the cornea. Rieger described patients with congenital iris abnormalities including iris hypoplasia, corectopia, and polycoria, now referred to as Rieger anomaly, in 1935[2] (Figure 2). Rieger anomaly, associated with systemic findings, such as dental, facial bone defects including maxillary hypoplasia, umbilical abnormalities, or pituitary involvement is known as Rieger syndrome.[3][4] The combination of Axenfeld anomaly and Reiger syndrome is known collectively as Axenfeld-Rieger syndrome.
Figures 1, 2. Note in 1 and 2 the posterior embryotoxon, a white line anterior to the limbus in the cornea which is the abrnormal premature termination of Descemet’s membrane with the trabecular meshwork. In figure 2, note the corectopia and area of atrophy commonly seen in ARS (Images are Courtesy of Vanderbilt Eye Institute and Scott K. Schultz, MD.).
Genetics
Axenfled-Rieger syndrome is autosomal dominant in most cases, but it can also occur sporadically.[5] It has complete penetrance with variable expressivity and is associated with a 50% risk of glaucoma.[3][5][6] ARS is a genetically heterogeneous group of abnormalities as a result of mutations in at least 4 different gene loci.
- Type 1 (PITX2 gene mutations)
- Clinical Findings:
- Abnormalities in the anterior segment of the eye (e.g., iris hypoplasia, corectopia)
- Dental anomalies (e.g., microdontia, hypodontia)
- Redundant periumbilical skin
- Craniofacial anomalies
- Cardiovascular defects
- Clinical Findings:
- Type 1 (PITX2 gene mutations)
- Type 2 (Gene likely located on chromosome 13)
- Clinical Findings:
- Abnormalities in the anterior segment of the eye
- Glaucoma
- Developmental delay and other systemic anomalies may be present but less specific compared to type 1 and type 3
- Clinical Findings:
- Type 2 (Gene likely located on chromosome 13)
- Type 3 (FOXC1 gene mutations)
- Clinical Findings:
- Abnormalities in the anterior segment of the eye (e.g., Axenfeld-Rieger anomaly)
- Glaucoma
- Hearing loss
- Heart defects
- Kidney anomalies
- Clinical Findings:
- Type 3 (FOXC1 gene mutations)
- General Role of PITX2 and FOXC1 proteins
- Transcription factors binding to DNA
- Control the activity of other genes
- Active in developing eyes and other body parts before birth
- Important for embryonic development, particularly in forming structures in the anterior segment of the eye
- General Role of PITX2 and FOXC1 proteins
Mutations in PITX2 on chromosome 4q25, FOXC1 on 6p25, PAX6 on 11p13, and FOXO1A on 13q14 have been associated with formation of ARS.[7][8][9][10] FOXC1 and PITX2 both encode for transcription factors which regulate expression of downstream genetic targets by binding to specific DNA sequences and activating transcription. In mouse models, both FOXC1 and PITX2 are expressed in the ocular structures affected in ARS during embryogenesis.[11][12] Cases of ARS with mutations in CYP1B1 have also been reported.[13]
Epidemiology/Pathophysiology
This disorder is seen is approximately 1/200,000 live births.[14] There is no sex predilection. Most cases are diagnosed during infancy or childhood; however, glaucoma typically occurs in late childhood or adulthood.[3][4]
Defects in differentiation, migration, or arrested development of neural crest cells in the anterior chamber, facial bones, teeth, cardiovascular system, and periumbilical skin are considered to be the etiological basis for the systemic and ocular findings characteristic of ARS.
Histologically, patients with ARS have been found to have a monolayer of endothelial-like cells with a Descemet-like membrane extending from the cornea, across the anterior chamber and angle structures onto the surface of the iris. The membrane is typically found in the quadrant with associated the ectropion uveae/corectopia and the iris atrophy is found in the opposite quadrant.[4]
It is important to recognize that 8-15% of the normal population may have a subtle, mild form of posterior embryotoxon without other sequelae, including glaucoma.[15] The posterior embryotoxon in ARS may be more dramatic and associated with other anterior segment findings, as described above.[16]
Clinical Characteristics
As mentioned, Axenfeld-Rieger syndrome is a bilateral, heterogeneous condition and may include developmental abnormalities in the anterior chamber angle, iris, and trabecular meshwork. Corectopia, polycoria, ectropion uveae, posterior embryotoxon, and increased intraocular pressure are common ophthalmologic findings with ARS. Photophobia may be a symptom resulting from the pupillary and iris abnormalities.
