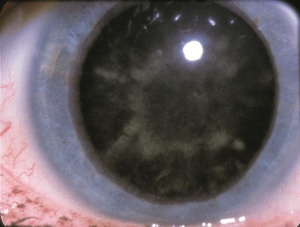
Lowe Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Lowe syndrome (LS) or oculocerebrorenal syndrome of Lowe (OCRL) is a rare disorder characterized by multiple features occurring mainly in males. Its prevalence is approximately 1 in 500,000. Its systemic manifestations include mental retardation, hypotonia, and kidney dysfunction in the form of Fanconi syndrome.[1] Some patients with LS also have bleeding disorders, with normal to low platelet count and impaired platelet function.[2] Ocular manifestations can include congenital cataracts, corneal keloid, and infantile glaucoma.[3]
Etiology and Epidemiology
Lowe Syndrome is an X-linked recessive disorder with the key mutation in the OCRL gene. The prevalence is almost exclusively in males and has been estimated at 1 in 500,000 in the general population and 1 to 10 males per 1 million people in the United States. A male may inherit mutation from their mother who has a mutated OCRL gene copy, but the mutation can also occur spontaneously without any previous family history.
Risk Factors
Male gender is a risk factor as well as having a mother as a carrier of the OCRL mutation.
Pathophysiology
The key mutation in LS is in the OCRL gene on chromosome Xq25-26, which encodes for an inositol 5-phosphatase enzyme (OCRL-1).[4] The enzyme is primarily found within clathrin-coated pits on the cell surface as well as within cellular organelles, particularly endosomes and the Golgi apparatus. Its function is to convert phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 4-phosphate.[5] OCLR-1’s function is essential for cellular processes, such as protein trafficking, cell signaling, cell motility, and actin cytoskeleton polymerization. Faulty protein trafficking may explain defects at the level of the kidney. The mechanisms behind the ocular abnormalities are poorly understood but altered OCRL-1 activity and localization may be needed for epithelial cell movement and ability to differentiate within the eye.
Diagnosis
The definitive diagnosis of Lowe Syndrome is based on genetic testing and physical exam findings upon birth.
History
Lowe Syndrome should be suspected in a newborn male patient with congenital cataracts, central hypotonia, and central nervous system delay. Family history may be positive for male relatives with similar findings. Mothers of affected children (carriers) may have radial cortical lenticular opacities in the shape of snowflakes found on ophthalmologic examination.
Physical Examination
Ophthalmological Abnormalities
- Cataracts: Bilateral dense cataracts can be noted on ultrasound even at 20 weeks gestation and are present at birth. Posterior lenticonus is a common association but can be found in other disorders, such as retinoblastoma, Down Syndrome, Alport syndrome, etc.[6]
- Glaucoma: Occurs in around 50% of patients, presenting with increased intraocular pressure and possible buphthalmos. Distorted anatomical configuration of the angle as noted via gonioscopy. Decreased visibility of both scleral spur and a narrow ciliary body band is noted. Despite intervention, the glaucoma is often aggressive requires surgical, rather than medical, management.
- Nystagmus: May be a result of aphakia and possible retinal abnormalities from the genetic mutation. This usually develops early in life, even with early and uncomplicated surgery. [7]
- About 25-35% of patients also have strabismus or corneal/conjunctival keloids which can further reduce visual prognosis. [8]
Nervous System Abnormalities
- Severe hypotonia is often present at birth with loss of deep tendon reflexes. This may complicate vital functions such as breathing at birth and feeding difficulties due to weak or ineffective sucking.
- Achievement of motor milestones may be delayed . Independent ambulation occurs in approximately 70% of patients by age 6 to 13 years, but some require the use of a wheelchair for mobility.[7]
- Seizures can vary in type and severity. More than 50% of affected adults are epileptic.[9]
- Intellectual disability can range from mild to severe.
- Patients are prone to developing maladaptive behaviors such as temper tantrums, irritability, and compulsive behavior. Patients with low vision are particularly susceptible.[10]
Kidney Abnormalities
- Fanconi Syndrome: the key dysfunction in Lowe Syndrome often develops with age and may be not present with symptoms at birth. Low molecular weight proteinuria may be the most sensitive marker for renal involvement of LS.
- Failure to thrive may present due to mineral wasting. Renal phosphate wasting increases the risk for rickets and osteomalacia.
- Chronic renal failure often develops as time progresses. By the fourth decade of life, most patients have Stage 4-5 chronic kidney disease (CKD).
Musculoskeletal Abnormalities
- Common musculoskeletal manifestations are osteopenia, tenosynovitis, and debilitating arthropathy.
- Patients present with stunted growth and short stature.
- Scoliosis has been found in LS patients.
Diagnostic procedures
Imaging
Neurologic findings may warrant neuroimaging. Typical T2-weighted MRI findings include periventricular and deep hyperintense lesions. Mild ventriculomegaly may be present as well. [11]
Laboratory test
A definitive diagnosis of LS can be made through detection of reduced inositol polyphosphate-5-phosphatase activity of OCRL-1 in cultured skin fibroblasts. In addition, molecular genetic analysis can be performed for OCLR, which accurately detects more than 95% of affected males. For antenatal testing, ultrasound may reveal the presence of fetal cataracts, and maternal serum and amniotic fluid may have elevated levels of alpha-fetoprotein. Neurologic and musculoskeletal findings may warrant electroencephalography or electromyography. Serologic testing may reveal metabolic acidosis, elevated creatinine, reduced GFR, hypokalemia, vitamin D deficiency, and elevated CPK and liver transaminases. Urinalysis may reveal aminoaciduria, hypercalciuria, and proteinuria.
