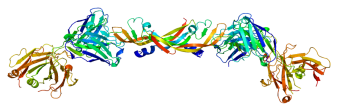Pseudophakic Cystoid Macular Edema (Irvine-Gass Syndrome)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Pseudophakic cystoid macular edema (CME), also known as Irvine-Gass syndrome, is one of the most common causes of visual loss after cataract surgery.[1][2] Phacoemulsification and small incision cataract surgery have significantly reduced the incidence of pseudophakic CME, but because cataract surgery is the most commonly performed surgery in the United States,[3] pseudophakic CME remains a commonly encountered morbidity.
Disease Entity
International Classification of Diseases (ICD)
ICD-9-CM 362.53 Cystoid macular degeneration, cystoid macular edema
ICD-10-CM H35.359 Cystoid macular degeneration, unspecified eye
History
In 1953, Irvine described a cystoid macular edema (CME) that specifically arose after cataract surgery.[4] Gass and Norton subsequently studied the characteristics of the new disease entity with fluorescein angiography.[5][6]
Definitions
- Angiographic pseudophakic CME: seen on fluorescein angiography (FA)
- Clinical pseudophakic CME: associated with decreased visual acuity
- Acute pseudophakic CME: within 6 months
- Chronic pseudophakic CME: over 6 months[1]
- Optical coherence tomography (OCT) definitions have also been proposed[7]
Incidence
- Angiographic CME after intracapsular cataract extraction: As high as 60%[1]
- Angiographic CME after extracapsular cataract extraction: 15% to 30%[1]
- Clinical CME after small incision phacoemulsification: 0.1% to 2.35%[8][9]
- OCT evidence of CME after small incision phacoemulsification: 4% [10] to 11%[11], but also reported to be as high as 41%[12]
Most patients with CME found via angiography or OCT will not have visual changes. Furthermore, most patients with clinical CME will experience spontaneous improvement by 3 to 12 months.[12]
Cost Analysis
Schmier et al. conducted a study of 139,759 Medicare beneficiaries who underwent modern cataract surgery, and determined that total ophthalmic payments were 47% ($1,092) higher for those who developed pseudophakic CME, compared to those who did not (P < .0001).[13]
Pathophysiology
The pathogenesis of pseudophakic CME is thought to be multifactorial. However, the major etiology appears to be inflammatory mediators that are upregulated in the aqueous and vitreous humors after surgical manipulation. Inflammation breaks down the blood-aqueous and blood-retinal-barriers, which leads to increased vascular permeability.[14] Eosinophilic transudate accumulates in the outer plexiform and inner nuclear layers of the retina to create cystic spaces that coalesce to form larger pockets of fluid.[1] In chronic CME, lamellar macular holes and subretinal fluid may also form.
Risk Factors
Surgical Complications[1][15]
- Vitreous loss
- Vitreous traction at incision sites
- Vitrectomy for retained lens fragments
- Iris trauma
- Posterior capsule rupture
- Intraocular lens dislocation
- Early postoperative capsulotomy
- Iris-fixated intraocular lenses
- Anterior chamber intraocular lenses
Diabetic Macular Edema
Diabetics confer a unique challenge in cataract surgery. Cataract surgery was historically thought to accelerate progression of diabetic retinopathy (DR), but recent prospective studies may not support this theory.[16][17] However, postoperative macular edema usually develops in those with a prior history of diabetic macular edema (DME).[18] If the patient actively had DME at the time of surgery, it rarely resolves on its own, even in eyes with prior vitrectomy.[19] For these reasons, DME and severe DR should be stabilized before undergoing cataract extraction.
Uveitis
Cataract surgery was historically considered a very high-risk procedure with many complications for patients with uveitis. However, the advent of modern surgical techniques is allowing patients with uveitis, who often develop cataract prematurely, opportunities for intraocular lens placement. Belair and Kim et al. demonstrated that incidence of pseudophakic CME on OCT at 1 month post-operatively was 12% for eyes with uveitis and 4% for controls (P = .2), and 8% and 0%, respectively, at 3 months (P = .08).[10] Eyes treated perioperatively with oral corticosteroids had a 7-fold reduction in CME (P = .05), while those with active inflammation within 3 months of surgery had a 6 fold increased risk of developing CME (P = .04). Such studies indicate that adequate preoperative and postoperative control of inflammation is paramount for successful cataract extraction.