The most commonly recognized clinical manifestations of ARS are the iris corectopia/atrophy, and posterior embryotoxon (figures 1,2). Typically, the remainder of the cornea is clear. Occasional patients have megalocornea or microcornea. In some cases, the posterior embryotoxon may not be visible with the slit lamp examination. Shields published a case series of 24 patients with ARS, in which 5 patients had posterior embryotoxon visible only with gonioscopy.[3]
The iris strands adherent to the posterior embryotoxon can range from fine threadlike strands to broad bands of iris tissue. Likewise, the iris stroma may be grossly abnormal including generalized atrophy with corectopia, ectropion uveae, and often is similar in clinical appearance to iridocorneal endothelial syndrome (ICE).[4] Systemically, patients with ARS will commonly have mild craniofacial dysmorphism, dental abnormalities, and redundant umbilical skin. The facial abnormalities include hypertelorism, telecanthus, maxillary hypoplasia, and a broad, flat nasal bridge. Dental abnormalities include microdontia, oligodontia, or hypodontia. In addition, patients may have hypospadias, anal stenosis, pituitary abnormalities, growth retardation, and cardiac valvular abnormalities.[5][17][18][19][20] Abnormalities of the pituitary gland and other surrounding areas are less common, but more serious findings. Cases of empty sella syndrome[21] and arachnoid cysts have been reported.[22] Growth hormone deficiency and short stature have also been described.[22]
Glaucoma
Glaucoma is seen in approximately 50% of the cases with ARS. Development arrest of the neural crest cells with their retention in the anterior chamber angle during gestation results in incomplete development of the trabecular meshwork or Schlemm canal. The extent of iris defects and iris stands in the angle do not correlate well with the severity of glaucoma. However, the high iris insertion appears to be more pronounced in eyes with glaucoma.[3] Although glaucoma may present in early infancy, most cases occur during adolescence or early adulthood.
Medical Management
Medical management of congenital and childhood glaucoma is generally used as an adjunct to surgical interventions. Aqueous suppressants, including beta-blockers[23][24] and carbonic anhydrase inhibitors[25] have been shown to be safe and effective. These medications can have side-effects, especially in children with smaller volume of distribution for the drugs; therefore, these patients should be monitored closely. Prostaglandin analogues may be used to lower IOP (intraocular pressure).[26][27] Alpha-2 agonists, especially brimonidine, is contraindicated in children less than 2 years of age secondary to their association with potentially serious apnea, bradycardia, hypotension, hypotonia, and CNS (central nervous system) depression in this population.[28] Apraclonidine should be used with caution for the same considerations but seems to be safer than brimonidine.[29] For further discussion see: Congenital_Or_Infantile_Glaucoma
Surgical Management
As in congenital glaucoma, surgical intervention is more efficacious than medical management in ARS.[30]However, achieving long term surgical success in congenital and developmental glaucoma is also difficult and complications are common.[31][32][33][34][35][36][37] Surgical options include, goniotomy, trabeculotomy, trabeculectomy with or without anitfibrotic agents, aqueous shunt procedures, or cyclodestructive procedures. In a retrospective review of pediatric glaucoma by Bussieres et al, 40% of patients with ARS had goniotomy, 30% had trabeculotomy, and 2% required glaucoma drainage devices.[31] Most of those patients required 1.5 surgeries per eye.[31]
In general, goniotomy and trabeculotomy are less successful interventions in developmental glaucoma than other pediatric glaucomas, presumably because of the angle dysgenesis and other developmental abnormalities associated with this group of glaucomas.[38][39]
