All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Peters anomaly is one disease in a constellation of diseases that causes corneal opacity due to dysgenesis of the anterior segment during development. Peters anomaly can cause devastating corneal opacity in an infant leading to severe amblyopia. Diagnosis involves careful anterior segment exam as well as testing for other systemic findings which would suggest Peters Plus syndrome. Treatment involves surgical intervention to establish a clear visual axis with either corneal transplant or optical iridectomy.
Disease Entity
Coloboma and other anomalies of anterior segment ICD-9 743.44
Disease
Peters anomaly is one disease in a constellation of diseases that causes corneal opacity due to anterior segment dysgenesis (ASD) during development. An estimate of 44-60 cases of Peters anomaly are reported in the United States annually.[1] It has been known over the years as primary mesodermal dysgenesis of the cornea, congenital anterior synechiae, posterior keratoconus and anterior chamber cleavage syndrome[2]. Peters anomaly affects the iris, corneal endothelium, and Descemet’s membrane, leading to Peters type I. Peters type II in addition features lens abnormalities and tend to be bilateral. 60% of those with Peters anomaly have bilateral involvement. In both forms, opacification of the cornea leads to an amblyogenic effect on an infant's developing vision. Peters Plus syndrome includes short stature, developmental delay, dysmorphic facial features, cardiac, genitourinary, and central nervous system malformations. These systemic findings are seen in up to 60% of patients. Bilateral Peters is more strongly associated with systemic malformations (71.8%) as compared to unilateral Peters anomaly (36.8%).[3] Peters is also associated with many other ocular pathologies including glaucoma, sclerocornea, corectopia, iris hypoplasia, cataract, ICE syndrome, aniridia, iris coloboma, persistent fetal vasculature and microcornea.
Etiology
Peters anomaly falls within the spectrum of anterior segment dysgenesis, which includes other diseases such as Axenfeld-Rieger syndrome. There have been multiple genetic loci that have been identified as causes for Peters anomaly including PAX6,[4] PITX2, PITX3, FOXC1,[5] FOXE3, CYP1B1,[6] MAF and MYOC.[7][8] The variety of genes involved contributes to the significant degree of phenotypic variability found in ASD. Reported chromosomal abnormalities in chromosomes 4,[9] 13, 11, and 20 have been reported to contribute to Peters. Peters can occur sporadically, but autosomal dominant and recessive inheritance have been reported[10].
In addition, lab studies on mice have shown that a deficiency of heparan sulfate leads to improper neural crest TGF-β2 signaling leading to ASD2.[11] Peters Plus syndrome is an autosomal recessive congenital disorder affecting beta-1,3-galactosyltransferase-like glycosyltransferase gene(B3GALTL) on chromosome 13.[12][13]
Risk Factors
Premature infants are at highest risk for development of ASD, including Peters anomaly. In addition, a deficiency of heparan sulfate can lead to abnormal neural crest development in utero. Fetal alcohol syndrome has been reported as a cause of Peters. Intrauterine infection and teratogenic exposures during pregnancy may also be associated with Peters. [14]
General Pathology
Histologically, in Peters type I, the cornea at the area of opacity has an an endothelium with underlying iridocorneal synechiae that extend from the iris collarette to the border of the corneal opacity. There are diverse histological changes in Descemet’s membrane. Most commonly, the Descemet’s membrane of the cornea is absent, but there has also been a reported case of a “multiple-layer” structure of Descemet’s.[15] Keratolenticular adhesions to the posterior cornea are also seen in some cases and can be visualized with slit lamp biomicroscopy or ultrasound biomicroscopy (UBM). Thinning, thickening, or absence of Bowman’s membrane and defects in the posterior stroma can occur, including residual fibrosis in the opacified stroma and central concave defect in the posterior corneal stroma (posterior ulcer) with disorderly stromal lamellae in the ulcer bed.[16] The opacification often involves the central cornea; however, it can also affect the entire cornea. Peters type II also features lens abnormalities that can be seen histologically.
The American Academy of Ophthalmology's Pathology Atlas contains a virtual microscopy image of Peters Anomaly.[17]
Pathophysiology
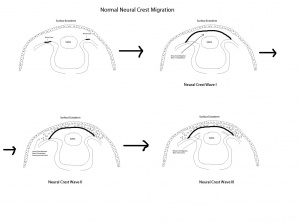
The critical embryological event that is associated with Peters is the formation of the anterior chamber. Normal corneal development depends on neural crest migration which occurs in 3 distinctive waves during embryogenesis to produce the structures of the anterior chamber. This typically occurs during the 7th week of gestation.