Pediatric uveitis patients pose unique challenges. Sijssens et al. reported that CME developed in 3 of 19 (16%) of aphakic, and 1 of 29 (3%) pseudophakic eyes at 1 year (P = .286), and 7 of 19 (37%) and 2 of 20 (10%) cumulatively at 2 years (P = .065), of children with juvenile idiopathic arthritis-associated uveitis.[20] Pseudophakic eyes also were not at increased risk of other complications, and visual acuity results were superior to aphakic eyes, indicating that intraocular lens placement may be warranted in well-selected cases.
Glaucoma Medications
Topical glaucoma medications as a risk factor for pseudophakic CME is still controversial. Miyake et al. demonstrated in several clinical trials and cellular studies that preoperative and postoperative topical glaucoma medications, specifically latanoprost and timolol, may increase the incidence of pseudophakic CME[21]. It appears that a commonly used preservative, benzalkonium chloride, is cytotoxic and stimulates inflammatory responses.[21] The effects were prevented by concomitant use of NSAID drops. Arcieriet al. also reported the results of a randomized trial that enrolled 80 subjects, showing that aphakic and pseudophakic glaucoma patients developed more anterior chamber flare if they were randomized to take bimatoprost 0.03%, latanoprost 0.005%, or travoprost 0.004%, compared to placebo (P < .02), and 6 of those patients developed angiographic CME (4 were treated with latanoprost [P = .03]), which resolved after discontinuing the prostaglandin analogues, and being treated with topical diclofenac.[22] A recent small series of pseudoexfoliative eyes that underwent uncomplicated phacoemulsification and developed pseudophakic CME on latanoprost, showed that the CME resolved with discontinuation of latanoprost.[23] However, in a recent retrospective comparative series by Law et al., 700 eyes with glaucoma that underwent cataract surgery did not have a higher incidence of clinical pseudophakic CME compared to 553 non-glaucomatous eyes (5.14% and 5.79%, respectively [P = .618]), and the use of preoperative and postoperative glaucoma medications was not associated with clinical CME (P > .05).[24] It is uncertain whether the results would have differed if angiographic CME were the endpoint.
Other Risk Factors
In a retrospective study of 1659 cataract surgeries, Henderson et al. showed, while not the primary endpoint of the study, that histories of retinal vein occlusion (RVO), epiretinal membrane (ERM), and prostaglandin analogs were also associated with pseudophakic CME.[8] RVO causes macular edema,[25] but interestingly, patients with preexisting macular edema were excluded from the study, indicating that RVOs may induce physiologic compromise of retinal vascular in the setting of intraocular surgery. Similarly, while ERMs predispose eyes to CME, CME can also be a cause of ERM formation.
Diagnosis
History and Clinical Presentation
The occurrence of pseudophakic CME peaks at approximately 4-6 weeks postoperatively. The most common presentation is blurry vision. Less common presentations include central scotomas, metamorphopsia, and mild photophobia.
On biomicroscopy, retinal thickening and loss of the foveal depression is usually appreciated. These findings are best observed using a fundus contact lens, and red-free light may aide in demonstrating cystic changes. In severe or chronic cases, optic disc swelling and/or a lamellar hole may also be seen. Splinter hemorrhages may also be present. However, biomicroscopy may not show any abnormalities in 5-10% of eyes, making ancillary imaging studies important.[26]
Imaging Studies
On fluorescein angiography, pseudophakic CME is characterized by retinal telangiectasis, capillary dilatation, and leakage from perifoveal capillaries in the early phase frames, and perifoveal hyperfluorescent spots classically described as a “petalloid” pattern in late phase frames, representing fluorescein accumulation in cystic spaces. Cystoid changes may also be apparent in the fovea and extramacular areas. Optic nerve staining is also commonly seen, and helps to distinguish pseudophakic CME from other causes of CME such as diabetic macular edema. FA is the gold standard in diagnosing pseudophakic CME, but treatment responses would be more conveniently monitored by biomicroscopy, visual acuity and OCT.
Optical coherence tomography (OCT) (image to the left) allows high-resolution cross-sectional imaging of the macula. Pseudophakic CME on OCT is characterized by loss of the foveal depression, retinal thickening, and cystic hyporeflective areas within the macula. However, OCT has not replaced FA as the gold standard in diagnosing pseudophakic CME, because FA can also rule out other causes of CME such as diabetic macular edema and retinal vein occlusion.
Optical coherence tomography angiography (OCTA) is a relatively new technology that represents a noninvasive way of visualizing the retinal vasculature. In a recent retrospective study, Sacconi et al.[27] found that OCTA in pseudophakic CME showed disruption of parafoveal capillary arcade and cystoid spaces in the deep capillary plexus. Patients with pseudophakic CME showed a larger foveal avascular zone and reduced vessel density of full-thickness retina and DCP.
