BostonSight PROSE (Prosthetic Replacement of the Ocular Surface Ecosystem) and Scleral Contact Lenses
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Scleral Lenses
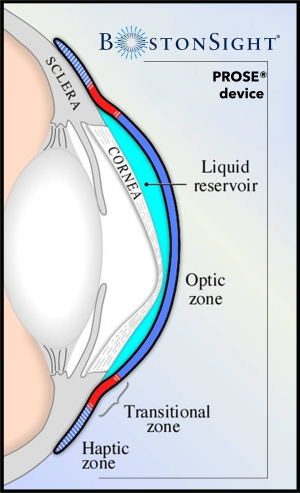
Scleral lenses are rigid gas permeable lenses that vault the cornea while landing gently on the sclera and overlying conjunctival tissue. Prior to application, the lens bowl is typically filled with either buffered or non-buffered preservative-free normal saline (0.9% sodium chloride). In this way, scleral lenses feature a fluid reservoir (also termed the tear lens reservoir) which is the fluid-filled space between the back surface of the lens and the front of the cornea. When evaluating the lens fit, this space is referred to as the corneal clearance.
The design of scleral lenses typically consists of three parts: an optical zone, a limbal/transitional zone, and a landing/haptic zone:[1]
- Optical Zone: The central portion of the lens which vaults the cornea and contains the refractive power and base curve. The optical zone is typically 9-10 mm in diameter.
- Limbal/Transitional Zone: The area of the lens that marks the transition between the optical zone and landing zone and provides the vault over the limbal area
- Landing/Haptic Zone: The area of the lens that lands on the scleral and overlying conjunctival anatomy. Categorized as spherical (symmetrical) or toric (asymmetrical).
Note: spherical and toric in this sense is in reference to the lens landing zone architecture and not the refractive power of the lens. Some degree of toricity is often required
especially with larger lenses due to the asymmetry seen in scleral anatomy.[2] [3] The aim is to fit tangentially to the conjunctival and scleral surface (fitting “in
alignment”) in order to evenly distribute the weight of the lens across the landing zone.[1]
The diameter of scleral lenses is generally larger than corneal or corneo-scleral rigid gas permeable lenses. Scleral lenses are differentiated from other lens modalities by a full landing on the sclera, with the following two subcategories:[1]
- Mini-scleral: Previously defined via diameter: 15 to 18 mm. Modern definition based on horizontal visible iris diameter (HVID): ≤ 6mm larger than the HVID.
- Full or true scleral: Previously defined via diameter: >18 mm. Modern definition based on HVID: > 6mm larger than the HVID.
In regard to the fitting characteristics of scleral lenses, though centration is preferred, it is not uncommon to have slight decentration, especially inferior-temporally.[1] Movement of the lens on the eye should be minimal to none when appropriate alignment of the haptics is achieved. Though movement is intended to be minimal, it is important to ensure the haptics are not too tight, which can create a seal-off effect and hinder tear exchange. It is important that tear exchange occurs with lens wear in order to maintain circulation of oxygenated tears, hence the term, fluid-ventilated scleral lenses. Features that are thought to improve oxygenated exchange include the addition of channels to promote fluid-ventilation and air-ventilation (via fenestration).[1]
The sagittal depth of the lens, also known as the “sag”, refers to the depth of the lens measured at a specific chord length. Increasing the sagittal depth will in turn increase the corneal clearance, i.e. an appropriate adjustment to make in situations with inadequate clearance and apical touch. Decreasing the sagittal depth will in turn decrease the corneal clearance, i.e. an appropriate adjustment to make in situations of excessive corneal clearance. Of note, scleral lenses settle into the conjunctival tissue over the first several hours of wear, typically resulting in a reduction in corneal clearance by ~100-150 microns; amount of settling however varies depending on the diameter of the lens, with larger diameter lenses typically settling less.[4] When picking the sag of the lens, the fitter should take this expected settling of the lens into account.
Prosthetic replacement of the ocular surface ecosystem (PROSE) treatment
Introduction
Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) is an iterative and integrated medical treatment model that restores vision, promotes healing, reduces symptoms, and improves the quality of life for patients with complex corneal disease. The prosthetic devices used in PROSE treatment are approved by the US Food and Drug Administration (FDA) for therapeutic use for the management of a distorted corneal surface (from corneal degenerations, corneal dystrophies, and corneal scarring from surgery, infection, or trauma) and for therapeutic use in eyes with ocular surface disease (from dry eye, limbal stem cell deficiency, disorders of the skin [i.e. ectodermal dysplasia], neurotrophic keratitis, and corneal exposure).[5] Approval from the FDA was first obtained in 1994 and has been updated as recently as 2016.[5]
PROSE treatment has been reported to be effective and safe in managing a variety of ocular conditions including, but not limited to, ocular surface disease, corneal ectasia, and post-surgical corneas.[6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] The diameter of devices used in PROSE treatment can range between 13 and 23 mm. PROSE devices have a central optic zone, a transitional zone, and a peripheral haptic zone.[23] The computer assisted and software designed device is made using a computerized lathe machine in-house at the BostonSight manufacturing laboratory in Needham, Massachusetts.
