Diabetic Macular Edema
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Definition
Diabetic macular edema (DME) is the accumulation of excess fluid in the extracellular space within the retina in the macular area, typically in the inner nuclear layer, outer plexiform, Henle’s fiber layer, and subretinal space.[1][2]
Pathophysiology

Chronic hyperglycemia-related accumulation of advanced glycated end products disrupts the blood–retina barrier (BRB), leading to endothelial cell junction breakdown and pericyte loss. The inner BRB is composed of endothelial cells in the retinal capillaries, while the outer BRB is composed of retinal pigment epithelium cells. When the BRB is altered, interstitial fluid is accumulated within and underneath the retina through leakage of molecules dependent on intact cell-to-cell junctions (Figure 1).[3]
Evidence also shows that DME has an inflammatory component, with several chemokines and cytokines involved in its development. These include vascular endothelial growth factor (VEGF), interleukins (ILs), matrix metalloproteinases, and tumor necrosis factor (TNF). Upregulation of multiple pathways leads to increased inflammation, oxidative stress, and vascular dysfunction.[4] Significant changes are seen in the neurovascular unit, altering the homeostasis between astrocytes, ganglion cells, Müller cells, retinal vascular endothelial cells, and amacrine cells.[5] Retinal vascular permeability changes also involve the kallikrein-kinin system, which induces vasorelaxation via bradykinin and nitric oxide.[6][7][8]
Natural History
DME can develop at any stage of diabetic retinopathy (DR), from mild nonproliferative diabetic retinopathy (NPDR) to proliferative diabetic retinopathy, but is more frequent as the severity of DR increases. DME occurring near to or at the fovea is more likely to result in blurred vision and metamorphopsia. When the DME involves or threatens the fovea, the risk of moderate visual loss (defined as a ≥3-line decrease in visual acuity (VA), equivalent to a doubling of the visual angle) increases; in the Early Treatment of Diabetic Retinopathy Study (ETDRS) study, visual loss decreased by 24% over 3 years without treatment.[9] The disease course is variable, with some eyes having chronic persistent DME spanning several years, while other eyes have rapid spontaneous resolution, although the risk of recurrence is always present.
Prevalence
The Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) found that the 25-year incidence of DME in people with type 1 diabetes mellitus (T1DM) was 29%.[10] The Diabetes Control and Complications Trial (DCCT) reported that 27% of people with T1DM had DME within 9 years of diabetes onset.[11] The WESDR also found that 25.4% of people with type 2 DM who used insulin and 13.9% of those who did not use insulin had DME.[10] Yau et al. estimated the global prevalence of DME to be 6.8% among people with DM. In the United States, prevalence rates are between 2.7% to 3.8%, with non-Hispanic White people less likely to have DME than non-Hispanic Black people.[12][13][14]
Risk Factors
Risk factors for DME and DR are similar. These include a longer DM duration, poor control of DM with elevated glycolated hemoglobin A1c (HbA1c) levels, hypertension, and hyperlipidemia. Other secondary risk factors include impaired renal function and the use of thiazolidinediones.[15][16][17]
Prevention
Primary prevention of DME involves intensive control of DM, blood glucose and lipid levels, hypertension, and other systemic risk factors. The Diabetic Retinopathy Clinical Research (DRCR) Protocol W investigated whether aflibercept injections in eyes with baseline moderate-to-severe NPDR could prevent the eventual development of center-involved DME (ci-DME) with vision loss.[18] Vision loss was defined as a ≥10-letter decrease in VA at 1 visit or a 5-to 9-letter decrease at 2 consecutive visits, with the decrease in vision attributed to the ci-DME. The 2-year cumulative probability of developing ci-DME with vision loss was 4.1% in patients given aflibercept vs 14.8% of patients given sham injections. However, there was no significant difference in mean VA between the treatment groups. From baseline to the 2-year checkup, eyes treated with sham injections had a mean (SD) change of −2.0 (6.1) letters, while eyes treated with aflibercept had a mean −0.9 (5.8) letter change (adjusted mean difference 0.5 letters; 97.5% CI, −1.0 to 1.9 letters; P = 0.47]. However, at present the use of anti-VEGF injections for the prevention of ci-DME is not the standard of care.[18]
Diagnosis
History
DME is suspected in patients with any level of DR who present with blurred vision or metamorphopsias. A detailed history including the approximate date of onset of diabetes, the use of insulin versus oral antihyperglycemic agents, and the quality of metabolic control (e.g., HbA1c level) should be elicited. Any associated medical problems such as hypertension, hypercholesterolemia, renal disease, and thyroid disease should be identified, along with a thorough review of medications. It should be noted that mild to extensive DME may be present without symptoms evident to the patient.
Physical Examination
Patients suspected of having DME may undergo a detailed biomicroscopic examination with a slit lamp and indirect ophthalmoscope. Historically, DME classifications were based on the 1980s-era ETDRS definitions of clinically significant macular edema (CSME). The specific criteria for diagnosing CSME were:
- Retinal thickening at or within 500 μm of the center of the fovea
- Hard exudates at or within 500 μm of the center of the fovea if adjacent to an area of retinal thickening
- Retinal thickening of at least 1 disc area, any portion of which is within 1500 μm (approximately 1 disc diameter) from the center of the fovea
Thus, CSME, as defined by the ETDRS, is a clinical diagnosis made by slit-lamp examination using a contact lens. While clinical examinations remain essential for the full evaluation of DME, optical coherence tomography (OCT) is now routinely used to complement physical examination in the diagnosis of DME. The management of DME is currently based on the central subfoveal thickness (CST) on the macular OCT.
Signs
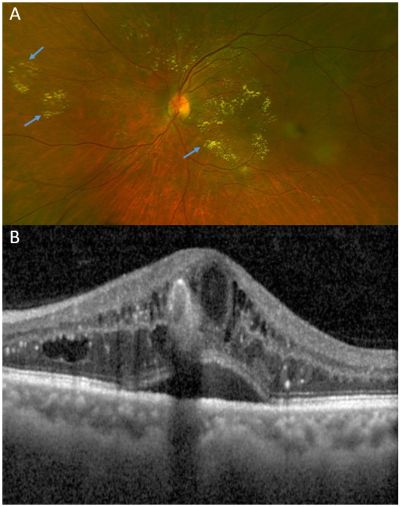
Macular thickening with or without hard exudates may be seen on stereo biomicroscopy. However, some eyes may present without apparent signs of retinal thickening on clinical examination, despite significant OCT-observed DME. Thickening can occur in various patterns, including focal, multifocal, and diffuse areas of retina thickening. Despite these terms being frequently used, there are no well-established standard, consistently used definitions.[19] In the ETDRS guidelines, focal leakage resulting from microaneurysms may be treated with a fluorescein angiography–guided focal laser, while diffuse capillary leakage is from a more widespread breakdown of the BRB and may be treated with grid laser.[20] Hard exudates in various patterns may also be seen, including circinate rings and focal aggregations of exudates (Figures 2–3). Hard exudates consist of lipoprotein residues of serous leakage from damaged vessels, and can serve as biomarkers for DME.