Differential Diagnosis
The differential diagnosis of pseudophakic CME is broad, and patients with underlying ocular and systemic conditions that predisposes them to other causes of CME may confer diagnostic challenges. To list a few, CME is also seen in:
- Diabetic macular edema
- Retinal vein occlusion
- Radiation retinopathy
- Hypertensive retinopathy
- Uveitidies
- Topical prostaglandins
- Retinal dystrophies
- Choroidal tumors
- Leukemia
- Chronic renal failure
- Of special note, it is important not to misdiagnose pseudophakic CME with choroidal neovascularization seen in age-related macular degeneration (AMD). Many patients undergoing cataract surgery is also within the age range for developing AMD.
Prophylaxis and Treatment
There is no standardized treatment or prophylactic protocol for pseudophakic CME, because there is a lack of strong randomized clinical trials and comparative effectiveness studies. The lack of large studies is perhaps due to the fact that most cases of acute pseudophakic CME spontaneously resolve. However, the treatment of chronic pseudophakic CME remains a challenge. The most recent studies are discussed below.
The management of pseudophakic CME is based on its pathogenesis. In the inflammation cascade, cell membrane lipids are converted to arachidonic acid by phospholipase A2, and prostaglandins are then formed by cyclooxygenases (COX). Corticosteroids reduce inflammation by inhibiting phospholipase A2, and nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit COX. The 2 critical COX isoforms are COX-1 and COX-2, and the later is the major isoform expressed in the retina.[12]
NSAIDs
Topical NSAIDs are the mainstay of perioperative pseudophakic CME prophylaxis. Topical administration is thought to have better ocular penetration than systemic routes, and side effects profiles are benign. Ketorolac 0.4% (Acular, Allergan, Irvine, CA), diclofenac 0.1% (Voltaren, Bausch & Lomb, Tampa, FL), bromfenac 0.09% (Xibrom/Bromday, Ista Pharmaceuticals, Irvine, CA), and nepafenac 0.1% (Nevanac, Alcon, Fort Worth, TX) are FDA approved for postoperative inflammation, although not for CME. Nepafenac is a prodrug that is converted by intraocular hydrolases into its active form, amfenac, and has been reported to have superior corneal penetration and posterior segment activity in rabbit models.[28] However, its superiority compared to other NSAIDs has not been proven yet in clinical studies for macular edema.
Prophylaxis
Recent studies assessing the efficacy of topical NSAIDs in the prophylaxis of pseudophakic CME have compared topical corticosteroid monotherapy to combination therapy with NSAIDs. In a multicenter masked randomized clinical trial (RCT), Wittpenn et al. showed that clinical CME developed in 5 of 278 patients who received perioperative prednisolone, and in 0 of 268 who also received ketorolac (P = .032).[29] Yavas et al., in an unmasked RCT of 189 patients, reported angiographic pseudophakic CME in 0% who received preoperative and postoperative topical indomethacin, 15% who received indomethacin postoperatively only, and 33% of controls, who received the standard topical steroids and antibiotics (P < .001).[30] Postoperative visual acuity was highest in the first group (P < .001) also. Almeida et al. also used OCT in an unmasked RCT of 98 patients to show that ketorolac prophylaxis resulted in lower total macular volume compared to controls at 1 month after surgery (P = .009), with 46.1% (P = .030) macular swelling reduction.[31] Wolf et al. showed in a review of 450 patients that those prophylactically treated with nepafenac 0.1% in addition to prednisolone (%) had no cases of clinical pseudophakic CME, while 5 patients developed CME in the group without nepafenac treatment (P = .0354).[32]
The Miyake Eye Hospital group in Japan has recently conducted several comparative effectiveness trials between NSAID and corticosteroid prophylaxis. Results of one double-masked RCT of 50 patients demonstrated that patients randomized to receive fluorometholone were more likely to develop angiographic CME than those who received diclofenac (P = .08 at 2 weeks, P = .001 at 5 weeks), and also demonstrated that the fluorometholone group had more anterior flare (P = .001 at 1 week, P = .025 at 2 weeks) and transient lower choroidal blood volume (P = .022) and flow (P = .003).[33] In another multicenter study with 142 subjects, the incidence of angiographic pseudophakic CME 5 weeks postoperatively was 18.8% in those treated with topical diclofenac 0.1%, compared to 58.0% in those treated with topical betamethasone 0.1% (P < 0.001).[34] The same group also recently showed in a double-masked single center RCT of 59 patients that those randomized to nepafenac prophylaxis had significantly lower rates of angiographic CME at 5 week postoperatively (P < .0001), with faster visual acuity recovery (P = .0395).[35]
Treatment
There is minimal evidence regarding treatment of acute pseudophakic CME. A small RCT by Heier et al. compared the efficacies of topical ketorolac, prednisolone and the combination of the two in 28 patients who developed CME within 21 to 90 days after cataract surgery.[36] The combination therapy resulted in superior visual acuity outcomes compared to monotherapies of either agent. These data interestingly suggested that NSAIDs and corticosteroids may work synergistically.