The BostonSight® PROSE Clinic and BostonSight Network Clinics are built on a medical model centering around PROSE treatment. PROSE Providers are optometrists that have completed the BostonSight® PROSE Clinical Fellowship focusing on device design, customization, and management and co-management of patients with complex corneal diseases. PROSE Providers work closely with an ophthalmologist on staff in order to co-manage complex corneal pathologies and together ensure proper physiological endpoints and ocular surface function.
Goals of Treatment
The three main goals of PROSE treatment are as follows and the patient may have one or more of the following goals.
Improve visual function
This is typically the goal for patients with irregular astigmatism from various pathologies, including, but not limited to, corneal ectasia (keratoconus, post-laser assisted in-situ keratomileusis [LASIK] ectasia, pellucid marginal degeneration), post-radial keratotomy, post-keratoplasty, and corneal scarring.
This sometimes can overlap with the goal of improving comfort for patients with debilitating ocular surface disease who have difficulty functioning visually due to extreme discomfort, photophobia, and pain.
Improve ocular comfort
This is typically the goal for patients with corneal pathology who are symptomatic with dryness, discomfort, photophobia, and/or pain. This includes, for example, patients with keratoconjunctivitis sicca in the setting of Sjogren’s syndrome, chronic ocular graft-versus-host-disease, Stevens-Johnson syndrome, ocular cicatricial pemphigoid, limbal stem cell deficiency, and corneal neuropathy or neuralgia.
Support of the ocular surface
This is typically the goal for patients who have severe ocular surface disease where the integrity of the ocular surface is compromised. This includes patients with a history of or with current epithelial erosions, persistent epithelial defects, or those who need protection from the anatomy of the lids, i.e. keratinization or entropion. This can be secondary to Stevens-Johnson syndrome (SJS), severe keratoconjunctivitis sicca, neurotrophic keratitis, or exposure keratopathy, for example. PROSE treatment provides an ideal microenvironment that promotes healing and protects from possible progression leading to corneal melt, corneal perforation, infection, and/or other sight-threatening complications.
Indications
Irregular Corneas
PROSE treatment is used as a refractive treatment for individuals with corneal conditions that cause irregular astigmatism. As a rigid prosthetic device with a fluid reservoir, devices used in PROSE allow the masking of corneal irregularities to provide a clarity and quality of vision that cannot be provided by conventional means. PROSE has been reported to be effective and safe in cases of corneal ectasia, i.e. keratoconus, keratoglobus, post-keratoplasty, post-radial keratotomy.[7] [8] [9] [10] [11] PROSE is reported to be used in cases of corneal degenerations, such as Salzmann’s nodular degeneration and Terrien’s degeneration, as well as corneal dystrophies.[9] [12] Improvement in visual acuity with PROSE treatment and scleral lenses has decreased the rate of keratoplasty.[24] [25] By doing so, the use of devices used in PROSE and other scleral lenses can significantly reduce the cost, effort, and morbidity related to the maintenance of corneal grafts. PROSE can also be used in cases of largely asymmetric refractive errors.
Ocular Surface Disease
PROSE treatment is used in cases of ocular surface disease to protect and support the ocular surface to reduce symptoms, promote healing, and prevent ocular surface breakdown. This is relevant in keratoconjunctivitis sicca, Stevens-Johnson syndrome, ocular cicatricial pemphigoid, Sjogren’s syndrome, rheumatoid arthritis, ocular graft-versus-host disease, and more.[6] [13] [14] [15] [16] PROSE can also be used to treat non-specific dry eye, corneal neuropathy, neurotrophic keratitis, and exposure keratopathy.[17] [18] In addition, PROSE treatment related case studies in the literature include management of patients with descemetocele, limbal stem cell deficiency, aniridic keratopathy, and vernal keratoconjunctivitis.[19][20] [21] [22] PROSE treatment provides a therapeutic benefit for patients with these pathologies by providing an ideal microenvironment that allows constant lubrication of the ocular surface as well as provides protection from desiccation and mechanical trauma from the eyelids.
Design and Customizations
Designing with Spline Functions
PROSE devices consists of a modifiable base curve radius, inner optic zone diameter, and external optic zone diameter. Modification of the corneal vault (sagittal height) is independent of the base curve. The lens design has junction-less curvatures as it is designed using spline functions, which allow for detailed modifications at various points along the front and back surface of the lens. PROSE treatment is unique in that PROSE practitioners directly manipulate the spline functions when designing the device, allowing advanced control of the design by the practitioner and a unique process of customization that is currently unavailable in other marketed scleral lens designs.