Symptoms
DME may present with decreased visual acuity (VA), metamorphopsia, changes in color perception, and difficulty reading, although it may also present asymptomatically.
Vision and DME
Studies show a poor to modest correlation between VA and CST on OCT, within the range of 0.3–0.5.[21][22] Over 2 years, only around 12%–14% of the change in VA can be attributed to the change in thickness. In a study by the DRCR Network, the slope of the best fit line shows an approximately 4.4 letter improvement (95% CI, 3.5–5.3) for every 100-micron decrease in CST at baseline.[22] Interestingly, on follow-up after treatment with macular laser photocoagulation, there were some eyes with paradoxical improvement in VA with increased CST (7-17% at different time points) and paradoxical worsening of VA with decreased CST (18-26% at different time points). These findings highlight how OCT measurements cannot be used as a perfect surrogate for visual acuity; however, in clinical and research settings, the technology remains an important tool. The DRCR Network has recommended a 10% change in CST to indicate a real change that can be considered in clinical decision-making.
Diagnostic Procedures
Optical Coherence Tomography (OCT)

Within recent years, OCT has quickly become an important ancillary procedure in diagnosing and treating DME. Three basic structural changes can be seen on OCT: retinal swelling, cystoid macular edema, and subretinal fluid (Figure 4). Macular scans can quickly and accurately identify even subtle areas of thickening, along with quantitative metrics for different areas. Changes in the anatomic distribution of DME can be monitored over time, especially the fluid’s relationship to the fovea. This information has clinical and research implications for the evaluation and management of DME. The International Council of Ophthalmology's guidelines for diabetic eye care, published in 2018, adopted the clinical entities of center-involved DME (ci-DME) and non-center–involved DME (non-ciDME) for the evaluation of macular fluid (Figure 5), as follows:
- Center-involved DME: Retinal thickening in the macula that involves the central subfield zone (1 mm in diameter)
- Non-center–involved DME: Retinal thickening in the macula that does not involve the central subfield zone (1 mm in diameter)
Aside from the location of the swelling, the DRCR Network has given recommendations on CST treatment thresholds based on sex-matched standards.[23] The thresholds were different for each OCT machine; since thickness measurements cannot be compared between different devices, each device has its own normative database and algorithms. In DRCR Protocol T, treatment eligibility thresholds per machine were:
- Heidelberg Spectralis: 320 μm for men and 305 μm for women
- Zeiss Cirrus OCT: 305 μm for men and 290 μm for women
- Zeiss Stratus OCT:250 μm for both men and women
Moreover, OCT is a more sensitive method for objective evaluation of vitreomacular interface abnormalities (VMIA), which include vitreomacular adhesion (VMA), vitreomacular traction (VMT), and epiretinal membrane (ERM). Identifying VMIA is crucial when diagnosing the etiology of macular edema, whether primarily from DME, from secondary causes of VMIA, or combined mechanism macular edema.

OCT Biomarkers for DME
DME's different OCT features have been associated with disparate prognostic outcomes and treatment responses.
- Disorganization of the retinal inner layers (DRIL) is thought to represent damaged cells within the inner retinal layers, indicating a disruption in the normal visual pathway from the photoreceptors to the ganglion cells.[24] DRIL is identified on OCT when there is disruption of the demarcating interface lines between the ganglion cell-inner plexiform complex, inner nuclear layer (INL), outer plexiform layer (OPL), and outer nuclear layer. Eyes with DRIL have a worse baseline and final VA despite anti–vascular endothelial growth factor (VEGF) injections and have an almost 8× greater risk for poor visual recovery. In contrast, the resolution of DRIL results in better visual outcomes (Figure 6).[25][26][27] DRIL was better correlated with VA than either glycemic status and CST.[24]
- External limiting membrane (ELM) and ellipsoid zone (EZ) disruption strongly correlate with baseline and final BCVA. At the same time, some studies found that restoration of the EZ was a requirement for good visual recovery (Figure 7).[28][29][30][31][32] The ELM connects a row of zonular adherents to the photoreceptor cell bodies, while the EZ represents the isthmus between the outer and inner segments of photoreceptors.[33]
- DME associated with VMIA, notably ERM and VMT, has poorer visual outcomes (Figure 8).[34][35] VMIA have been observed in 7%–16% of eyes with DME, with an annual incidence rate of up to 4.5%.[36][37] The READ-3 trial showed that eyes with VMIA still had a good response to anti-VEGF therapy.[38] Presence of posterior vitreous detachment (PVD) has been associated with improved visual outcomes. Nasrallah et al. reported that DME was present in 55% of eyes with an attached hyaloid, compared with only 20% of eyes with PVD.[39] Hikishi et al. reported only a 25% rate of spontaneous resolution in eyes with attached posterior hyaloid, vs a 55% resolution rate in eyes with PVD.[40] The role of the vitreous and VMIA in DME, which may be related to changes in transvitreal oxygenation, hyalocyte proliferation from vitreous remnants on the ILM, and the present growth factors in the premacular hyaloid, is still being investigated.[41][42][43][44]
 Figure 7: External limiting membrane and ellipsoid zone disruption. A: DME with large cystic spaces (blue arrow) and exudates (green arrows). Despite anti-VEGF treatment, DME persisted. The EZ and ELM were still intact, with BCVA of 20/32. B: Five years after photo A, extensive ELM and EZ disruption (red bracket) developed which involved the fovea. A few chronic parafoveal cysts can still be seen. BCVA dropped to 20/63.
Figure 7: External limiting membrane and ellipsoid zone disruption. A: DME with large cystic spaces (blue arrow) and exudates (green arrows). Despite anti-VEGF treatment, DME persisted. The EZ and ELM were still intact, with BCVA of 20/32. B: Five years after photo A, extensive ELM and EZ disruption (red bracket) developed which involved the fovea. A few chronic parafoveal cysts can still be seen. BCVA dropped to 20/63.
Fluorescein Angiography

Fluorescein angiography (FA) is performed to identify leaking microaneurysms or capillaries to help guide laser treatment, and to identify areas of retinal ischemia. Leakage seen with FA is not synonymous with retinal edema. Focal DME is characterized by focal leakage from microaneurysms or capillaries; in contrast, diffuse DME is characterized by poorly demarcated areas of capillary leakage (Figure 9). Recently, there has been a decreasing trend in the use of FA in the management of DME, likely due to the procedure being more invasive and time-consuming than OCT.[45] Contraindications to the use of FA include pregnancy and allergy associated with the contrast dye.
Laboratory Testing
Primary laboratory testing for DME should involve checking HbA1c, blood pressure, and lipid levels. Secondary tests can include hemoglobin (anemia exacerbates diabetic retinopathy and may be associated with diabetic nephropathy); both fasting and postprandial blood sugar; urea, creatinine, and urine microalbumin levels, and a thyroid panel.
Differential Diagnosis

Other causes of DME include retinal vein occlusion, ruptured macroaneurysm, Irvine-Gass syndrome, radiation retinopathy, hypertensive retinopathy, subfoveal choroidal neovascularization, retinal vein occlusion, and VMIA. OCT and FA ancillary diagnostics can help to identify these differential diagnoses (Figures 10–11).