Trabeculectomy with mitomycin C is associated with successful IOP lowering effect in 82-95% of cases and long-term success around 59% at 2-year follow-up.[32][33] However, this intervention is associated with risk of late post-operative endophthalmitis in 7-8% of cases.[33][34]
Glaucoma drainage devices have been reported to have success rates of 70-90% with long term success reported to be 58-63% at 2-year follow-up.[34][35][36][37][40] The rate of endophthalmitis is low with this procedure, 2.9%,[37] but surgical revision may have to be performed for other associated complications such as dislocation, tube-cornea touch, or erosion.[34][35][36] In addition, concomitant medical therapy is often necessary to augment IOP control with glaucoma drainage devices, and re-operation may be necessary.[30][35][36] In surgically refractory pediatric glaucomas, specifically developmental glaucomas, glaucoma drainage devices are likely to be successful where trabeculectomy has a relatively poor chance of success.[32][36] Cyclodestructive procedures are usually reserved for refractory glaucomas after other options have been exhausted because of low reported success rates, frequent need for re-treatment, and high complication rates.[30]
Other Considerations
As patients with ARS age, they may experience significant and debilitating glare or photophobia resulting from the sometimes progressive iris atrophy, polycoria, and corectopia. In these circumstances painted or tinted contact lenses may be beneficial in reducing these symptoms.
Differential Diagnosis
ICE (Iridocorneal Endothelial) Syndrome
Spectrum of disorders characterized by varying degrees of corneal edema, glaucoma, and iris abnormalities. Encompassed by three variations (1) Chandler’s Syndrome, (2) essential iris atrophy, (3) Cogan-Reese (iris nevus). ICE is typically unilateral, has female predominance, and manifests in early adulthood. Pathologically, it appears to be an acquired disease where endothelial cells acquire features of epithelial cells. Chandler’s syndrome is produced when the pathologic changes are confined to the inner corneal surface with dysfunction of the endothelial pump resulting in corneal edema. Essential iris atrophy is produced when the abnormal endothelium proliferates onto the iris surface with subsequent contractile membranes resulting in pupil corectopia, iris atrophy, polycoria. If the malformed and dysfunctional endothelium spreads across the anterior chamber angle, peripheral anterior synechiae develop and result in glaucoma. Cogan-Reese (iris nevus) is manifested by multiple pigmented iris nodules caused by contraction of the endothelial membranes on the surface of the iris. The unilateral nature, corneal endothelial changes, manifestation in middle age, female predominance, and lack of systemic abnormalities differentiate ICE from ARS.[4][41]
Peters' Anomaly
Characterized by a central corneal opacity associated with an absence of Descemet’s membrane and endothelial layers as well as variable degrees of iridocorneal adhesions from the border of the corneal opacity. [42] Around 60% of cases are bilateral and can be associated with congenital glaucoma, aniridia, and microcornea. Peters' can also be associated with systemic abnormalities including developmental delay, heart defects, hearing loss, CNS defects, gastrointestinal and genitourinary defects.
Peters' anomaly is usually sporadic, but it can be inherited in an autosomal dominant or recessive pattern caused by PAX6, PITX2, CYP1B1, or FOXC1 genes. Although there are many similarities, the significant corneal changes differentiate Peters' from ARS.[43]
Aniridia (Iris Hypoplasia)
A bilateral condition with variable appearance of the iris from a rudimentary stump to mild iris atrophy.[44] Aniridia is also characterized by pannus extending onto the central cornea from stem-cell deficiency, cataract, foveal hypoplasia, decreased vision, or nystagmus. Inheritance of aniridia is typically autosomal dominant, but 30% can be sporadic mutation in PAX6.