Materials
PROSE devices are made of fluorosilicone acrylate polymers of varying oxygen permeability (85 to 180 x 10-11 cm2 mL O2/s mL mmHg [ISO/Fatt]). Oxygen permeability is determined by the thickness of the lens in a relationship defined as Dk/t.[1] Utilizing a proprietary CAD-CAM software, PROSE Providers can customize materials and lens thickness to increase oxygen permeability. High Dk materials are often used in corneas with endothelial compromise, typically with post-surgical corneas. Unfortunately, some materials with higher Dk can have poorer wettability of the front surface of the lens, which can be visually debilitating for some patients. Newer materials and the addition of post-manufacturing hydrophilic coatings can help improve wettability. This is considered when selecting the material of a lens for a patient.
Front surface eccentricity
Front surface eccentricity (FSE) is a value that defines the asphericity of the front surface of the lens.[26] Higher eccentricity values indicate a more rapid rate of flattening of the front surface of the lens from the center toward the periphery.[26] Incorporating FSE into the design of devices used in PROSE treatment can reduce residual higher-order aberrations, especially spherical aberration and/or coma.[26][27] Studies also show that FSE can improve both high contrast and low contrast visual acuity in PROSE treatment patients.[27][28] [29] In patients with corneal ectasia, FSE is hypothesized to compensate for aberrations from the posterior surface of the ectatic cornea.[27] It is also posited that FSE compensates for poor alignment of the optical axes of the eye with the optical portion of PROSE devices.[27] FSE is most often used in patients with corneal ectasia but can also be beneficial for patients with other corneal conditions.[26]
Meridional Specific Designs
PROSE devices can have spherical, toric, or quadrant-specific landing zones, with most lenses being either toric or quadrant-specific. PROSE devices are unique in that they allow for specifications and unique customization in up to 8 independent meridians of the lens. This feature allows the practitioner more flexibility to fit around the asymmetry of the patient’s eye, when compared to currently marketed scleral lenses that often allow specifications in only 2 meridians with a limited number of manufacturers also allowing quadrant specific designs.[1] Additionally, in the design of current commercially available scleral lenses, typically only one spherical base curve exists for the lens. In PROSE devices, the base curve can be changed in one meridian independently from other meridians. This can be helpful in vaulting obstacles in the fit within the optical zone that are unable to be modified via the use of spline functions and/or when designing fenestrated devices.
SmartChannels™
SmartChannels™ are proprietary radial groves added into the back surface of the lens’ haptic. These are designed and uniquely customized by PROSE Providers using a proprietary CAD-CAM software, FitConnect™, and are added during manufacturing. These can extend from the edge or the outer haptic area into the limbal zone. Addition of channels allows for fluid-ventilation of the tear lens reservoir, allowing oxygenated tear exchange underneath the device.[13] Blinking forces can act as a pumping mechanism to increase tear exchange through these channels.[13] It is thought that the addition of channels can reduce the suction underneath the lens.[13] A maximum of 4 channels can be applied and they can be customized for location, width, depth, and length. Channels are also used to vault anatomical obstacles in the conjunctival or scleral anatomy, including pingueculas, blebs, and nodules.
Fenestrations
With fenestrated lenses, one or several small (0.25-0.50mm diameter) holes are drilled into the lens to allow air ventilation. The goal is twofold: to reduce suction and to allow for an increase of oxygenated exchange. Fenestrations can be added in the haptic portion of the lens, the optic zone portion, or a combination of both. Fitting of fenestrated lenses is typically more involved than non-fenestrated lenses and may present certain fitting challenges. By nature of the design, air bubbles are introduced into the fluid reservoir, when the fenestration is placed in the optic zone of the lens. Commonly, small bubbles occur without significant issues, however large bubbles can compromise vision, increase glare at night, create discomfort, and/or lead to desiccation of the ocular surface. Unique features of fenestrated lenses typically include an intentionally low corneal clearance and the use of more viscous solutions such as Refresh® Celluvisc® along with saline in the lens bowl in an attempt to prevent bubble formation.[1]
PROSE treatment also allows unique customizations when it comes to fenestrated devices. The proprietary software used to design PROSE devices, allows for independent customization of the optic zone in up to 8 meridians. This enables accurate and precise customization of the contour of the optic zone vaulting the cornea to better accommodate the size and location of the air bubble that arises as a result of a fenestration addition in the optic zone. This level of customization leads to an overall improvement in comfort and visual outcomes. Fenestrations are typically used in patients who develop corneal edema in non-fenestrated design and in cases of significant endothelial dysfunction or compromise, i.e. post-surgical and post-keratoplasty corneas.
Evaluation of Customization and Function
Customization Process
PROSE Providers use diagnostic fitting sets and initiate a treatment based off a parent trial device of a certain diameter and sagittal depth. A slit lamp biomicroscope is used to evaluate the customization of the trial PROSE device on the eye. An over-refraction is performed over the device to determine the visual potential. The PROSE Provider will then use the data from the over-refraction and the customization assessment of the trial device to make modifications and additions in order to create the first customized PROSE device for the patient’s eye.