Management
Management of DME includes strict control of diabetes, blood glucose, hypertension, and hypercholesterolemia.[46] This can be achieved by pharmacologic methods or by nonpharmacologic methods, such as diet modification, weight loss, and exercise.
Pharmacotherapy
At present, anti-VEGF agents are the first-line treatment for DME. The FDA approved ranibizumab for DME in 2012, aflibercept in 2014, and brolucizumab and faricimab in 2022. Since 2005, intravitreal bevacizumab has been used off-label for ocular conditions.
DRCR Network Anti-VEGF Treatment Algorithm
The DRCR anti-VEGF treatment algorithm states that 6 monthly injections should be given unless VA is 20/20 or better and CST is <320 μm for men or <305 μm for women (as measured by the Heidelberg Spectralis), in which case treatment may be withheld starting on the 4th month. At the 6th visit, if stability in vision or CST has been achieved (based on the previous 2 visits' findings) or DME has resolved, further treatment may be withheld. Stability is defined as:
- Lack of improvement
- No BCVA increase of ≥5 letters (approximately 1 line on a Snellen chart)
- No decrease in OCT-measured CST ≥10% (per OCT imaging)
- No worsening
- BCVA decreases by ≥5 letters (approximately 1 line on a Snellen chart) in the setting of persistent DME
- OCT-measured CST increases by ≥10%
If there is worsening on follow-up, anti-VEGF treatment is resumed. In general, eyes showing stability may be followed up in 3–4 months, or sooner as needed. Withholding injections does not require 20/20 vision or a dry macula, only evidence of stability over the previous 2 or more visits. DRCR Protocol I issued the first anti-VEGF as-needed treatment algorithm for DME, as long-term data show that even without using a monthly treatment protocol, eyes with persistent DME often maintain good BCVA over the long term in clinical trial settings.[47] Outside of trial settings, the 5-year extension phase of Protocol T showed that CST remained stable among eyes given anti-VEGF injections; however, mean BCVA worsened between the 2- and 5-year time points.[48] On average, patients are given 6–8 injections in the first year, 2–4 injections in the second year, and 0–1 injections beginning the third year.[49]
Clinical Considerations for Treating DME With Anti-VEGF Agents
Observation is recommended for eyes with ci-DME and VA of 20/25 or better. This recommendation is based on the findings of DRCR Protocol V, where patients were randomized to receive aflibercept injections, macular laser, or no treatment (observation). At the 2-year endpoint, the mean BCVA was 20/20, and the rates of ≥5-letter vision loss were similar among all 3 groups (16%–19%). Given the risks of ocular infection and the higher costs associated with intravitreal injections, observation is a viable option in these patients if they can reliably follow up and receive appropriate therapy when there is clinical deterioration. In contrast, among eyes with ci-DME presenting with 20/50 vision or worse, Protocol T study results showed that aflibercept was superior to both ranibizumab and bevacizumab, with area-under-the-curve analyses showing better visual outcomes over 1 year.[50] However, the superiority of aflibercept over ranibizumab was not maintained at the 2-year follow-up visit.[23] Bevacizumab thinned the retina the least; however, all 3 medications had similar visual outcomes among eyes with baseline VA of 20/40 or better.
Not all treatments result in complete resolution of DME. In Protocol I, 40% of eyes still showed persistent DME (defined as never having a CST <250 μm through 6 months, as seen on time-domain OCT) after 2 years.[47] Eyes given bevacizumab were more likely to have persistent DME than eyes given aflibercept. The percentage of eyes with complete resolution increased with each ranibizumab or aflibercept injection.[51] At the 3-year follow-up visit, eyes with chronic persistent DME still had a significant (7-letter) mean improvement in VA over baseline, though lower than the 13-letter mean improvement in eyes with complete DME resolution.[52] Importantly, only 3.4% of eyes with chronic persistent DME lost ≥2 lines of vision over 2 years regardless of the type of anti-VEGF treatment they received.[53] To summarize, in clinical trial settings, a substantial proportion of eyes with persistent DME still had ≥2 lines of improvement in vision over the long term, with very few eyes developing considerable vision loss.
For eyes that do not respond optimally to one anti-VEGF agent, switching to another is a treatment option. The switch is typically from bevacizumab to either ranibizumab or aflibercept, or from ranibizumab to aflibercept. The rationale for switching is that aflibercept has 100 times more affinity for VEGF-A than bevacizumab or ranibizumab, while concurrently binding placental growth factor and VEGF-B.[54] Aflibercept also has a longer half-life and can negate more cytokines that promote DME development. Most studies looking at switching were retrospective and showed a significant reduction in CST; however, visual outcomes varied.[55][56][57][58][59][60][61][62][63][64][65][66][67][68] Some eyes may be delayed responders to anti-VEGF treatment, and need more than the initial 3–6 monthly injections to show significant improvement, though they eventually catch up with immediate responders.[69][70] It may be viable to continue treatment with the same medication if there is at least a trend of continued improvement, as bevacizumab and ranibizumab are more cost-effective options. Caution is warranted for switching, as any clinical improvement may be due to continued treatment among delayed responders instead of due to a shift in medications.
DRCR protocol AC was a randomized controlled trial evaluating the relative efficacy of administering aflibercept monotherapy vs bevacizumab first with a switch to aflibercept (step therapy) in eyes with suboptimal response despite treatment. A total of 312 eyes with moderate vision loss from ci-DME were enrolled. Over a 2-year period, the mean improvement in VA was 14.0 letters in the bevacizumab-first group and 15.0 letters in the aflibercept-monotherapy group (adjusted difference, 0.8 letters; 95% CI, −0.9 to 2.5; P = 0.37). At 2 years, mean changes in VA and retinal CST were similar in the two groups.[71]
Steroids
Intravitreal steroids improve vision and decrease retinal thickness, as there is an inflammatory component to DME. Steroids have powerful anti-edematous and anti-inflammatory effects, as they decrease several pro-inflammatory mediators (IL-6, IL-8, TNF-α, monocyte chemotactic protein-1, intercellular adhesion molecule-1, VEGF, etc).[72] However, long-term results have not shown maintenance of initial clinical improvement. In DRCR Protocol I, although the group given intravitreal triamcinolone had a similar response with ranibizumab in the first 6 months, vision declined due to cataracts. Even with cataract surgery, the final vision did not recover to the levels seen in the group given ranibizumab.[47] In Protocol U, eyes with persistent DME despite at least 6 ranibizumab injections were randomized to continue ranibizumab monotherapy or shift to ranibizumab plus dexamethasone (Ozurdex).[73] At 6 months, the average visual gain was similar in both groups, although CST improvement was greater in the combination group. Phakic eyes given intravitreal steroids often develop cataracts needing surgery, and are at risk for intraocular pressure (IOP) elevations leading to glaucoma.