The first wave involves the formation of the corneal endothelium as the neural crest cells migrate between the surface ectoderm and the lens. In the second wave, peripheral neural crest cells migrate between the newly formed corneal endothelium and surface ectoderm to form the keratocytes that will lead to formation of corneal stroma. The final wave involves formation of the iris stroma. Any disruption of neural crest migration or separation including incomplete migration/differentiation of central corneal endothelium and Descemet membrane precursor cells or defective separation between the primitive lens and cornea can lead to an ASD.[14]
PAX6, PITX2, and FOX genes, mentioned above as possible genes associated with Peters, are homeobox genes that are involved with corneal development. However, the mechanisms of how these transcription factors interact at the periocular neural crest cell level to direct their differentiation into corneal endothelium are still under investigation. There are also reported cases of Peters that do not have mutations in these genes. [14]
Primary Prevention
Peters anomaly occurs as a result of an in-utero abnormality of multiple genetic loci that causes anterior segment dysgenesis. No primary prevention has been described for this disorder.
Diagnosis
Peters anomaly is diagnosed by anterior segment examination, which shows corneal opacification present at birth. B-scan ultrasonography or ultrasound biomicroscopy can be used to examine the anatomic relationship between the lens, iris and cornea. Ultrasound biomicroscopy is useful in detecting central corneal opacity, the absence of Descemet's membrane, and iridocorneal and keratolenticular adhesions. If full-thickness corneal transplantation is performed, then histopathologic findings of the cornea are a useful adjunctive tool in the diagnosis of Peters as well. Genetic testing of one of the aforementioned genes can help to confirm Peters anomaly, but it is classically diagnosed clinically.
History
Patients are often seen initially by the pediatrician and found to have an abnormal red reflex with a corneal leukoma. Nystagmus or abnormal vision may be noted by the pediatrician or guardian of the patient.
Patients should be screened for systemic malformations, including congenital cardiac defects, craniofacial dysplasia and skeletal, central nervous system, and urogenital anomalies.
Physical examination
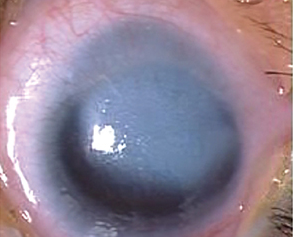
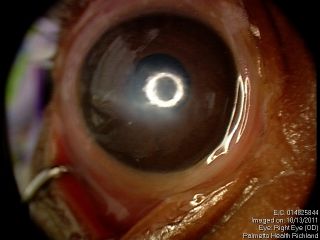
Anterior segment examination reveals an opacification on the cornea with underlying loss of endothelium and Descemet’s membrane and overlying corneal edema. Iris strands can often been seen attached to the area of opacified cornea. These strands may or may not be still attached to the body of the iris. Corectopia is often present in addition to a shallow anterior chamber. In addition, in type II Peters, the lens is typically adherent or closely abutting the cornea.
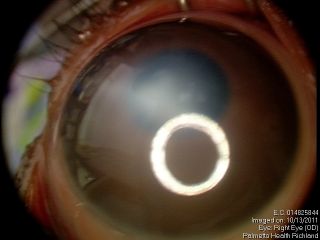
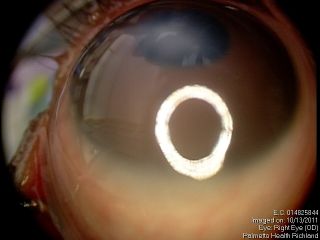
These figures show a corneal stromal opacity in a 1 week old infant. The anterior chamber is extremely shallow.
Signs
The most common sign is a corneal opacification seen at birth.
Symptoms
Decreased vision by way of blockage of the central visual axis due to the corneal opacification will lead to deprivation amblyopia. In addition, patient may suffer from glaucoma due to malformation of the angle structures as well as a shallow anterior chamber.
Clinical diagnosis
Peters is diagnosed by clinical features seen on anterior segment exam, histopathology findings, and ultrasound biomicroscopy. Other associated ocular anomalies such as glaucoma and sclerocornea may be present. Systemic abnormalities including congenital cardiac defects, craniofacial dysplasia and skeletal, central nervous system, and urogenital anomalies are more likely to be present in Peters plus.
Laboratory test
Genetic testing for aforementioned genes and chromosomes.