PROSE Providers personally modify or make changes to the device design via spline functions using a proprietary computer-assisted design software.[27] The design is then sent to high tech lathing machines that manufacture spherical and highly toric lenses onsite. The design of the first customized device is assessed on application and again after a few hours of wear time to allow for settling. Refinement of the design is an iterative process of incremental changes that are sequentially evaluated on the eye to achieve an optimal design and physiologic function.[23][30] Before PROSE devices are dispensed to the patient, highly trained technicians individually instruct patients on proper application and removal of PROSE devices, as well as proper cleaning, disinfection, and storage.
Customization Philosophy
An optimal customization of a PROSE device is determined by the following characteristics:
Adequate corneal clearance
The device is designed to clear the steepest point (the apex) of the cornea in addition to vaulting the limbal areas 360 degrees. Limbal vault is especially important as the limbus houses the stem cells thought to be responsible for corneal healing.[31] These limbal stem cells are thought to be compromised in cases of inadequate limbal clearance, which can result in limbal edema.[1]
Corneal clearance is assessed via slit lamp examination by comparing the thickness of the fluid-filled space to the known thickness of the device or to the corneal thickness of the patient. If needed, anterior segment optical coherence tomography (AS-OCT) can be used to measure the amount of corneal clearance more accurately.[32] Although there is no clear consensus and ideal corneal clearance has varied in reports, clearance between 200 to 500 microns is generally considered to be adequate.[1] [4][33]
Clearance must be monitored over time, especially in progressive diseases such as keratoconus. Progressive steepening and the corresponding change in corneal shape may cause inadequate corneal clearance leading to the cornea contacting the back surface of the device, which can result in corneal erosions and scarring. One of the challenges of device customization includes vaulting the apex without excessive vault elsewhere.
Adequate landing zone alignment
The optimal design also aims to rest over the conjunctival and scleral anatomy in alignment, so that the device is not too tight or too loose. An inadequate landing zone can manifest via compression or impingement of conjunctival vasculature, resulting in one or more of the following: rebound redness upon device removal, impression ring and/or edge staining on the conjunctiva after device removal, and/or a feeling of pressure or pinching during device wear. These are signs that the landing zone is too steep or too tight.
Alternatively, improper alignment can occur when the landing zone is too flat or too loose. The edge of the device lifts off the conjunctiva, causing edge awareness as the eyelid blinks over the edge. However, it is not uncommon that new wearers may have some degree of edge awareness in the beginning of device wear, which can require an adaptation period. A loose lens can also allow for more debris accumulation in the tear lens reservoir, sometimes resulting in complaints of fogging or haziness of vision (termed mid-day fogging as it usually occurs after several hours of wear).
Achieving landing zone alignment can be challenging when designing larger diameter devices and/or working with highly toric and asymmetric scleral anatomies, as it requires a larger amount of haptic toricity. Limitations may include the availability of lathes to manufacture devices of the appropriate toricity and ensuring the integrity of the design of a highly toric lens without areas vulnerable to breakage.
Adequate physiological function
PROSE Providers monitor the long-term ocular health of the patients as they habitually wear their PROSE devices. The aim for an adequate physiological function includes no signs of conjunctival or corneal staining related to device customization upon device removal, no rebound injection or pain on removal, and no signs of corneal or limbal edema or other compromise to the health of the ocular surface. A minimal or insignificant amount of any of the aforementioned aspects may be allowable with close monitoring.
An additional treatment goal PROSE Providers is to reduce suctional forces as much as possible by generally fitting larger diameter and looser lenses. Sometimes this involves the addition of channels and/or fenestrations. These fitting goals aim to reduce any seal-off effect that can occur while maintaining proper tear exchange as a PROSE device settles in the conjunctival tissue.
Optimize patient’s vision and comfort
PROSE devices are designed and customized to improve visual function and comfort for the patient.
A challenge to this goal includes the fact that, at times, debris in the fluid reservoir can temporarily deteriorate vision (mid-day fogging). With this in mind, PROSE devices are customized for each patient to reduce debris accumulation in the fluid reservoir without detriment to tear exchange. That being said, debris accumulation can occur even with an optimized fit for unknown reasons, especially in patients with ocular surface inflammation. In these cases, patients may have to remove and reapply their devices throughout the day to refresh and replenish the fluid within the device.
Other visual considerations during PROSE treatment and device customization include non-wetting or deposits on the front surface of device, more commonly seen in high Dk materials and sometimes necessitating additional coatings (such as Hydra-PEG®).