Landmark Pharmacotherapy Studies
Anti-VEGF Agents
- Bevacizumab: Bevacizumab is a recombinant humanized monoclonal immunoglobulin G1 (IgG1) antibody that binds VEGF. Bevacizumab is given off-label for the treatment of DME, and remains the most cost-effective treatment option among anti-VEGF medications.[74] In the BOLT study, intravitreal bevacizumab (1.25 mg), given at 6-week intervals, was reported to be more effective than modified ETDRS focal/grid laser in terms of VA improvement at 12 months.[75] In DRCR Protocol T, a comparison between bevacizumab, ranibizumab, and aflibercept, 1-year results showed that bevacizumab thinned the retina the least.[23] However, all 3 medications had similar visual outcomes among eyes with baseline vision of 20/40 or better.[53] A study from The Pan-American Collaborative Study Group found improvements in BCVA and reduced macular thickness (seen on OCT) at 24 months with intravitreal bevacizumab doses of 1.25 mg–2.5 mg.[76]
- Ranibizumab: Ranibizumab is a recombinant humanized IgG1 monoclonal antibody fragment that binds and inhibits VEGF-A.[77] It is FDA approved for the treatment of DME.
- DRCR Protocol I was the first definitive phase 3 study demonstrating the effectiveness of ranibizumab for DME treatment.[47] Four treatment arms were compared: ranibizumab with immediate grid/focal laser; ranibizumab with macular laser given only for at least 6 months of persistent DME; intravitreal triamcinolone with immediate macular laser; and macular laser with sham injections. Results showed that ranibizumab, given in as-needed treatment protocol, had efficacy superior to that of laser therapy. At the 1-year end point, eyes treated with ranibizumab gained an average of 8–9 letters, vs an average gain of only 3 letters seen with laser therapy. Protocol I revolutionized the treatment protocol for DME when it was published in 2011. Before this study, macular laser was consideredfirst-line therapy for DME, a treatment protocol originating from the original ETDRS papers first published in 1985.
- RISE and RIDE: The 2-year results for ranibizumab 0.3-mg monthly infections showed that 98% of patients maintained vision (lost <15 letters) and 34%–45% of patients gained at least 15 letters; the mean VA gain was 10.9–12.5 letters.[78] Only 45%–49% of patients needed macular laser, compared with 91%–94% in the control group. No additional benefit was seen with ranibizumab 0.5 mg monthly vs 0.3 mg monthly.[79]
- Aflibercept: Aflibercept is a soluble decoy receptor that binds VEGF-A, VEGF-B, and placental growth factor with high affinity.[79] It is termed a decoy receptor or a “VEGF-trap,” as VEGF mistakenly binds with aflibercept instead of the body’s native receptors, reducing VEGF’s activity. It is FDA approved for the treatment of DME. In the Da Vinci study, patients with DME were randomly assigned to 1 of 5 treatment regimens: aflibercept 0.5 mg every 4 weeks (0.5q4), 2 mg every 4 weeks (2q4), 2 mg every 8 weeks after 3 initial monthly doses (2q8), or 2 mg as needed after 3 initial monthly doses (2prn); or macular laser photocoagulation.[80] Mean improvements in BCVA in the aflibercept groups at week 52 were 11.0, 13.1, 9.7, and 12.0 letters for the 0.5q4, 2q4, 2q8, and 2prn regimens, respectively, vs −1.3 letters for the laser group. The proportion of eyes with gains in BCVA of ≥15 ETDRS letters at week 52 in the aflibercept groups were 40.9%, 45.5%, 23.8%, and 42.2%, respectively, vs 11.4% for laser. Mean reductions in CST in the aflibercept groups at week 52 were −165.4 μm, −227.4 μm, −187.8 μm, and −180.3 μm, respectively, vs −58.4 μm for laser.[79] The PHOTON trial evaluated the use of high-dose aflibercept (8 mg) aflibercept every 12 weeks (8q12) or 16 weeks (8q16) vs conventional dosing of 2 mg every 8 weeks (2q8).[81] When comparing BCVA gains and retinal thinning at 48 weeks, the 8-mg extended interval dosage was noninferior to conventional treatment. There was an increase of 9.2, 8.8, and 7.9 letters and a mean CST reduction of −165 μm, −172 μm, and −148 μm for the 2q8, 8q12, and 8q16 groups at 48 weeks, respectively. Patients in the high-dose group with a 50-μm increase in CST or a 10-letter loss from week 12 onwards had their dosing schedule shortened to either 12 or 8 weeks, but 93% of patients remained on dosing intervals of ≥12 weeks. There was no increase in hypertension in the high-dose group.
- Faricimab: Faricimab is a novel combined-mechanism inhibitor that binds both angiopoietin-2 and VEGF-A with high specificity and affinity. The 1-year results from the phase 2 BOULEVARD study showed that in patients given faricimab 6 mg, there was a statistically significant VA gain of 3.6 letters over that seen with ranibizumab.[82] Moreover, improvement in diabetic retinopathy severity, as well as reduced CST, was seen with faricimab; it also had a longer time to re-treatment than ranibizumab. There were no unexpected safety concerns. The phase 3 YOSEMITE and RHINE noninferiority trials compared faricimab with aflibercept in patients with DME.[83] The noninferiority primary endpoint of BCVA was achieved with faricimab given every 8 weeks. More than 50% of patients had good results with an every-16-weeks dosing regimen, and more than 70% had good results with faricimab given every 12 weeks or longer. These results demonstrate the potential of faricimab to decrease patients' treatment burden by extending the intraocular durability of anti-VEGF treatment. The FDA approved the use of faricimab for DME as 4 monthly loading doses followed by injections every 1–4 months, or as a loading dose of 6 monthly injections followed by injections every 2 months.
- Brolucizumab: Brolucizumab is a single-chain antibody fragment with a high affinity for VEGF. Brolucizumab’s low molecular weight of 26 kDa allows for more of the drug to be delivered intraocularly per injection, with the potential for increased durability and effective tissue penetration in the eye. The phase 3 KESTREL and KITE 52-week results found that brolucizumab 6 mg was noninferior to aflibercept in mean improvement of BCVA. More patients taking brolucizumab had CST <280 μm without persistent macular fluid than patients taking aflibercept, and >50% of the brolucizumab-treated patients were maintained on q12w dosing after the loading dose was given.[84] However, reports of retinal vascular occlusion (RVO) and retinal vasculitis with intraocular inflammation (IOI) have been reported.[85][86] Risk factors for developing these adverse reactions include a prior history of IOI or RVO in the 12 months before brolucizumab initiation, female sex, and same-day bilateral injections.[87][88] The FDA approved the use of brolucizumab for the treatment of DME in June 2022, as a 6-mg injection given every 8–12 weeks after a loading dose of 5 injections given every 6 weeks.
Steroids
- Triamcinolone: In Protocol B, triamcinolone (1 or 4 mg) preservative-free intravitreal injection was less effective and had more side effects than focal/grid photocoagulation at the 2-year follow-up visit.[89]
- Dexamethasone: In the 3-year phase 3 MEAD study, dexamethasone 0.7 mg biodegradable implant improved vision by at least 15 letters in 22% of patients.[90] At this dose, 41.5% of patients needed antiglaucoma medications, with 0.6% needing glaucoma surgery. Around 60% of eyes in the 0.7-mg implant group had cataract surgery. The 0.7-mg implant has been approved by the FDA for DME, and is effective for approximately 3–6 months.