Differential diagnosis
Peters anomaly is part of a spectrum of ASD, all of which have similar pathways of development. These include: Axenfeld-Rieger anomaly and syndrome, iridocorneal endothelial syndrome, posterior polymorphous dystrophy, congenital hereditary endothelial dystrophy, and congenital hereditary stromal dystrophy.
Peters anomaly is on the differential diagnosis for congenital corneal opacifications. The S.T.U.M.P.E.D. classification system can aid the differential.
S.T.U.M.P.E.D.
- Sclerocornea
- Tears in Descemet's
- Ulcers
- Metabolic
- Peters
- Endothelial dystrophy: Congenital hereditary endothelial dystrophy (CHED); Posterior polymorphous corneal dystrophy (PPCD)
- Dermoid
Management
General treatment
Treatments for Peters anomaly aim to clear the central visual axis to allow for visual maturation. Iridoplasty for reformation of iris and cataract extraction for those with lens involvement may also be considered.
Medical therapy
Medical therapy would include monitoring of glaucoma, since intraocular pressure is often elevated in Peters patients due to the dysgenesis of anterior segment structures including angle structures.
While waiting for a more definitive procedure, pharmacological pupillary dilation using topical phenylephrine is a reasonable option to expand the visual axis and reduce the likelihood of amblyopia in cases where the opacity is limited to the central cornea. The use of phenylephrine is limited due to the mandatory strong adherence and its vasopressor effect. Cycloplegics such as atropine and cyclopentolate are not recommended for this purpose because of the impairment of accommodation.
Treatment for spectacle correction and amblyopia treatment should be initiated as soon as possible. There is a high incidence of strabismus in patients with Peters anomaly.[18]
Medical follow up
Patients with Peters anomaly should be considered for surgery for clearing of the visual axis as soon as medically possible. Due to the systemic problems that can accompany Peters anomaly, including cardiovascular and central nervous system defects, consultation with genetics, cardiology, and neurosurgery should be considered. Close follow-up for management of glaucoma is recommended, with examinations under anesthesia as needed.
Surgery
Penetrating keratoplasty (PK) in infancy before 1 year of age has been the conventional treatment in Peters and is preferred if the opacity involves the whole cornea.[7]
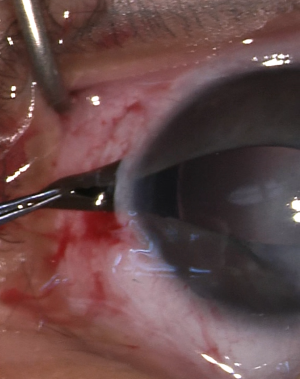
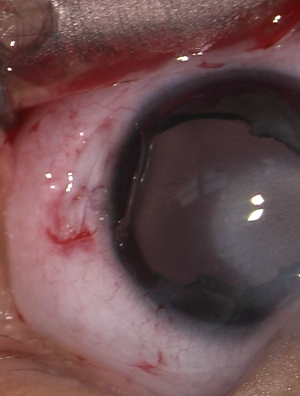
Optical iridectomy has been suggested as a safer alternative in cases with a clear peripheral corneal zone.[3] This procedure is shorter and technically easier than PK. It also avoids several of the possible complications of PK discussed below. Additionally, it is an alternative for use with high-risk PK patients, patients who cannot follow up, and patients in areas without trained pediatric corneal surgeons.
Optical iridectomy enlarges the pupillary aperture or creates a secondary visual axis by removing iris obstruction from the visual axis. During the procedure, an incision at the corneal limbus is made, and iris tissue at the intended new visual axis is externalized through the incision and transected. Alternatively, multiple radial incisions can be made at the pupillary sphincter in order to permanently dilate the pupil.
Surgical follow up
As with any corneal graft, frequent follow-up after PK is required to help prevent graft failure. In children, follow-up with frequent exam under anesthesia is warranted to check pressure as these infants will be on long-term immunosuppression with topical steroids and cyclosporine to avoid rejection. Complete suture removal within the first 2-3 months may be necessary as younger patients tend to have a more robust response with corneal neovascularization along the suture tracts.
Optical iridectomy requires less rigorous postoperative care and does not have the risk of graft rejection. Follow-up after optical iridectomy is required to ensure that the newly created visual axis remains patent and that proper refractive correction and amblyopia treatment are performed.