A metaanalysis of four RCTs evaluating the efficacy of ketorolac in the treatment of acute pseudophakic CME has been published in the literature.[37] Three of 4 studies did not have statistically significant benefits, but pooled together, the larger sample size resulted in significance.
Several studies have investigated NSAIDs as treatment for chronic pseudophakic CME, although there are no recent large studies. In 1991, Flach et al. reported a multicenter RCT involving 120 patients with chronic aphakic or pseudophakic CME.[38] Patients treated with ketorolac achieved better visual acuities compared to placebo after 30 days (P = .04), 60 days (P = .02), and 90 days (P = .01). In 2003, Rho compared the efficacy of topical ketorolac to topical diclofenac in a non-controlled study, and showed that the mean time for a 2-line improvement and resolution of chronic pseduophakic CME was comparable.[39]
Corticosteroids
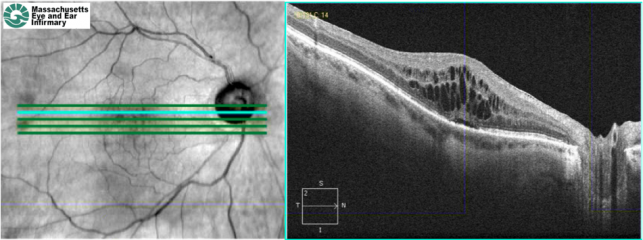
Spectral domain optical coherence tomography (SD-OCT) of a patient with chronic refractory pseudophakic CME. There is retinal thickening, cystic intraretinal lesions, and subretinal fluid.
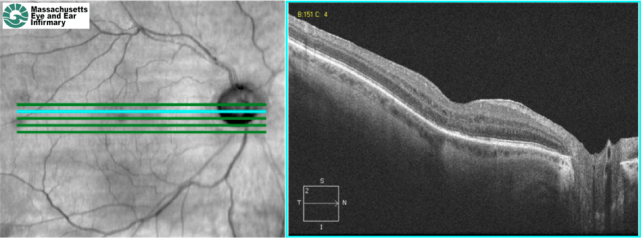
SD-OCT of the same patient 1 month after a sub-Tenon's injection of triamcinolone. The retinal thickening, cystic lesions, and subretinal fluid have improved (Images courtesy of Ivana Kim, MD, Massachusetts Eye and Ear Infirmary)
Topical
Topical corticosteroids are commonly used in the treatment of pseudophakic CME. Few studies, however, have examined their efficacy. Furthermore, its effects are often confounded by the concomitantly used and more efficacious topical NSAIDs.
Periocular
Periocular corticosteroids have been shown to be effective for pseudophakic CME refractory to topical treatments (see figures above). Thach et al. demonstrated in a series of 48 patients with refractory CME, that sub-Tenon’s injections improved visual acuity from 20/92 to 20/50 (P = 0.0001), and retrobulbar injections improved visual acuity from 20/97 to 20/58 (P = 0.035), and there was no statistical outcome difference between the two techniques.[40] More recently, Randazzo and Vinciguerra reported improved anatomic and visual outcomes in a patient with chronic PCME treated with sub-Tenon betamethasone 4 mg.[41]
Intravitreal
In 2003, Benhamou et al. reported the first study using 8 mg of intravitreal triamcinolone to treat 3 cases of refractory chronic pseudophakic CME, and showed that macular thickness and visual acuity improved, but the effects were transient.[42] Conwayet al. also reported on 8 patients with refractory (to topical and periocular corticosteroids) pseudophakic CME, who were treated with 4 mg of intravitreal triamcinolone.[43] The mean visual acuity at baseline was 20/200 (20/80-20/400), and 20/60 (20/25-20/100) 1 month after intravitreal triamcinolone, but the visual acuity in eyes that subsequently required multiple injections gradually decreased. Other case series also reported the transient benefits using 4 mg of intravitreal triamcinolone [5, 57, 58, 60].[6] [44][45][46]Jonas et al. reported in a series of 5 patients that 25 mg of triamcinolone can also be used safely. Jonas et al. reported in a series of 5 patients that 25 mg of triamcinolone can also be used safely.[47]
Ahmadabadi et al. recently reported one of the first controlled studies, where 41 diabetic eyes with moderate nonproliferative diabetic retinopathy were randomized to routine phacoemulsification or that with intravitreal triamcinolone at the end of the case, and showed that 4 eyes in the routine treatment group and 0 eyes in the treatment group developed pseudophakic CME (P = .059).[48] The treatment group had better visual acuity improvements at 1 month (P = .045), but the visual benefits were not significant at 6 months. Clearly, one of the major limitations of intravitreal corticosteroids is the transient effect that requires repeated injections.