- Fluocinolone acetonide: The fluocinolone acetonide (Iluvien) 0.19-mg non-biodegradable implant is a sustained-release device that is effective for up to 3 years when administered intraocularly. It is FDA approved to treat DME in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in IOP.[91][92] At the 0.2-μg/day dosage, BCVA improvement of at least 15 letters was found in 28.7% of patients, with 38.4% needing anti-glaucoma medications and 4.8% needing glaucoma surgery. The latter figure was reduced to 0% among eyes that did not have a history of IOP elevation with a prior steroid challenge. Complications associate with both the fluocinolone and the dexamethasone implants include cataracts and implant migration.
Laser Photocoagulation
Before anti-VEGF treatments for DME were approved, the standard treatment for CSME was macular laser photocoagulation. In “focal” CSME, a focal laser pattern is used to treat leaking microaneurysms identified on the FA that contribute to the retinal edema (Figure 12). In “diffuse” CSME, intraretinal leakage is noted on the FA from dilated retinal capillary beds or intraretinal microvascular abnormalities without isolated, discrete foci of leakage. A macular grid is done in cases of diffuse macular edema (Figure 13). Laser photocoagulation has been shown to decrease the risk of moderate visual loss (loss of ≥15 ETDRS letters) from 24% to 12% after 3 years.[9] After the laser treatment is administered, the patient will come back in 3 months for a follow-up examination. If residual CSME is noted, OCT and FA may be performed to evaluate the benefit and location of repeat laser treatment. With the FDA approval of anti-VEGF agents for DME, focal/grid laser is only indicated in patients with non-ciDME. Macular laser remains a viable treatment option for patients with DME, especially in resource-limited countries with decreased access to anti-VEGF agents.
Combined Therapy
- Intravitreal ranibizumab with laser: In Protocol I, intravitreal ranibizumab with prompt (within 1 week) or deferred (after 24 weeks) laser was more effective than focal/grid laser alone for the treatment of ci-DME.[47] Ranibizumab is injected intravitreally at baseline, followed by monthly injections for 4 months followed by the continuation of injections at 16 weeks if the OCT central subfield thickness is ≥250 um with VA worse than 20/20.
- Steroid with laser: Protocol B found that intravitreal triamcinolone (IVT) 4 mg plus focal/grid laser (given within a week of injection) was more effective than laser alone at 4 months. However, long-term benefit with this combined therapy was not seen, with mean BCVA better in the laser monotherapy group than the combined therapy group from the 16-month time point until the 2-year end point. Complications of steroid therapy included cataracts, ocular hypertension, and glaucoma. The difference in BCVA could not be attributed fully to the development of cataracts. In Protocol I, 6-month VA improvement in eyes given IVT with prompt laser was comparable to that seen in eyes given ranibizumab, but vision declined afterward until the 2-year time point.[47][93] A subgroup analysis in pseudophakic eyes given IVT showed that visual improvement was significantly better than in phakic eyes, with 1- and 2-year results comparable to pseudophakic eyes given ranibizumab.
- Intravitreal ranibizumab with peripheral targeted retinal photocoagulation (TRP): The DAVE study was a phase 1/2 clinical trial that compared ranibizumab plus peripheral TRP and ranibizumab monotherapy for reduce the number of required anti-VEGF injections.[94] Peripheral TRP was defined as retinal photocoagulation administered outside the macula to areas of retinal capillary nonperfusion identified on widefield FA. The nonperfused hypoxic retina is thought to upregulate hypoxia-inducible factors and cytokines, including VEGF and erythropoietin, with small studies suggesting that TRP can decrease the anti-VEGF treatment burden.[95][96][97][98][99] At the 3-year endpoint, there was no evidence that combined ranibizumab plus TRP reduced treatment burden or improved vision outcomes compared with ranibizumab alone.
Surgery
No well-constructed studies show a definitive benefit of pars plana vitrectomy (PPV) for managing DME. The theoretical basis for PPV as a treatment option comes from reports that it increases vitreous oxygenation in ischemia, leading to decreased VEGF production, and from the observation that DME is less common among eyes with PVD.[100][101][102][103][104][105] Vitreous viscosity also significantly decreases after PPV, which may bring about a greater diffusion of pro-inflammatory cytokines away from the macula.[106] Other studies suggest that PPV plus internal limiting membrane (ILM) peeling brings better resolution of the tractional forces at the vitreoretinal interface known to worsen DME. This procedure also prevents proliferating astrocytes from using the ILM as a scaffold, which may lead to ERM.[107] In a systematic review looking at PPV for DME, CST was significantly decreased by 102 μm, and a nonsignificant VA increase of 2 letters was observed.[108] However, the anatomic benefit was not maintained by the 12-month time point. A similar meta-analysis looking at PPV plus ILM peeling vs PPV alone showed no significant difference in postoperative vision and macular thickness between the treatments.[109] DRCR Protocol D, a prospective study of eyes with DME and VMT, found that at 6 months after surgery, 43% of eyes had a reduction in CST to <250 μm.[110] However, the median VA did not change at 6 months. In 38% of eyes, the median visual acuity improved by ≥10 letters, and in 22% the median VA decreased by ≥10 letters. A post hoc analysis of Protocol I showed that previously vitrectomized eyes with ci-DME given anti-VEGF treatments had no improved clinical outcomes compared with non-vitrectomized eyes.[111]
Treatment Complications
The complications listed below may arise from the various treatment modalities. The per-injection risk of developing complications is also listed below, when available.
Intravitreal Injections
- Endophthalmitis (0.019%–0.05%)[112][113][114]
- Intraocular inflammation (0.09%–0.4%)[115]
- Retinal tears/detachment (0.01%–0.08%)[116][117][118]
- Increase in IOP (for intravitreal steroids, IOP ≥21 mmHg in 45% at 1 month, 20% at 3 months, and 13% of eyes at 6 months post-injection.)[119]
- Cataract (common after intravitreal steroid injections. By year 3, 83% of patients given IVT needed cataract surgery.)[120]
- Subconjunctival hemorrhage (10%)[121]
- Vitreous hemorrhage
Laser Photocoagulation
- Subretinal fibrosis
- Extension of the laser scar into the fovea
- Choroidal neovascular membrane
- Paracentral scotoma
- Decreased visual acuity
Pars Plana Vitrectomy
- Retinal tears and retinal detachment
- Vitreous hemorrhage
- Elevated intraocular pressure
- Endophthalmitis
- Cataract
Prognosis
Clinical factors associated with better visual outcomes with anti-VEGF treatment include lower HbA1c, younger age, less severe diabetic retinopathy, absence of ERM, quick and consistent CST decreases, and absence of prior panretinal photocoagulation.[122][123] In terms of anatomic outcomes, eyes with hard exudates within the 6-mm foveal center had a larger CST decrease at the end of 1 year. The presence of exudates may be a marker of BRB abnormalities typical of DME responsive to anti-VEGF therapy. Eyes that lack exudates may have other underlying mechanisms of retinal thickening, including cystoid degeneration, traction, or ischemia. In contrast, eyes with high baseline CST (≥570 μm) had a significantly higher chance of developing persistent DME despite monthly ranibizumab therapy.[124] In terms of response to macular laser therapy, the ETDRS reported that worse clinical outcomes were associated with higher blood lipid levels, presence of hard exudates, and diffuse edema.[125]
Matsunaga et al. looked at eyes with DME treated with at least 1 dose of anti-VEGF agents that were then awere lost to follow-up (LTFU) for at least 6 months before returning to the clinic.[126] Vison worsened significantly at the initial return visit, to ~20/69 from ~20/52; however, at the 3-, 6-, and 12-month follow-up visits and the final checkup, vision recovered with no significant differences from baseline. OCT CST also showed similar trends. Gao et al. found that 25% of patients with NPDR and DME had no follow-up visits for at least 1 year after receiving 1 anti-VEGF injection.[127] Factors associated with being LTFU included Hispanic ethnicity (OR 1.7) and American Indian/Pacific Islander/multiple ethnicities (OR 2.6). Lower adjusted gross income and decreasing baseline vision were also factors significantly associated with LTFU.