Complications
PK complications include graft failure, infection, and steroid-induced glaucoma. More than 50% of patients with Peters anomaly have secondary glaucoma that often requires multiple surgeries. Corneal transplantation is associated with high and variable rates of graft failure (20-80%). This variability may be due to a wide phenotypic spectrum, variation in age, as well as other comorbid congenital ophthalmic anomalies affecting the results of PK.[3][7]
Optical iridectomy carries the risk of scar at the clear corneal zone which can complicate the chance of achieving a red reflex. A large opening in the iris also increases the potential for photophobia and glare. Overall, complication rates cited in the literature after optical iridectomy have been low. [3]
Prognosis
The prognosis for maintaining clear graft 2 years after PK is a low 22%.[19] Another study reported that even if the graft is clear, long-term visual acuity is 20/200 or worse.[20] Due to the young age of the patients, it is believed that immunologic rejection is the most common cause of graft failure. Graft failure rates have been reported to increase with the severity of disease and when combined with lensectomy and vitrectomy. Another study found higher rates of graft survival, while concluding that those with preexisting glaucoma have a poorer visual prognosis.[21]
Overall visual prognosis is poor after corneal graft with one study finding that less than one-third of eyes with Peters have visual acuity better than 20/400. In that same study, predictors of poor visual outcome included stromal vessels and large corneal grafts >8mm. [22]
PK at the youngest possible age would theoretically minimize the risk of amblyopia, but surgery should be weighed against the risk of general anesthesia for the initial surgery and subsequent EUAs as well as the higher rate of graft failure in younger patients. Rao et al. showed that age plays a role in the likelihood of graft failure. In children less than 6 months of age, PK was associated with poorer outcomes.[19] The decision regarding when to perform PK should consider both the rate of graft failure and the degree of amblyopia that has developed.[23]
Studies have indicated that optical iridectomy can be performed in children as young one-week-old. In addition, three studies showed visual improvement, and two of those also showed good intraocular pressure postoperatively.[24][25] In a small proportion of cases, patients who underwent optical iridectomy needed a follow-up surgery of PK. Jünemann et al. reported 47% of patients had achieved a visual acuity of 20/500 to 20/200 over a mean follow-up of 3.5 years.[26]
Additional Resources
- http://ghr.nlm.nih.gov/condition/peters-plus-syndrome
- http://rarediseases.info.nih.gov/GARD/Condition/7377/Peters_anomaly.aspx
- http://www.images.missionforvisionusa.org/anatomy/2006/12/what-is-peters-anomaly-of-cornea.html
References
- ↑ Kurilec JM, Zaidman GW. Incidence of Peters anomaly and congenital corneal opacities interfering with vision in the United States. Cornea. 2014;33(8):848-850.
- ↑ Kaufman HE, Barron BA, McDonald MB. The Cornea. 2nd edition. Butterworth-Heinemann, 1997.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Spierer O, Cavuoto KM, Suwannaraj S, McKeown CA, Chang TC. Outcome of optical iridectomy in Peters anomaly. Graefes Arch Clin Exp Ophthalmol. 2018;256(9):1679-1683.
- ↑ Zhang X, Tong Y, Xu W, Dong B, Yang H, Xu L, Li Y. Two novel mutations of the PAX6 gene causing different phenotype in a cohort of Chinese patients. Eye (Lond). 2011 Sep 9. doi: 10.1038/eye.2011.215
- ↑ Weisschuh N, Wolf C, Wissinger B, Gramer E. A novel mutation in the FOXC1 gene in a family with Axenfeld-Rieger syndrome and Peters anomaly. Clin Genet. 2008 Nov;74(5):476-8
- ↑ Vincent A, Billingsley G, Priston M, Glaser T, Oliver E, Walter M, Ritch R, Levin A, Heon E. Further support of the role of CYP1B1 in patients with Peters anomaly. Mol Vis. 2006 May 16;12:506-10.
- ↑ Jump up to: 7.0 7.1 7.2 Bhandari R, Ferri S, Whittaker B, Liu M, Lazzaro DR. Peters anomaly: review of the literature. Cornea. 2011;30(8):939-944.
- ↑ Mataftsi A, Islam L, Kelberman D, Sowden JC, Nischal KK. Chromosome abnormalities and the genetics of congenital corneal opacification. Mol Vis. 2011;17:1624-40. Epub 2011 Jun 17.
- ↑ Neilan E, Pikman Y, Kimonis VE. Peters anomaly in association with multiple midline anomalies and a familial chromosome 4 inversion. Ophthalmic Genet. 2006 Jun;27(2):63-5.