Additionally, the appropriate FSE value is selected to optimize vision for the patient. Trial devices of different eccentricities are available to determine what is best for each individual patient, as currently there is no way to definitively predict this without a trial. Often, patients with corneal ectasia may benefit from a higher FSE to allow for some degree of higher order aberration correction, whereas ‘normal’ corneas, such as those patients with ocular surface disease, may find FSE to disturb vision.[29]
Challenges and Complications with PROSE treatment and Scleral Lenses
There remain a few hurdles in manufacturing these custom-designed lenses, as this demands specialized equipment. The cost of the precision lathe machines is very high, which increases the cost of manufacturing these lenses significantly. Additionally, mastering the nuances of designing and achieving a well fitted, well- functioning PROSE device requires an optometrist with a high level of expertise and training. Although this is the case, PROSE treatment was shown to be cost-effective in regards to the health benefits for the patient.[34] Significant financial assistance, when qualified, is available through the non-profit charitable organization BostonSight which has been designing customized PROSE devices since 1992.
Complications with PROSE treatment appear to be no more or less than complications with scleral lenses in general. Adverse events in scleral lenses are reported in low incidence. On a review of published cases of microbial keratitis in scleral lens wearers, cases were typically associated with epithelial compromise (i.e. epithelial defects), poor lens hygiene and compliance, and patients currently immunosuppressed.[35] [36] One case report describes a scleral lens patient with recurrent non-infectious infiltrative keratitis, most likely due to improper contact lens maintenance and poor cleaning, that resolved after alterations to the care and cleaning schedule of the lens and the lens case.[37] Overall, incidence of microbial keratitis in scleral lens wearers remains to be defined but has been reported in literature as 0.5% and 1.6%.[38] [39]
Another consideration with scleral lenses is the possibility that corneal hypoxia that can occur with this lens system. The tear lens thickness as well as the thickness of the scleral lens itself presents a more significant barrier to oxygen transmission than other lens modalities. Additionally, the fit of the scleral lens system in regard to oxygenated tear exchange likely plays an important role in regard to oxygen transmission to the cornea. With modern high Dk scleral lenses, there is little clinical evidence of significant corneal edema during daily wear of scleral lenses in patients with adequate endothelial function.[35] [37] [40] [41] However, low grade corneal hypoxia and corneal edema is thought to occur with scleral lenses. This is taken into consideration during PROSE treatment, for all patients, but especially in patients with poor endothelial function (i.e. post-keratoplasty, keratoglobus, etc), often by modifying lens thickness and monitoring oxygenated tear exchange with addition of channels or fenestrations if needed.
One case series involving patients with ocular surface disease in PROSE treatment reported transient bullae during lens wear that resolved after lens removal.[42] These were hypothesized to occur due to weak connections in the corneal epithelium in the basement membrane and Bowman’s layer in patients with compromise corneas due to ocular surface disease.[42] Bullae can increase the risk of epithelial defects, which in turn could increase the risk of infection and other complications.[42]
Future Considerations
Higher Order Aberration Correcting PROSE Devices
Although scleral lenses correct for irregular astigmatism and some amount of higher order aberrations (HOA), residual higher order aberrations can exist for some patients, especially for those patients with corneal ectasia.[43] Attempts to correct the HOA may first involve using FSE although this does not work for all patients. Patients with residual HOA will often complain of visually debilitating glare, shadows, and multiple images. Correction of HOA via wavefront-guided scleral lenses is of increasing interest in practice and in development for PROSE devices. The general method involves measuring the pupil size as well as the rotation and decentration of the scleral lens on the eye, followed by capturing the wavefront of the patient via an aberrometer. The correction needed to neutralize the HOA within the patient’s wavefront can then be added into the device itself, including the compensation needed due to the lens decentration and rotation.[43][44] This allows for a largely stable and higher quality visual correction for certain patients.
PROSE Devices as a Method of Drug Delivery
Devices used in PROSE treatment are sometimes used off-label in overnight wear for the healing of persistent epithelial defects by acting as a fluid bandage lens. Overnight wear of PROSE can either consist of continuous wear or 12/12 wear. Continuous wear is when one lens is worn for 24 hours or more. Twelve/twelve wear schedule is when one lens is worn for 12 hours at a time, followed by removal, cleaning, disinfection and reapplication for another 12 hours of wear. Some practitioners will require a second device that is used after the first 12-hour period instead of cleaning and reapplying the same device. This allows for cleaning and peroxide-based disinfection of the second device, while the patient is wearing the other device for the first 12 hours. The patient then wears the second device for the second 12 hours of the day. Overnight wear of any contact lens, including scleral lenses, comes with an increased risk of infection and must be used sparingly, with caution and only in those situations not responding to standard means and with proper informed consent from the patient.