Some studies have shown increased risks of end-stage renal disease in patients with concurrent DME. Assessment of renal function should be strongly encouraged in patients with chronic DME.
Future Directions
Numerous pharmacotherapy trials for DME treatment are underway. The development of a long-acting anti-VEGF agent that could remain effective in the vitreous for multiple months or years would significantly decrease the treatment burden for patients needing monthly injections. Novel pharmacotherapies based on different mechanisms of action include anti-VEGF designed ankyrin repeat proteins, growth factor inhibitors, anti-inflammatory medications, hormone modulators, acetylcholine receptor blockers, insulin-like growth factor 1 receptor blockers, and neuroprotective/antiapoptotic agents.[128] Port delivery systems that are FDA approved for wet AMD are in development to treat diabetic retinopathy and DME.[129] These experimental therapies have the potential to significantly decrease the burden of treatment while restoring vision to patients with DME in the coming years ahead.
Artificial intelligence (AI) plus tele-ophthalmology models are being used to develop screening tools for diabetic retinopathy and DME. These models can detect the retinal complications of diabetes remotely, and if this technology is placed in the offices of internists and endocrinologists, it may allow for early detection and timely intervention.[130][131]
Further reading
- Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1-32
- Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010; 117(6):1064-1077.
- Early Treatment Diabetic Retinopathy Study: Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796-1806.
- Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12 month data: report 2. Ophthalmology. 2010; 117(6):1078-1086.e2.
- Schachat AP. A new approach to the management of diabetic macular edema. Ophthalmology. 2010;117(6):1059-1060.
References
- ↑ Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127(6):688-693.
- ↑ Yanoff M, Fine BS, Brucker AJ, et al. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28(suppl):505-511.
- ↑ Xu H-Z, Le Y-Z. Significance of outer blood–retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52(5):2160-2164.
- ↑ Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615-1625.
- ↑ Del Zoppo G. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267(2):156-171.
- ↑ Gao B-B, Clermont A, Rook S, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13(2):181-188.
- ↑ Jeppesen P, Aalkjær C, Bek T. Bradykinin relaxation in small porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2002;43(6):1891-1896.
- ↑ Parpura V, Basarsky TA, Liu F, et al. Glutamate-mediated astrocyte–neuron signalling. Nature. 1994;369(6483):744-747.
- ↑ Jump up to: 9.0 9.1 Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103:1796-1806.
- ↑ Jump up to: 10.0 10.1 Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy XV: the long-term incidence of macular edema. Ophthalmology. 1995;102(1):7-16.
- ↑ White NH, Sun W, Cleary PA, et al. Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes. 2010;59(5):1244-1253.
- ↑ Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmology. 2014;132(11):1334-1340.
- ↑ Wong TY, Klein R, Islam FA, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446-455.e441.
- ↑ Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
- ↑ Ishibazawa A, Nagaoka T, Takahashi K, et al. Association between diabetic macular edema and chronic kidney disease in patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2013;54(15):2400. Abstract.
- ↑ Hsieh Y-T, Tsai M-J, Tu S-T, et al. Association of abnormal renal profiles and proliferative diabetic retinopathy and diabetic macular edema in an Asian population with type 2 diabetes. JAMA Ophthalmology. 2018;136(1):68-74.
- ↑ Fong DS, Contreras R. Glitazone use associated with diabetic macular edema. Am J Ophthalmol. 2009;147(4):583-586.e581.
- ↑ Jump up to: 18.0 18.1 Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti–vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmology. 2021;139(7):701-712.
- ↑ Browning DJ, Altaweel MM, Bressler NM, et al. Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008;146(5):649-655.e646.
- ↑ Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology. 1987;94(7):761-774.
- ↑ Sun JK, Jampol LM. The Diabetic Retinopathy Clinical Research Network (DRCR.net) and its contributions to the treatment of diabetic retinopathy. Ophthalmic Res. 2019;62(4):225-230.
- ↑ Jump up to: 22.0 22.1 Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography–measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525-536.
- ↑ Jump up to: 23.0 23.1 23.2 Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
- ↑ Jump up to: 24.0 24.1 Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmology. 2014;132(11):1309-1316.
- ↑ Santos AR, Costa MÂ, Schwartz C, et al. Optical coherence tomography baseline predictors for initial best-corrected visual acuity response to intravitreal anti-vascular endothelial growth factor treatment in eyes with diabetic macular edema: the CHARTRES study. Retina. 2018;38(6):1110-1119.
- ↑ Radwan SH, Soliman AZ, Tokarev J, et al. Association of disorganization of retinal inner layers with vision after resolution of center-involved diabetic macular edema. JAMA Ophthalmology. 2015;133(7):820-825.
- ↑ Das R, Spence G, Hogg RE, et al. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmology. 2018;136(2):202-208.
- ↑ Maheshwary AS, Oster SF, Yuson RM, et al. The association between percent disruption of the photoreceptor inner segment–outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150(1):63-67.e61.
- ↑ Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina. 2010;30(5):774-780.
- ↑ Shin HJ, Lee SH, Chung H, et al. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):61-70.
- ↑ Lai K, Huang C, Li L, et al. Anatomical and functional responses in eyes with diabetic macular edema treated with “1+ PRN” ranibizumab: one-year outcomes in population of mainland China. BMC Ophthalmol. 2020;20(1):1-7.
- ↑ Mori Y, Suzuma K, Uji A, et al. Restoration of foveal photoreceptors after intravitreal ranibizumab injections for diabetic macular edema. Sci Rep. 2016;6(1):1-11.
- ↑ Bunt-Milam A, Saari JC, Klock IB, et al. Zonulae adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci. 1985;26(10):1377-1380.
- ↑ Ercalik NY, Imamoglu S, Kumral ET, et al. Influence of the epiretinal membrane on ranibizumab therapy outcomes in patients with diabetic macular edema. Arq Bras Oftalmol. 2016;79(6):373-375.
- ↑ Maryam AK, Tafgeh M, Mahmoud M, et al. Short term effect of intravitreal bevacizumab for diabetic macular edema associated with epiretinal membrane. Rom J Ophthalmol. 2018;62(3):212-216.