Differential diagnosis
Presence of cataracts with hypotonia requires ruling out mitochondrial disorders such as Leber congenital optic neuropathy (LHON), peroxisomal disorders like Zellweger syndrome, and congenital infections including but not limited to rubella or toxoplasmosis. Hypotonia is a feature of OCRL which may be seen in congenital myotonic dystrophies or congenital myopathy such as Muscle-Eye-Brain Disease. It is important to note that presence of renal pathology will generally rule out these other differential diagnoses. Joubert syndrome is another inherited cerebrorenal syndrome with characteristic cerebellar and brainstem abnormalities.
Management
General treatment
Eye Abnormalities
As with other forms of congenital cataracts, cataract surgery is recommended within the first three months of life to minimize deprivation amblyopia. Due to the increased risk of complications and need for further surgeries, infants are traditionally left aphakic. Aphakic spectacles or contacts lenses are important for visual development, but contact lens correction may be challenging due to behavioral difficulties as well as concurrent glaucoma and cornea problems. Children with Lowe's syndrome and glaucoma often require surgery to treat their glaucoma. There is no consensus on preferred glaucoma surgeries, but options include: goniotomy, trabeculotomy, and tube shunt procedures are the mainstay of management for glaucoma. Children should be screened every 6 months for glaucoma. If corneal keloids occur, they can sometimes be removed surgically but often recur more aggressively than before. There is no consistent proven therapy to eradicate corneal keloids.[12]
Systemic Abnormalities
Hypotonia requires early intervention with physical and occupational therapy. Maladaptive behaviors may be controlled by antipsychotics. Clomipramine, paroxetine, and risperidone have been shown to have some beneficial effects with nervous abnormalities. Rapamycin and statins are currently being investigated for their complementary action in improving symptoms in Lowe Syndrome.[13] Kidney pathology often presents in the form of renal tubular acidosis and may be treated with sodium bicarbonate or other alkali. Intravenous fluids may be required for infants that have resultant dehydration. Additionally, supplementation with vitamin D is essential to prevent occurrence of rickets with adjustment based on close monitoring of both parathyroid hormone and calcium levels.
Complications and Prognosis
- Prognosis is variable and early diagnosis is important to prevent life threatening complications due to renal pathology, hypotonia, or infection. According to the National Organization for Rare Diseases, kidney failure is the most common cause of reduced life expectancy of 30-40 years.[12] Other main causes are infections, respiratory illness, epilepsy or cardiorespiratory arrest.[9] Importantly, quality of life is highly dependent on the extent of nervous and renal compromise.
- Visual prognosis is overall poor in patients with Lowe Syndrome due to various visual abnormalities. Vision is rarely better than 20/100.
Additional Resources
- National Organization for Rare Disorders: Lowe Syndrome
- National Institutes of Health: Lowe Syndrome Genetics Home Reference
- Lowe Syndrome Association
- https://www.omim.org/entry/309000
References
- ↑ Schurman SJ, Scheinman SJ. Inherited cerebrorenal syndromes. Nat Rev Nephrol. 2009;5(9):529-538.
- ↑ Bura, A, de Matteis, MA, Bender, M, Swinkels, M, Versluis, J, Jansen, AJG, et al. Oculocerebrorenal syndrome of Lowe protein controls cytoskeletal reorganisation during human platelet spreading. Br J Haematol. 2023; 200(1): 87–99. https://doi.org/10.1111/bjh.18478
- ↑ Loi M. Lowe syndrome. Orphanet J Rare Dis. 2006;1(1).
- ↑ Erdmann KS, Mao Y, McCrea HJ, et al. A Role of the Lowe Syndrome Protein OCRL in Early Steps of the Endocytic Pathway. Dev Cell. 2007;13(3):377-390.
- ↑ Mehta ZB, Pietka G, Lowe M. The cellular and physiological functions of the lowe syndrome protein OCRL1. Traffic. 2014;15(5):471-487.
- ↑ Kaur, K, Gurnani, B. Lenticonus. StatPearls [Internet]. 2023.https://www.ncbi.nlm.nih.gov/books/NBK589671/
- ↑ 7.0 7.1 Lewis RA, Nussbaum RL, Brewer ED. Lowe Syndrome. 2001 Jul 24 [Updated 2019 Apr 18]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. Available from: https://www.ncbi.nlm.nih.gov/sites/books/NBK1480/
- ↑ Ma X, Ning K, Jabbehdari S, et al. Oculocerebrorenal syndrome of Lowe: Survey of ophthalmic presentations and management. European Journal of Ophthalmology. 2020;30(5):966-973. doi:10.1177/1120672120920544
- ↑ 9.0 9.1 Lloyd, C. (2022). Cataract. Black, GCM, Ashworth, JL. (Eds.) Clinical Ophthalmic Genetics and Genomics. (p. 133-136) Elsevier.
- ↑ Kenworthy L, Charnas L. Evidence for a discrete behavioral phenotype in the oculocerebrorenal syndrome of Lowe. Am J Med Genet. 1995;59(3):283-290.
- ↑ Bokenkamp A, Ludwig M. The oculocerebrorenal syndrome of Lowe: an update. Pediatr Nephrol. 2016. 31(12): 2201–2212
- ↑ 12.0 12.1 https://rarediseases.org/rare-diseases/lowe-syndrome/
- ↑ Madhivanan K, Ramadesikan S, Hsieh WC, et al. Lowe syndrome patient cells display mTOR- and RhoGTPase-dependent phenotypes alleviated by rapamycin and statins. Hum Mol Genet. 2020 Jun 27;29(10):1700-1715. doi: 10.1093/hmg/ddaa086