Drug Delivery Systems
Sustained drug delivery systems (DDS) have been developed to address this limitation of intravitreal corticosteroid injections. Ozurdex (Allergan, Irvine, CA) is an injectable, biodegradable intravitreal DDS that provides sustained release of preservative-free dexamethasone, a potent corticosteroid.[49] In the United States, Ozurdex is FDA-approved for treatment of macular edema secondary to retinal vein occlusions, diabetic macular edema, and non-infectious posterior uveitis.[50] A Phase II study subgroup analysis investigated its efficacy in the treatment of persistent macular edema resulting from uveitis or pseudophakic CME.[51] Twenty-seven patients with refractory pseudophakic CME were recruited, and randomized to receive dexamethasone DDS 350 ug, dexamethasone DDS 700 ug, or observation. Eight patients showed at least a 10-letter improvement at day 90 and maintained the improvement at day 180. Significance testing was performed with combined data from pseudophakic CME and uveitis patients: an improvement in best corrected visual acuity (BCVA) of at least 10 letters at day 90 was seen in 42% in the 350 ug group (P = .117), and 54% in the 700 ug group (P = .029). Increased intraocular pressure was reported in 7 patients (P = .079), which was managed by observation or pressure-lowering medications. Additionally, a new micro-insert of fluocinolone acetonide (Yutiq, Eyepoint Pharmaceuticals, Watertown, MA) was introduced in 2019; it is FDA-approved for non-infectious posterior uveitis and will likely offer another avenue for treatment in affected patients.
Anti-VEGF Treatments
Anti-vascular endothelial growth factor (VEGF) therapy has revolutionized our treatment of exudative macular degeneration, and its efficacy is being tested with promising results in other forms of ocular neovascularization and now CME, including DME and pseudophakic CME. VEGF is well known to be a key mediator of angiogenesis, but it also plays an important role in the inflammation and capillary permeability that causes CME. Bevacizumab (Avastin, Genentech, South San Francisco, CA) is a humanized monoclonal antibody that inhibits VEGF-A (structure of VEGF-A images to the left). Arevalo et al. reported on a series of 28 eyes that received at least one 1.25 mg or 2.5 mg injection of intravitreal bevacizumab as the primary treatment of chronic pseudophakic CME.[52] During the mean follow-up of 32 weeks, 21 eyes (71%) improved BCVA by 2 or more Early Treatment of Diabetic Retinopathy Study (ETDRS) lines. The mean baseline BCVA and central macular thickness were 20/160 and 466 microns, which improved to 20/63 and 265 microns, respectively (both P < 0.0001). Eight eyes required a second injection, and 4 a third. In a 6 month follow-up study, now with 110 eyes and a mean of 6.3 months of follow-up, the visual and anatomic benefits were still observed, although 16 (21%) eyes required a second injection, and 6 (8%) a third.[53] Similar results were seen at the 24 month follow-up study, when the investigators also noted no outcome differences between the 1.25 mg and 2.50 mg dosing.[54]
Most other studies have focused on the treatment of chronic pseudophakic CME that has been refractory to other treatments. In 2008, Spitzer et al. showed that 1.25 mg of intravitreal bevacizumab did not significantly improve visual outcomes in a series of 16 eyes with refractory pseudophakic CME, although there was slight decrease in retinal thickness.[55] However, Arevalo et al. subsequently reported a series of 36 eyes with chronic pseudophakic CME refractory to topical, periocular, systemic and intravitreal treatments, and showed that 26 eyes (72%) demonstrated improvement of BCVA by at least 2 ETDRS lines, and mean baseline BCVA and central macular thickness improved from 20/200 and 500 microns, respectively, to 20/80 and 286 microns, respectively, at 12 months, after a mean of 2.7 injections per eye (P < 0.0001).[56] Another series of 10 patients that received 1.25 mg of bevacizumab also reported promising visual and anatomic outcomes, with follow up of 6 months.[57] “Triple therapy” with intravitreal triamcinolone, intravitreal bevacizumab and topical NSAIDs has been shown to be effective as well, although the effects of the intravitreal medications were transient.[58]
Carbonic Anhydrase Inhibitors
Oral carbonic anhydrase inhibitors (CAIs) may be considered in refractory pseudophakic CME. CAIs are thought to improve the pumping action of the retinal pigment epithelium, to decrease intraretinal fluid. Encouraging results have been reported in small series and case reports, but their use is may be limited by a severe side effect profile.[14] Topical CAIs have not yet been investigated in pseudophakic CME .