Aniridia is associated with a 50-75% risk of glaucoma, commonly resulting from rotation of the rudimentary iris into the anterior chamber angle obstructing aqueous outflow and may not occur until the second decade of life. One must consider WAGR (Wilms tumor, aniridia, genitourinary abnormalities, and mental retardation), an autosomal dominant form seen in 13% of patients with aniridia. Gillespie syndrome, an autosomal recessive form of aniridia, is associated with cerebellar ataxia and mental retardation seen in 2% of patients with aniridia. The conreal pannus, foveal hypoplasia, and iris hypoplasia differentiate aniridia from ARS.[45]
Congenital Ectropion Uveae
Congenital displacement or dragging of the posterior pigmented epithelium of the pupil caused by contraction of myofibroblasts over the papillary margin.[46] Possibly a component of ARS, ICE, neurofibromatosis, facial hemihypertophy, Prader-Willi syndrome, but can be seen as an isolated finding. Congenital ectropion uveae can also be seen with rubeosis iridis. Unilateral congenital iris ectropion; smooth, cryptless iris surface; high iris insertion; dysgenesis of the anterior chamber angle; glaucoma is consistent with the diagnosis of congenital iris ectropion syndrome.[47][48]
Ectopia Lentis et Pupillae
A rare, bilateral, autosomal recessive condition characterized by displacement of the pupil, typically inferotemporally, associated with subluxation of the lens, usually in the opposite direction. This condition is also characterized by microsperophakia, miosis, and poor papillary dilation. Pathologically thought to be secondary to a defect in neuroectodermally derived tissues including the zonules, as well as the dilator muscle and pigmented layer of the pupil. Glaucoma is typically not a feature of ectopia lentis et pupillae.[49]
Oculodentodigital Dysplasia
An autosomal dominant disorder characterized by pigmentary retinopathy, iris coloboma, congenital cataract, glaucoma, microcornea, microphthalmos, thin nose with narrow nostrils and hypoplastic alae, abnormality of the fourth and fifth fingers, and hypoplastic dental enamel. The lack of angle changes differentiates this entity from ARS.[50]
Summary
Axenfled-Rieger syndrome is an autosomal dominant disorder with high penetrance, but variable expressivity associated with ocular as well as systemic developmental abnormalities secondary to genetic defects resulting from derangement in neural crest cell differentiation and migration. The most common ocular manifestations include a spectrum of posterior embryotoxon, iris atrophy, corectopia, and ectropion uveae with 50% of patients developing glaucoma. Systemic findings include mild craniofacial dysmorphism, dental and cardiovascular abnormalities, and redundant umbilical skin. Defects in PITX2, PAX6, FOXC1, and FOXO1A have been reported to cause ARS. Management primarily includes surgical management of the glaucoma, prevention of amblyopia, and possibly the use of painted contact lenses for associated photophobia. Life-long monitoring of these patients is important.
Additional Resources
- Genetic and Rare Diseases Information Center (GARD). Axenfeld-Rieger syndrome. The Genetic and Rare Diseases (GARD) Information Center. https://rarediseases.info.nih.gov/diseases/5701/axenfeld-rieger-syndrome Accessed 29 March 2019.
- Yahoo! groups: Reiger syndrome support groups
- Facebook: Axenfled-Rieger Syndrome support group
References
- ↑ Axenfeld TH. Embryotoxon cornea posterius. Klin Monatsbl Augenheilkd. 1920;65:381-382.
- ↑ Rieger H. Beitrage zur Kenntnis seltener Missbildungen der Iris, II: uber Hypoplasie des Irisvorderblattes mit Verlagerung und entrundung der Pupille. Albrecht von Graefes Arch Klin Exp Ophthalmol. 1935;133:602-635.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Shields MB. Axenfeld-Rieger syndrome. A theory of mechanism and distinctions from the iridocorneal endothelial syndrome. Trans Am Ophthalmol Soc. 1983;81:736–84.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Shields MB. Axenfeld-Rieger and Iridocorneal Endothelial syndromes: Two spectra of Disease With Striking Similarities and Differences. J of Glaucoma. 2001;10(suppl 1):S36-S38.
- ↑ Jump up to: 5.0 5.1 5.2 Alward WLM: Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol 2000;130:107-115.
- ↑ Hjalt TA, Semina EV: Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med 2005;7:1-17.