- ↑ Berker N, Alanay Y, Elgin U, Volkan-Salanci B, Simsek T, Akarsu N, Alikasifoglu M. A new autosomal dominant Peters' anomaly phenotype expanding the anterior segment dysgenesis spectrum. Acta Ophthalmol. 2009 Feb;87(1):52-7
- ↑ Iwao K, Inatani M, Matsumoto Y, Ogata-Iwao M, Takihara Y, Irie F, Yamaguchi Y, Okinami S, Tanihara H. Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF-B2 signaling. J Clin Invest. 2009 Jul;119(7):1997-2008
- ↑ Aliferis K, Marsal C, Pelletier V, Doray B, Weiss MM, Tops CM, Speeg-Schatz C, Lesnik SA, Dollfus H. A novel nonsense B3GALTL mutation confirms Peters plus syndrome in a patient with multiple malformations and Peters anomaly. Ophthalmic Genet. 2010 Dec;31(4):205-8.
- ↑ Shimizu R, Saito R, Hoshino K, Ogawa K, Negishi T, Nishimura J, Mitsui N, Osawa M, Ohashi H. Severe Peters Plus syndrome-like phenotype with anterior eye staphyloma and hypoplastic left heart syndrome:proposal of a new syndrome. Congenit Anom (Kyoto). 2010 Sep;50(3):197-9. Epub 2010 Jun 24.
- ↑ Jump up to: 14.0 14.1 14.2 Mannis MJ, Holland EJ, Kumar P, Hammersmith KM, Eagle RC. Congenital Corneal Opacities: Diagnosis and Management. In: Cornea. London: Elsevier; 2022:1-208.
- ↑ Ni, Wei et al. “A novel histopathologic finding in the Descemet's membrane of a patient with Peters Anomaly: a case-report and literature review.” BMC ophthalmology vol. 15 139. 23 Oct. 2015.
- ↑ Yanoff M, Kataguiri P, Duker JS, Kenyon KR, Wadia HP, Vasaiwala RA. Congenital Corneal Anomalies. In: Opthalmology. Elsevier; 2019:172-176.
- ↑ Ocular Pathology Atlas. American Academy of Ophthalmology Web site. https://www.aao.org/resident-course/pathology-atlas. Published 2016. Accessed January 4, 2017.
- ↑ Najjar DM, Christiansen SP, Bothun ED, Summers CG. Strabismus and amblyopia in bilateral Peters anomaly. J AAPOS. 2006 Jun;10(3):193-7.
- ↑ Jump up to: 19.0 19.1 Rao KV, Fernandes M, Gangopadhyay N, Vemuganti GK, Krishnaiah S, Sangwan VS. Outcome of penetrating keratoplasty for Peters anomaly. Cornea. 2008;27(7):749-753.
- ↑ Chang JW, Kim MK, Kim JH, Kim SJ, Wee WR, Yu YS. Long-term visual outcomes of penetrating keratoplasty for Peters anomaly. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):953-958.
- ↑ Zaidman GW, Flanagan JK, Furey CC. Long-term visual prognosis in children after corneal transplant surgery for Peters anomaly type I. Am J Ophthalmol. 2007 Jul;144(1):104-108.
- ↑ Yang LL, Lambert SR, Drews-Botsch C, Stulting RD. Long-term visual outcome of penetrating keratoplasty in infants and children with Peters anomaly. J AAPOS. 2009 Apr;13(2):175-80.
- ↑ Basdekidou C, Dureau P, Edelson C, De Laage De Meux P, Caputo G. Should unilateral congenital corneal opacities in Peters anomaly be grafted? Eur J Ophthalmol. 2011 Feb 4
- ↑ Zaidman GW, Rabinowitz Y, Forstot LS. Optical iridectomy for corneal opacities in Peter’s anomaly. Journal of Cataract & Refractive Surgery. 1998;24(5):719–22.
- ↑ Chang TC, Cavuoto KM. Shallowing of the Anterior Chamber During Optical Iridectomy for Peters Anomaly. JAMA Ophthalmology. 2018;136(10):1199.
- ↑ Jünemann A, Gusek GC, Naumann GO. Optische Sektoriridektomie: Eine Alternative zur perforierenden Keratoplastik bei Petersscher Anomalie [Optical sector iridectomy: an alternative to perforating keratoplasty in Peters anomaly]. Klin Monbl Augenheilkd. 1996;209(2-3):117-124.