Immunomodulatory Therapy
Recent small pilot studies have started to examine subcutaneous interferon alpha (IFN-a, Imgenex, San Diego, CA)[59] and intravitreal infliximab (Remicade, Centocor Ortho Biotech, Horsham, PA) with mixed results.[60][61]
Surgical Treatments
Laser Vitreolysis
Neodymium:YAG laser anterior vitreolysis can release vitreous incarceration in the cataract incision wounds that complicate pseudophakic CME. Steinert et al. reported a series of 29 patients with vitreous incarceration and acute PCME, where 55% improved 2 or more lines of visual improvement.[62] Smaller series with similar results have also been published.[44][45] However, the results may have been confounded with post-laser topical anti-inflammatory medication and spontaneous resolution.
Pars Plana Vitrectomy
Pars plana vitrectomy may be considered when pseudophakic CME is complicated by vitreoretinal traction, and/or if the CME is unresponsive to other treatments.[9] Vitrectomy may also theoretically reduce the concentration of inflammatory mediators and growth factors. In a 1985 randomized trial of 68 eyes with chronic aphakic CME, Fung found that eyes that underwent vitrectomy had better visual outcomes compared to controls (P < .01).[63] However, the use of corticosteroids in the treatment group may have confounded the results. Furthermore, the results may not necessarily apply to pseudophakic CME, and few patients are left aphakic after modern cataract extractions.
Harbour et al. in 1995 reviewed 24 patients with refractory pseudophakic CME due to vitreous adhesions to anterior segment structures who were treated with vitrectomy, and reported that the mean BCVA improved from 20/190 preoperatively, to 20/52 postoperatively (P < 0.001).[46] Pendergast et al. subsequently reported in a series of 23 patients with chronic refractory pseudophakic CME without vitreous abnormalities, that vitrectomy improved median BCVA from 20/200 to 20/60 (P < .0001), although some eyes did not improve.[64] Further studies are clearly needed to better define the role of vitrectomy in pseudophakic CME.
Additional Resources
- American Academy of Ophthalmology http://www.aao.org
- American Society of Cataract and Refractive Surgery http://www.ascrs.org
- European Society of Cataract and Refractive Surgeons http://www.escrs.org
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 1998;96:557-634.
- ↑ Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol 2012;23(1):26-32.
- ↑ Feldman BH, Heersink S. Cataract. EyeWiki. https://eyewiki.aao.org/Cataract. Accessed 11/12/2011.
- ↑ Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol 1953;36:599-619.
- ↑ Gass JDM, Norton EWD. Fluorescein studies of patients with macular edema and papilledema following cataract extraction. Trans Am Ophthalmol Soc 1966;64:232-249.
- ↑ 6.0 6.1 Gass JDM, Norton EWD. Cystoid macular edema and papilledema following cataract extraction. Arch Ophthalmol 1966;76:646-661.
- ↑ Kim SJ, Belair ML, Bressler NM, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina 2008;28(6):870-876.
- ↑ 8.0 8.1 Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg 2007;33(9):1550-1558.
- ↑ 9.0 9.1 Loewenstein A, Zur D. Postsurgical cystoid macular edema. Dev Ophthalmol 2010;47:148-159.
- ↑ 10.0 10.1 Belair ML, Kim SJ, Thorne JE, et al. Incidence of cystoid macular edema after cataract surgery in patients with and without uveitis using optical coherence tomography. Am J Ophthalmol 2009;148(1):128-135.
- ↑ Perente I, Utine CA, Ozturker C, et al. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res 2007;32(3):241-247.
- ↑ 12.0 12.1 12.2 Shelsta HN, Jampol LM. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina 2011;31(1):4-12.
- ↑ Schmier JK, Halpern MT, Covert DW, Matthews GP. Evaluation of costs for cystoid macular edema among patients after cataract surgery. Retina 2007;27(5):621-628.
- ↑ 14.0 14.1 Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin 2010;50(1):139-153.
- ↑ Cohen SM, Davis A, Cukrowski C. Cystoid macular edema after pars plana vitrectomy for retained lens fragments. J Cataract Refract Surg 2006;32(9):1521-1526.