- ↑ Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2(3):232-239.
- ↑ Semina EV, Reiter R, Leysens NJ, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996; 14(4):392-399.
- ↑ Nishimura DY, Swiderski RE, Alward WL, et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet. 1998; 19(2):140-147.
- ↑ Phillips JC, del Bono EA, Haines JL, et al. A second locus for Rieger syndrome maps to chromosome 13q14. Am J Hum Genet. 1996;59(3):613-619.
- ↑ Berry FB, Lines MA, Oas JM et al: Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld –Rieger syndrome and anterior segment dysgenesis. Hum Mol Genet 2006;15: 905– 919.
- ↑ Smith RS, Zabaleta A, Kume T et al. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet 2000;9:1021-1032.
- ↑ Tanwar M, Dada T, Dada R. Axenfeld-Rieger Syndrome Associated with Congenital Glaucoma and Cytochrome P4501B1 Gene Mutations. Case Rep Med. 2010;2010:212656. doi:10.1155/2010/212656
- ↑ Agarwal P, Jain K, Sandesh S, Chopra S. Axenfeld-Rieger Syndrome: Rare Case Presentation and Overview. J Maxillofac Oral Surg. 2020;19(3):364-369. doi:10.1007/s12663-019-01307-9
- ↑ Ho DK, Levin AV, Anninger WV, Piccoli DA, Eagle RC Jr. Anterior Chamber Pathology in Alagille Syndrome. Ocul Oncol Pathol. 2016;2(4):270-275. doi:10.1159/000446804
- ↑ Burian HM, Rice MH, Allen L. External visibility of the Schlemm’s canal. Arch Ophthalmol. 1957;57:651-658.
- ↑ Alkemade, P. Dysgenesis Mesodermalis of the Iris and the Cornea. Van Gorcum, Assen, The Netherlands. 1969.
- ↑ Tümer Z, Bach-Holm D. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet. 2009 Dec;17(12):1527-39. Epub 2009 Jun 10.
- ↑ Tsai JC, Grajewski AL. Cardiac valvular disease and Axenfeld-Rieger syndrome. Am J Ophthalmol. 1994 Aug 15;118(2):255-6.
- ↑ Fitch N, Kaback M.The Axenfeld syndrome and the Rieger syndrome. J Med Genet. 1978 Feb;15(1):30-4.
- ↑ Shields MB, Buckley E, Klintworth GK, et al. Axenfeld–Rieger syndrome. A spectrum of developmental disorders. Surv Ophthalmol. 1985;29:387–409. doi: 10.1016/0039-6257(85)90205-X.
- ↑ Jump up to: 22.0 22.1 Reis LM, Maheshwari M, Capasso J, et al. Axenfeld-Rieger syndrome: more than meets the eye [published online ahead of print, 2022 Jul 26]. J Med Genet. 2022;jmedgenet-2022-108646. doi:10.1136/jmg-2022-108646
- ↑ Boger WP, Walton DS: Timolol in uncontrolled childhood glaucoma. Ophthalmology. 1981;88:253–288.
- ↑ Hoskins HD, Hetherington J, Magee SD, et al: Clinical experience with timolol in childhood glaucoma. Arch Ophthalmol. 1985;103:1163–1165.
- ↑ Portellos M, Buckley EG, Freedman SF: Topical versus oral carbonic anhydrase inhibitor therapy for pediatric glaucoma. Journal of American Association of Pediatric Ophthalmology and Strabismus 1998;2:43–47.
- ↑ Enyedi LB, Freedman SF, Buckley EG: The effectiveness of latanoprost for the treatment of pediatric glaucoma. Journal of American Association of Pediatric Ophthalmology and Strabismus. 1999;3:33–39.
- ↑ Black AC, Jones S, Yanovitch TL, et al. Latanoprost in pediatric glaucoma--pediatric exposure over a decade. J AAPOS. 2009;13(6):558-62.
- ↑ Carlsen JO, Zabriskie NA, Kwon YH, et al: Apparent central nervous system depression in infants after the use of topical brimonidine. Am J Ophthalmol. 1999;128:255–256.