The use of PROSE treatment as a therapeutic means for healing persistent epithelial defects with the use of prophylactic antibiotic in the lens bowl has been published in case series with successful results.[45] [46] Case series have also reported that PROSE treatment was successfully used as a drug delivery system for treatment of infiltrative corneal ulceration via the addition of preservative-free, fortified antibiotics in the fluid reservoir.[36][47]
Another study also showed improvement in corneal neovascularization in patients who applied a drop of 1% preservative-free bevacizumab solution to the reservoir of PROSE device twice daily.[48]
Scleral lenses may also play a role in procedural drug delivery. In one publication, scleral lenses were used to assist in corneal collagen crosslinking (CXL). After removal of the corneal epithelium, the modified scleral lens was filled with riboflavin and applied in order to allow for efficient penetration of the riboflavin into the corneal stroma and the anterior chamber before the CXL procedure.[49] This may prove to be beneficial and less burdensome than the conventional method of riboflavin delivery before the CXL procedure (manual instillation of riboflavin solution drops every 2 minutes for 30 minutes).[49]
Image-Guided PROSE Devices
The process of an image-guided fit consists of obtaining several images of the eye using specialized instruments, of which there are several methods in development. Optical coherence tomography, scheimpflug imaging, Fourier transform profilometry, or other modalities are currently being utilized.8 The data from the images captured of the scleral and corneal shape can then be converted and extrapolated to create a design of a determined diameter.
Optical coherence topography (OCT) to measure certain parameters of the corneal and scleral shape has been used to aid in the design of a device. This was done in a study with PROSE devices ranging in diameter from 18.5mm to 20mm with reported success and repeatability.[23]
Image-guided fitting may be beneficial in the future as it has the potential to reduce the number of visits and the number of devices needed to be manufactured in the customization process. However, the use of image-guided fitting is still under development and presents specific challenges. Instillation of sodium fluorescein dye on the eye is sometimes required which may be irritating to the eye. Exposure of the ocular surface to the extent necessary to acquire complete data to the desired diameter may prove challenging particularly for certain anatomy (i.e. prominent brow, inset eyes). Extended eye exposure during imaging may be uncomfortable for those with significant ocular surface disease. Technology that uses stitching of many pictures in various gazes may prove less accurate.
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Van Der Worp E. A Guide to Scleral Lens Fitting (2nd Edition). Pacific Univ Common Knowl. Published online 2015:1-64. http://commons.pacificu.edu/mono/10/
- ↑ Jacobs DS. Update on scleral lenses. Curr Opin Ophthalmol. Published online 2008. doi:10.1097/ICU.0b013e328302cc4f
- ↑ Visser ES, Visser R, Van Lier HJJ. Advantages of toric scleral lenses. Optom Vis Sci. Published online 2006. doi:10.1097/01.opx.0000214297.38421.15
- ↑ Jump up to: 4.0 4.1 Kauffman MJ, Gilmartin CA, Bennett ES, Bassi CJ. A Comparison of the short-term settling of three scleral lens designs. Optom Vis Sci. Published online 2014. doi:10.1097/OPX.0000000000000409
- ↑ Jump up to: 5.0 5.1 U.S. Food and Drug. 510(k) Premarket Notification for BostonSight PD Prosthetic Device. Published 2016. Accessed May 21, 2020. https://www.accessdata.fda.gov/cdrh_docs/pdf16/K161461.pdf
- ↑ Jump up to: 6.0 6.1 Papakostas TD, Le HG, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of stevens-johnson syndrome/toxic epidermal necrolysis. Ophthalmology. Published online 2015. doi:10.1016/j.ophtha.2014.08.015
- ↑ Jump up to: 7.0 7.1 Lee JC, Chiu GB, Bach D, Bababeygy SR, Irvine J, Heur M. Functional and visual improvement with prosthetic replacement of the ocular surface ecosystem scleral lenses for irregular corneas. Cornea. Published online 2013. doi:10.1097/ICO.0b013e3182a73802
- ↑ Jump up to: 8.0 8.1 Rathi VM, Dumpati S, Mandathara PS, Taneja MM, Sangwan VS. Scleral contact lenses in the management of pellucid marginal degeneration. Contact Lens Anterior Eye. Published online 2016. doi:10.1016/j.clae.2015.11.005