- ↑ Rashid S, Young LH. Progression of diabetic retinopathy and maculopathy after phacoemulsification surgery. Int Ophthalmol Clin 2010;50(1):155-166.
- ↑ Shah AS, Chen SH. Cataract surgery and diabetes. Curr Opin Ophthalmol 2010;21(1):4-9.
- ↑ Jiramongkolchai K, Lalezary M, Kim SJ. Influence of previous vitrectomy on incidence of macular oedema after cataract surgery in diabetic eyes. Br J Ophthalmol 2011;95(4):524-529.
- ↑ Horozoglu F, Yanyali A, Aytug B, et al. Macular thickness changes after phacoemulsification in previously vitrectomized eyes for diabetic macular edema. Retina 2011:[Epub ahead of print].
- ↑ Sijssens KM, Los LI, Rothova A, et al. Long-term ocular complications in aphakic versus pseudophakic eyes of children with juvenile idiopathic arthritis-associated uveitis. Br J Ophthalmol 2010;94(9):1145-1149.
- ↑ 21.0 21.1 Miyake K, Ibaraki N, Goto Y, et al. ESCRS Binkhorst lecture 2002: Pseudophakic preservative maculopathy. J Cataract Refract Surg 2003;29(9):1800-1810.
- ↑ Arcieri ES, Santana A, Rocha FN, et al. Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol 2005;123(2):186-192.
- ↑ Panteleontidis V, Detorakis ET, Pallikaris IG, Tsilimbaris MK. Latanoprost-Dependent Cystoid Macular Edema Following Uncomplicated Cataract Surgery in Pseudoexfoliative Eyes. Ophthalmic Surg Lasers Imaging 2010:1-5.
- ↑ Law SK, Kim E, Yu F, Caprioli J. Clinical cystoid macular edema after cataract surgery in glaucoma patients. J Glaucoma 2010;19(2):100-104.
- ↑ Comer GM. Retinal vein occlusion. EyeWiki. https://eyewiki.aao.org/Retinal_Vein_Occlusion. Accessed 11/12/2011.
- ↑ Desai P. The National Cataract Surgery Survey: II. Clinical outcomes. Eye (Lond) 1993;7 ( Pt 4):489-494.
- ↑ Sacconi R, Corbelli E, Carnevali A, et al. Optical coherence tomography angiography in pseudophakic cystoid macular oedema compared to diabetic macular oedema: qualitative and quantitative evaluation of retinal vasculature. Br J Ophthalmol. 2018 Dec;102(12):1684-1690.
- ↑ Gamache DA, Graff G, Brady MT, et al. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation 2000;24(4):357-370.
- ↑ Wittpenn JR, Silverstein S, Heier J, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol 2008;146(4):554-560.
- ↑ Yavas GF, Ozturk F, Kusbeci T. Preoperative topical indomethacin to prevent pseudophakic cystoid macular edema. J Cataract Refract Surg 2007;33(5):804-807.
- ↑ Almeida DR, Johnson D, Hollands H, et al. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. J Cataract Refract Surg 2008;34(1):64-69.
- ↑ Wolf EJ, Braunstein A, Shih C, Braunstein RE. Incidence of visually significant pseudophakic macular edema after uneventful phacoemulsification in patients treated with nepafenac. J Cataract Refract Surg 2007;33(9):1546-1549.
- ↑ Miyake K, Nishimura K, Harino S, et al. The effect of topical diclofenac on choroidal blood flow in early postoperative pseudophakias with regard to cystoid macular edema formation. Invest Ophthalmol Vis Sci 2007;48(12):5647-5652.
- ↑ Asano S, Miyake K, Ota I, et al. Reducing angiographic cystoid macular edema and blood-aqueous barrier disruption after small-incision phacoemulsification and foldable intraocular lens implantation: multicenter prospective randomized comparison of topical diclofenac 0.1% and betamethasone 0.1%. J Cataract Refract Surg 2008;34(1):57-63.
- ↑ Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2011;37:1581-8.
- ↑ Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000;107(11):2034-2038;discussion 2039.
- ↑ Yilmaz T, Cordero-Coma M, Gallagher MJ. Ketorolac therapy for the prevention of acute pseudophakic cystoid macular edema: a systematic review. Eye (Lond). 2011 Nov [Epub ahead of print]
- ↑ Flach AJ, Jampol LM, Weinberg D, et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. Am J Ophthalmol. 1991;112(5):514-519. doi:10.1016/s0002-9394(14)76851-5
- ↑ Rho DS. Treatment of acute pseudophakic cystoid macular edema: Diclofenac versus ketorolac. J Cataract Refract Surg 2003;29(12):2378-2384.