- ↑ Wright TM, Freedman SF. Exposure to topical apraclonidine in children with glaucoma. J Glaucoma. 2009;18(5):395-8.
- ↑ Jump up to: 30.0 30.1 30.2 Beck AD. Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am. 2001;14(3):501-12.
- ↑ Jump up to: 31.0 31.1 31.2 Bussières JF, Therrien R, Hamel P, et al. Retrospective cohort study of 163 pediatric glaucoma patients. Can J Ophthalmol. 2009;44(3):323-7.
- ↑ Jump up to: 32.0 32.1 32.2 Beck AD, Wilson WR, Lynch MG, et al: Trabeculectomy with adjunctive mitomycin C in pediatric glaucoma. Am J Ophthalmol. 1998;126:648–657.
- ↑ Jump up to: 33.0 33.1 33.2 Sidoti, PA, Belmonte SJ, Liebmann JM, et al: Trabeculectomy with mitomycin–C in the treatment of pediatric glaucomas. Ophthalmology. 2000;107:422–429.
- ↑ Jump up to: 34.0 34.1 34.2 34.3 Coleman AL, Smyth RJ, Wilson MR, et al: Initial clinical experience with the Ahmed glaucoma valve implant in pediatric patients. Arch Ophthalmol. 1997;115:186–191.
- ↑ Jump up to: 35.0 35.1 35.2 35.3 Eid TE, Katz LJ, Spaeth GL, et al: Long–term effects of tube–shunt procedures on management of refractory childhood glaucoma. Ophthalmology. 1997;104:1011–1016.
- ↑ Jump up to: 36.0 36.1 36.2 36.3 36.4 Englert JA, Freedman SF, Cox TA: The Ahmed valve in refractory pediatric glaucoma. Am J Ophthalmol. 1999;127:34–42.
- ↑ Jump up to: 37.0 37.1 37.2 Djodeyre MR, Peralta Calvo J, Abelairas Gomez J. Clinical evaluation and risk factors of time to failure of Ahmed Glaucoma Valve implant in pediatric patients. Ophthalmology. 2001;108(3):614-20.
- ↑ Luntz MH: Congenital, infantile, and juvenile glaucoma. Ophthalmology. 1979;86:793–802.
- ↑ Taylor RH, Ainsworth JR, Evans AR, et al: The epidemiology of pediatric glaucoma: The Toronto experience. Journal of American Association of Pediatric Ophthalmology and Strabismus. 1999;3:308–315.
- ↑ Chen TC, Bhatia LS, Walton DS. Ahmed valve surgery for refractory pediatric glaucoma: a report of 52 eyes. J Pediatr Ophthalmol Strabismus. 2005;42(5):274-83; quiz 304-5.
- ↑ External Disease and Cornea, Section 8. Basic and Clinical Science Course, AAO. Pg. 300-301;2009-2010.
- ↑ Jat NS, Tripathy K. Peters Anomaly. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 22, 2022.
- ↑ External Disease and Cornea, Section 8. Basic and Clinical Science Course, AAO. Pg. 292-294;2009-2010.
- ↑ Tripathy K, Salini B. Aniridia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 22, 2022.
- ↑ Glaucoma, Section 10. Basic and Clinical Science Course, AAO. Pg. 162;2009-2010.
- ↑ Sridhar U, Tripathy K. Iris Ectropion Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 22, 2022.
- ↑ Pediatric Ophthalmology and Strabismus, Section 6. Basic and Clinical Science Course, AAO. Pg. 169-170;2009-2010.
- ↑ Ophthalmic Pathology and Intraocular Tumors, Section 4. Basic and Clinical Science Course, AAO. Pg. 186;2009-2010.
- ↑ Pediatric Ophthalmology and Strabismus, Section 6. Basic and Clinical Science Course, AAO. Pg. 305-306;2009-2010.
- ↑ Retina and Vitreous, Section 12. Basic and Clinical Science Course, AAO. Pg. 256;2009-2010.