- ↑ Kim NR, Kim YJ, Chin HS, et al. Optical coherence tomographic patterns in diabetic macular oedema: prediction of visual outcome after focal laser photocoagulation. Br J Ophthalmol. 2009;93(7):901-905.
- ↑ Chang C-K, Cheng C-K, Bai C-H, et al. Development of vitreomacular interface abnormality in patients with diabetic macular edema. Taiwan J Ophthalmol. 2012;2(3):93-98.
- ↑ Sadiq MA, Soliman MK, Sarwar S, et al. Effect of vitreomacular adhesion on treatment outcomes in the Ranibizumab for Edema of the Macula in Diabetes (READ-3) study. Ophthalmology. 2016;123(2):324-329.
- ↑ Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95(10):1335-1339.
- ↑ Hikichi T, Fujio N, Akiba J, et al. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology. 1997;104(3):473-478.
- ↑ Stefánsson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79(5):435-440.
- ↑ Stolba U, Binder S, Gruber D, et al. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140(2):295-301.
- ↑ Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51(4):364-380.
- ↑ Bu S-C, Kuijer R, Li X-R, et al. Idiopathic epiretinal membrane. Retina. 2014;34(12):2317-2335.
- ↑ Bailey C, Sparrow J, Grey R, et al. The national diabetic retinopathy laser treatment audit. I. Maculopathy. Eye. 1998;12(1):69-76.
- ↑ Tripathy K, Raj Sharma Y, Chawla R, et al. Recent advances in management of diabetic macular edema. Curr Diabetes Rev. 2015;11(2):79-97.
- ↑ Jump up to: 47.0 47.1 47.2 47.3 47.4 47.5 Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609-614.
- ↑ Glassman AR, Wells III JA, Josic K, et al. Five-year outcomes after initial aflibercept, bevacizumab, or ranibizumab treatment for diabetic macular edema (Protocol T Extension Study). Ophthalmology. 2020;127(9):1201-1210.
- ↑ Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375-381.
- ↑ Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203.
- ↑ Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmology. 2018;136(3):257-269.
- ↑ Bressler SB, Ayala AR, Bressler NM, et al. Persistent macular thickening after ranibizumab treatment for diabetic macular edema with vision impairment. JAMA ophthalmology. 2016;134(3):278-285.
- ↑ Jump up to: 53.0 53.1 Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
- ↑ Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171-185.
- ↑ Do DV, Nguyen QD, Vitti R, et al. Intravitreal aflibercept injection in diabetic macular edema patients with and without prior anti–vascular endothelial growth factor treatment: outcomes from the phase 3 program. Ophthalmology. 2016;123(4):850-857.
- ↑ Ehrlich R, Dan I, Deitch I, et al. The effectiveness of intravitreal ranibizumab in patients with diabetic macular edema who have failed to respond to intravitreal bevacizumab. Ophthalmologica. 2016;235(3):133-136.
- ↑ Hanhart J, Chowers I. Evaluation of the response to ranibizumab therapy following bevacizumab treatment failure in eyes with diabetic macular edema. Case Rep Ophthalmol. 2015;6(1):44-50.
- ↑ Katz G, Moisseiev E, Goldenberg D, et al. Ranibizumab for persistent diabetic macular edema after bevacizumab treatment. Eur J Ophthalmol. 2017;27(2):210-214.
- ↑ Lee JH, Lee WK, Kim SE. Short-term outcomes of switching to ranibizumab therapy for diabetic macular edema in patients with persistent fluid after bevacizumab therapy. J Ocul Pharmacol Ther. 2016;32(10):659-664.
- ↑ Lim LS, Ng WY, Mathur R, et al. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715-1718.
- ↑ Rahimy E, Shahlaee A, Khan MA, et al. Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol. 2016;164:118-127.e112.
- ↑ Shah CP, Heier JS. Aflibercept for diabetic macular edema in eyes previously treated with ranibizumab and/or bevacizumab may further improve macular thickness. Ophthalmic Surg Lasers Imaging Retina. 2016;47(9):836-839.
- ↑ Wood EH, Karth PA, Moshfeghi DM, et al. Short-term outcomes of aflibercept therapy for diabetic macular edema in patients with incomplete response to ranibizumab and/or bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):950-954.
- ↑ Ashraf M, Souka AA, ElKayal H. Short-term effects of early switching to ranibizumab or aflibercept in diabetic macular edema cases with non-response to bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):230-236.
- ↑ Bahrami B, Hong T, Zhu M, et al. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1133-1140.
- ↑ Klein KA, Cleary TS, Reichel E. Effect of intravitreal aflibercept on recalcitrant diabetic macular edema. Int J Retina Vitreous. 2017;3(1):1-7.
- ↑ Mira F, Paulo M, Henriques F, et al. Switch to aflibercept in diabetic macular edema patients unresponsive to previous anti-VEGF therapy. J Ophthalmol. 2017;2017:5632634.
- ↑ Fechter C, Frazier H, Marcus WB, et al. Ranibizumab 0.3 mg for persistent diabetic macular edema after recent, frequent, and chronic bevacizumab: the ROTATE trial. Ophthal Surg Lasers Imaging Retina. 2016;47(11):1-18.
- ↑ Ferris FL, Maguire MG, Glassman AR, et al. Evaluating effects of switching anti–vascular endothelial growth factor drugs for age-related macular degeneration and diabetic macular edema. JAMA Ophthalmology. 2017;135(2):145-149.
- ↑ Pieramici DJ, Wang P-W, Ding B, et al. Visual and anatomic outcomes in patients with diabetic macular edema with limited initial anatomic response to ranibizumab in RIDE and RISE. Ophthalmology. 2016;123(6):1345-1350.
- ↑ Jhaveri CD, Glassman AR, Ferris III FL, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387(8):692-703.
- ↑ Whitcup SM, Cidlowski JA, Csaky KG, et al. Pharmacology of corticosteroids for diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59(1):1-12.
- ↑ Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR network phase 2 randomized clinical trial. JAMA Ophthalmology. 2018;136(1):29-38.
- ↑ Ross EL, Hutton DW, Stein JD, et al. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmology. 2016;134(8):888-896.
- ↑ Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study): 12-month data: report 2. Ophthalmology. 2010;117(6):1078-1086. e1072.
- ↑ Arevalo JF, Sanchez JG, Wu L, et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116(8):1488-1497.e1481.
- ↑ Krispel C, Rodrigues M, Xin X, et al. Ranibizumab in diabetic macular edema. World J Diabetes. 2013;4(6):310-318.
- ↑ Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789-801.
- ↑ Jump up to: 79.0 79.1 79.2 Sharma YR, Tripathy K, Venkatesh P, et al. Aflibercept: how does it compare with other anti-VEGF drugs. Austin J Clin Ophthalmol. 2014;1(3):8.
- ↑ Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658-1665.
- ↑ Brown DM, on behalf of the PHOTON study investigators. Intravitreal aflibercept injection 8 mg for DME: 48-week results from the phase 2/3 PHOTON trial. Presented at: American Academy of Ophthalmology Annual Meeting; September 30-October 3, 2022; Chicago, IL. https://investor.regeneron.com/static-files/da20405e-b843-402e-855b-d824a15dec60.