- ↑ Thach AB, Dugel PU, Flindall RJ, et al. A comparison of retrobulbar versus sub-Tenon's corticosteroid therapy for cystoid macular edema refractory to topical medications. Ophthalmology 1997;104(12):2003-2008.
- ↑ Randazzo A, Vinciguerra P. Chronic macular edema medical treatment in Irvine-Gass syndrome: case report. Eur J Ophthalmol 2010;20(2):462-465.
- ↑ Benhamou N, Massin P, Haouchine B, et al. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol 2003;135(2):246-249.
- ↑ Conway MD, Canakis C, Livir-Rallatos C, Peyman GA. Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular edema. J Cataract Refract Surg 2003;29(1):27-33.
- ↑ 44.0 44.1 Tchah H, Rosenberg M, Larson RS, Lindstrom RL. Neodymium: YAG laser vitreolysis for treatment and prophylaxis of cystoid macular oedema. Aust N Z J Ophthalmol 1989;17(2):179-183.
- ↑ 45.0 45.1 Katzen LE, Fleischman JA, Trokel S. YAG laser treatment of cystoid macular edema. Am J Ophthalmol 1983;95(5):589-592.
- ↑ 46.0 46.1 Harbour JW, Smiddy WE, Rubsamen PE, et al. Pars plana vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol 1995;120(3):302-307.
- ↑ Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide for pseudophakic cystoid macular edema. Am J Ophthalmol 2003;136(2):384-386.
- ↑ Ahmadabadi HF, Mohammadi M, Beheshtnejad H, Mirshahi A. Effect of intravitreal triamcinolone acetonide injection on central macular thickness in diabetic patients having phacoemulsification. J Cataract Refract Surg 2010;36(6):917-922.
- ↑ London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: Indications and evidence. Adv Ther 2011;28(5):351-366.
- ↑ United States Food and Drug Administration. Label and approval history. http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/index.cfm. Accessed 11/13/2011
- ↑ Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol 2009;147(6):1048-1054, 1054 e1041-1042.
- ↑ Arevalo JF, Garcia-Amaris RA, Roca JA, et al. Primary intravitreal bevacizumab for the management of pseudophakic cystoid macular edema: pilot study of the Pan-American Collaborative Retina Study Group. J Cataract Refract Surg 2007;33(12):2098-2105.
- ↑ Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007;114(4):743-750.
- ↑ Arevalo JF, Sanchez JG, Wu L, et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology 2009;116(8):1488-1497.
- ↑ Spitzer MS, Ziemssen F, Yoeruek E, et al. Efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J Cataract Refract Surg 2008;34(1):70-75.
- ↑ Arevalo JF, Maia M, Garcia-Amaris RA, et al. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology 2009;116(8):1481-1487.
- ↑ Barone A, Russo V, Prascina F, Delle Noci N. Short-term safety and efficacy of intravitreal bevacizumab for pseudophakic cystoid macular edema. Retina 2009;29(1):33-37.
- ↑ Warren KA, Bahrani H, Fox JE. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina 2010;30(2):260-266. This is the first study to examine triple therapy with intravitreal triamcinolone, intravitreal bevacizumab, and a topical NSAID.
- ↑ Deuter CM, Gelisken F, Stubiger N, et al. Successful treatment of chronic pseudophakic macular edema (irvine-gass syndrome) with interferon alpha: a report of three cases. Ocul Immunol Inflamm 2011;19(3):216-218.
- ↑ Wu L, Fernando Arevalo J, Hernandez-Bogantes E, Roca JA. Intravitreal infliximab for refractory pseudophakic cystoid macular edema: results of the Pan-American Collaborative Retina Study Group. Int Ophthalmol 2010; Apr 8 [Epub ahead of print]
- ↑ Giganti M, Beer PM, Lemanski N, et al. Adverse events after intravitreal infliximab (Remicade). Retina 2010;30(1):71-80.
- ↑ Steinert RF, Wasson PJ. Neodymium:YAG laser anterior vitreolysis for Irvine-Gass cystoid macular edema. J Cataract Refract Surg 1989;15(3):304-307.
- ↑ Fung WE. Vitrectomy for chronic aphakic cystoid macular edema. Results of a national, collaborative, prospective, randomized investigation. Ophthalmology 1985;92(8):1102-1111.
- ↑ Pendergast SD, Margherio RR, Williams GA, Cox MS, Jr. Vitrectomy for chronic pseudophakic cystoid macular edema. Am J Ophthalmol 1999;128(3):317-323.