- ↑ Jump up to: 9.0 9.1 9.2 Mahadevan R, Fathima A, Rajan R, Arumugam AO. An ocular surface prosthesis for keratoglobus and terrien’s marginal degeneration. Optom Vis Sci. Published online 2014. doi:10.1097/OPX.0000000000000200
- ↑ Jump up to: 10.0 10.1 Mian SZ, Agranat JS, Jacobs DS. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) treatment for complications after LASIK. Eye Contact Lens. Published online 2016. doi:10.1097/ICL.0000000000000303
- ↑ Jump up to: 11.0 11.1 Rosenthal P. The Boston Lens and the management of keratoconus. Int Ophthalmol Clin. Published online 1986. doi:10.1097/00004397-198602610-00014
- ↑ Jump up to: 12.0 12.1 Chiu GB, Bach D, Theophanous C, Heur M. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) scleral lens for Salzmann’s nodular degeneration. Saudi J Ophthalmol. Published online 2014. doi:10.1016/j.sjopt.2014.06.001
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. Published online 2005. doi:10.1097/01.ICL.0000152492.98553.8D
- ↑ Jump up to: 14.0 14.1 Rosenthal P, Cotter J. The Boston scleral lens in the management of severe ocular surface disease. Ophthalmol Clin North Am. Published online 2003. doi:10.1016/S0896-1549(02)00067-6
- ↑ Jump up to: 15.0 15.1 Jacobs DS, Rosenthal P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. Published online 2007. doi:10.1097/ICO.0b013e318155743d
- ↑ Jump up to: 16.0 16.1 Romero-Rangel T, Stavrou P, Cotter J, Rosenthal P, Baltatzis S, Foster CS. Gas-permeable scleral contact lens therapy in ocular surface disease. Am J Ophthalmol. Published online 2000. doi:10.1016/S0002-9394(00)00378-0
- ↑ Jump up to: 17.0 17.1 Chahal JS, Heur M, Chiu GB. Prosthetic Replacement of the Ocular Surface Ecosystem Scleral Lens Therapy for Exposure Keratopathy. Eye Contact Lens. Published online 2017. doi:10.1097/ICL.0000000000000265
- ↑ Jump up to: 18.0 18.1 Gervasio KA, Godfrey KJ, Marlow ED, Lee MN, Lelli GJ. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) Versus Standard of Care for Postsurgical Lagophthalmos and Exposure Keratopathy: Trends in Visual Outcomes. In: Ophthalmic Plastic and Reconstructive Surgery. ; 2019. doi:10.1097/IOP.0000000000001233
- ↑ Jump up to: 19.0 19.1 Xu M, Randleman JB, Chiu GB. Long-Term Descemetocele Management With Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) Treatment. Eye Contact Lens. Published online 2020. doi:10.1097/ICL.0000000000000602
- ↑ Jump up to: 20.0 20.1 Kojima T, Hasegawa A, Nakamura T, Isogai N, Kataoka T, Ichikawa K. Five-year PROSE treatment for aniridic keratopathy. Optom Vis Sci. Published online 2016. doi:10.1097/OPX.0000000000000942
- ↑ Jump up to: 21.0 21.1 Kim KH, Deloss KS, Hood CT. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) for Visual Rehabilitation in Limbal Stem Cell Deficiency. Eye Contact Lens Sci Clin Pract. Published online 2020. doi:10.1097/icl.0000000000000685
- ↑ Jump up to: 22.0 22.1 Rathi VM, Sudharman Mandathara P, Vaddavalli PK, Dumpati S, Chakrabarti T, Sangwan VS. Fluid-filled scleral contact lenses in vernal keratoconjunctivitis. Eye Contact Lens. Published online 2012. doi:10.1097/ICL.0b013e3182482eb5
- ↑ Jump up to: 23.0 23.1 23.2 Le HGT, Tang M, Ridges R, Huang D, Jacobs DS. Pilot study for OCT guided design and fit of a prosthetic device for treatment of corneal disease. J Ophthalmol. Published online 2012. doi:10.1155/2012/812034
- ↑ Koppen C, Kreps EO, Anthonissen L, Van Hoey M, Dhubhghaill SN, Vermeulen L. Scleral Lenses Reduce the Need for Corneal Transplants in Severe Keratoconus. Am J Ophthalmol. Published online 2018. doi:10.1016/j.ajo.2017.10.022
- ↑ Deloss KS, Fatteh NH, Hood CT. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) scleral device compared to keratoplasty for the treatment of corneal ectasia. Am J Ophthalmol. Published online 2014. doi:10.1016/j.ajo.2014.07.016
- ↑ Jump up to: 26.0 26.1 26.2 26.3 Gumus K, Gire A, Pflugfelder SC. The impact of the boston ocular surface prosthesis on wavefront higher-order aberrations. Am J Ophthalmol. Published online 2011. doi:10.1016/j.ajo.2010.10.027
- ↑ Jump up to: 27.0 27.1 27.2 27.3 27.4 Hussoin T, Le HG, Carrasquillo KG, Johns L, Rosenthal P, Jacobs DS. The effect of optic asphericity on visual rehabilitation of corneal ectasia with a prosthetic device. Eye Contact Lens. Published online 2012. doi:10.1097/ICL.0b013e3182657da5
- ↑ Mahadevan R, Arumugam AO, Madhumathi, Ganesan N, Siddireddy JS. Clinical visual performance of different front surface eccentricity in PROSE. Contact Lens Anterior Eye. Published online 2012. doi:10.1016/j.clae.2012.08.021