- ↑ Sahni J, Patel SS, Dugel PU, et al. Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126(8):1155-1170.
- ↑ Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet. 2022;39(10326):741-755.
- ↑ Brown DM, Emanuelli A, Bandello F, et al. KESTREL and KITE: 52-week results from two Phase III pivotal trials of brolucizumab for diabetic macular edema. Am J Ophthalmol. 2022;238:157-172.
- ↑ Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis. 2020;4(4):269-279.
- ↑ Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345-1359.
- ↑ Khanani AM, Zarbin MA, Barakat MR, et al. Safety outcomes of brolucizumab in neovascular age-related macular degeneration: results from the IRIS Registry and Komodo Healthcare Map. JAMA Ophthalmology. 2021;140(1):20-28.
- ↑ Enríquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmology. 2021;139(4):441-448.
- ↑ Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447-1459.e1410.
- ↑ Boyer DS, Yoon YH, Belfort Jr R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914.
- ↑ Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125-2132.
- ↑ Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626-635.e622.
- ↑ Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e1035.
- ↑ Brown DM, Ou WC, Wong TP, et al. Targeted retinal photocoagulation for diabetic macular edema with peripheral retinal nonperfusion: three-year randomized DAVE trial. Ophthalmology. 2018;125(5):683-690.
- ↑ Muqit MM, Marcellino GR, Henson DB, et al. Optos‐guided pattern scan laser (Pascal)‐targeted retinal photocoagulation in proliferative diabetic retinopathy. Acta Ophthalmol. 2013;91(3):251-258.
- ↑ Reddy S, Hu A, Schwartz SD. Ultra wide field fluorescein angiography guided targeted retinal photocoagulation (TRP). Semin Ophthalmol. 2009;24(1):9-14.
- ↑ Suñer IJ, Peden MC, Hammer ME, et al. RaScaL: a pilot study to assess the efficacy, durability, and safety of a single intervention with ranibizumab plus peripheral laser for diabetic macular edema associated with peripheral nonperfusion on ultrawide-field fluorescein angiography. Ophthalmologica. 2015;233(2):89-95.
- ↑ Takamura Y, Tomomatsu T, Matsumura T, et al. The effect of photocoagulation in ischemic areas to prevent recurrence of diabetic macular edema after intravitreal bevacizumab injection. Invest Ophthalmol Vis Sci. 2014;55(8):4741-4746.
- ↑ Wessel MM, Nair N, Aaker GD, et al. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694-698.
- ↑ Lee SS, Ghosn C, Yu Z, et al. Vitreous VEGF clearance is increased after vitrectomy. Invest Ophthalmol Vis Sci. 2010;51(4):2135-2138.
- ↑ Simpson AR, Dowell NG, Jackson TL, et al. Measuring the effect of pars plana vitrectomy on vitreous oxygenation using magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2013;54(3):2028-2034.
- ↑ Sivaprasad S, Ockrim Z, Massaoutis P, et al. Posterior hyaloid changes following intravitreal triamcinolone and macular laser for diffuse diabetic macular edema. Retina. 2008;28(10):1435-1442.
- ↑ Stefansson E, Landers MB 3rd, Wolbarsht M. Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc. 1981;79:307-334.
- ↑ Stefansson E, Landers MB 3rd, Wolbarsht ML. Vitrectomy, lensectomy, and ocular oxygenation. Retina. 1982;2(3):159-166.
- ↑ Stefansson E, Novack R, Hatchell D. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31(2):284-289.
- ↑ Lee B, Litt M, Buchsbaum G. Rheology of the vitreous body. Part I: viscoelasticity of human vitreous. Biorheology. 1992;29(5-6):521-533.
- ↑ Gandorfer A, Messmer EM, Ulbig MW, et al. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20(2):126-133.
- ↑ Jackson TL, Nicod E, Angelis A, et al. Pars plana vitrectomy for diabetic macular edema: a systematic review, meta-analysis, and synthesis of safety literature. Retina. 2017;37(5):886-895.
- ↑ Nakajima T, Roggia MF, Noda Y, et al. Effect of internal limiting membrane peeling during vitrectomy for diabetic macular edema: systematic review and meta-analysis. Retina. 2015;35(9):1719-1725.
- ↑ Diabetic Retinopathy Clinical Research Network Writing Committee. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117(6):1087-1093.e3.
- ↑ Bressler SB, Melia M, Glassman AR, et al. Ranibizumab plus prompt or deferred laser for diabetic macular edema in eyes with vitrectomy prior to anti-vascular endothelial growth factor therapy. Retina. 2015;35(12):2516.
- ↑ Mason JO III, White MF, Feist RM, et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina. 2008;28(4):564-567.
- ↑ Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28(10):1395-1399.
- ↑ Diago T, McCannel CA, Bakri SJ, et al. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina. 2009;29(5):601-605.
- ↑ Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56(2):95-113.
- ↑ Singerman LJ, Masonson H, Patel M, et al. Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol. 2008;92(12):1606-1611.
- ↑ Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
- ↑ Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e55.
- ↑ Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109(5):920-927.
- ↑ Diabetic Retinopathy Clinical Research Network. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmology. 2009;127(3):245-251.
- ↑ Ladas ID, Karagiannis DA, Rouvas AA, et al. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29(3):313-318.
- ↑ Bressler SB, Odia I, Maguire MG, et al. Factors associated with visual acuity and central subfield thickness changes when treating diabetic macular edema with anti–vascular endothelial growth factor therapy: an exploratory analysis of the Protocol T randomized clinical trial. JAMA Ophthalmology. 2019;137(4):382-389.
- ↑ Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130(9):1153-1161.
- ↑ Halim MS, Afridi R, Hasanreisoglu M, et al. Differences in the characteristics of subjects achieving complete, partial, or no resolution of macular edema in the READ-3 study. Graefes Arch Clin Exp Ophthalmol. 2021;259(10):2941-2948.
- ↑ Chew EY, Klein ML, Ferris FL, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114(9):1079-1084.
- ↑ Matsunaga DR, Salabati M, Obeid A, et al. Outcomes of eyes with diabetic macular edema that are lost to follow-up after anti–vascular endothelial growth factor therapy. Am J Ophthalmol. 2022;233:1-7.
- ↑ Gao X, Obeid A, Aderman CM, et al. Loss to follow-up after intravitreal anti–vascular endothelial growth factor injections in patients with diabetic macular edema. Ophthalmol Retina. 2019;3(3):230-236.
- ↑ Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375-1394.
- ↑ Holekamp NM, Campochiaro PA, Chang MA, et al. Archway randomized phase 3 trial of the Port Delivery System with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2022;129(3):295-307.
- ↑ Kuklinski EJ, Henry RK, Shah M, et al. Screening of diabetic retinopathy using artificial intelligence and tele-ophthalmology. J Diabetes Sci Technol. 2023 Nov;17(6):1724-1725.
- ↑ Vought R, Vought V, Shah M, et al. EyeArt artificial intelligence analysis of diabetic retinopathy in retinal screening events. Int Ophthalmol. 2023;43(12):4851-4859.