- ↑ Jump up to: 29.0 29.1 Jagadeesh D, Mahadevan R. Visual performance with changes in eccentricity in PROSE device: A case report. J Optom. Published online 2014. doi:10.1016/j.optom.2013.04.001
- ↑ Carrasquillo KG. What Makes a Scleral Lens Fit Physiological? A Case Report. J Ophthalmol Clin Res. Published online 2018. doi:10.24966/ocr-8887/100041
- ↑ Schornack MM. Limbal stem cell disease: Management with scleral lenses. Clin Exp Optom. Published online 2011. doi:10.1111/j.1444-0938.2011.00618.x
- ↑ Gemoules G. A novel method of fitting scleral lenses using high resolution optical coherence tomography. Eye Contact Lens. Published online 2008. doi:10.1097/ICL.0b013e318166394d
- ↑ Sonsino J, Mathe DS. Central vault in dry eye patients successfully wearing scleral lens. Optom Vis Sci. Published online 2013. doi:10.1097/OPX.0000000000000013
- ↑ Shepard DS, Razavi M, Stason WB, et al. Economic Appraisal of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. Published online 2009. doi:10.1016/j.ajo.2009.07.012
- ↑ Jump up to: 35.0 35.1 Walker MK, Bergmanson JP, Miller WL, Marsack JD, Johnson LA. Complications and fitting challenges associated with scleral contact lenses: A review. Contact Lens Anterior Eye. Published online 2016. doi:10.1016/j.clae.2015.08.003
- ↑ Jump up to: 36.0 36.1 Kalwerisky K, Davies B, Mihora L, Czyz CN, Foster JA, Demartelaere S. Use of the Boston ocular surface prosthesis in the management of severe periorbital thermal injuries: A case series of 10 patients. Ophthalmology. Published online 2012. doi:10.1016/j.ophtha.2011.08.027
- ↑ Jump up to: 37.0 37.1 Bruce AS, Nguyen LM. Acute red eye (non-ulcerative keratitis) associated with mini-scleral contact lens wear for keratoconus. Clin Exp Optom. Published online 2013. doi:10.1111/cxo.12033
- ↑ Schornack MM. Scleral lenses: A literature review. Eye Contact Lens. Published online 2015. doi:10.1097/ICL.0000000000000083
- ↑ Zimmerman AB, Marks A. Microbial keratitis secondary to unintended poor compliance with scleral gas-permeable contact lenses. Eye Contact Lens. Published online 2014. doi:10.1097/ICL.0b013e318273420f
- ↑ Bergmanson JPG, Ezekiel DF, Van der Worp E. Scleral contact lenses and hypoxia Theory versus practice. Contact Lens Anterior Eye. Published online 2015. doi:10.1016/j.clae.2015.03.007
- ↑ Michaud L, van der Worp E, Brazeau D, Warde R, Giasson CJ. Predicting estimates of oxygen transmissibility for scleral lenses. Contact Lens Anterior Eye. Published online 2012. doi:10.1016/j.clae.2012.07.004
- ↑ Jump up to: 42.0 42.1 42.2 Isozaki VL, Chiu GB. Transient corneal epithelial bullae associated with large diameter scleral lens wear: A case series. Contact Lens Anterior Eye. Published online 2018. doi:10.1016/j.clae.2018.05.002
- ↑ Jump up to: 43.0 43.1 Marsack JD, Ravikumar A, Nguyen C, et al. Wavefront-guided scleral lens correction in keratoconus. Optom Vis Sci. Published online 2014. doi:10.1097/OPX.0000000000000275
- ↑ Sabesan R, Johns L, Tomashevskaya O, Jacobs DS, Rosenthal P, Yoon G. Wavefront-guided scleral lens prosthetic device for keratoconus. Optom Vis Sci. Published online 2013. doi:10.1097/OPX.0b013e318288d19c
- ↑ Khan M, Manuel K, Vegas B, Yadav S, Hemmati R, Al-Mohtaseb Z. Case series: Extended wear of rigid gas permeable scleral contact lenses for the treatment of persistent corneal epithelial defects. Contact Lens Anterior Eye. Published online 2019. doi:10.1016/j.clae.2018.09.004
- ↑ He X, Donaldson KE, Perez VL, Sotomayor P. Case Series: Overnight Wear of Scleral Lens for Persistent Epithelial Defects. Optom Vis Sci. Published online 2018. doi:10.1097/OPX.0000000000001162
- ↑ Zhai H, Bispo PJM, Kobashi H, Jacobs DS, Gilmore MS, Ciolino JB. Resolution of fluoroquinolone-resistant Escherichia coli keratitis with a PROSE device for enhanced targeted antibiotic delivery. Am J Ophthalmol Case Reports. Published online 2018. doi:10.1016/j.ajoc.2018.09.006
- ↑ Lim M, Jacobs DS, Rosenthal P, Carrasquillo KG. The boston ocular surface prosthesis as a novel drug delivery system for bevacizumab. Semin Ophthalmol. Published online 2009. doi:10.1080/08820530902802013
- ↑ Jump up to: 49.0 49.1 Soares RT, Novo NF, França WM. Evaluation of a Modified Scleral Contact Lens as a Riboflavin Delivery Device for Corneal Collagen Crosslinking. Open J Ophthalmol. Published online 2017. doi:10.4236/ojoph.2017.74036