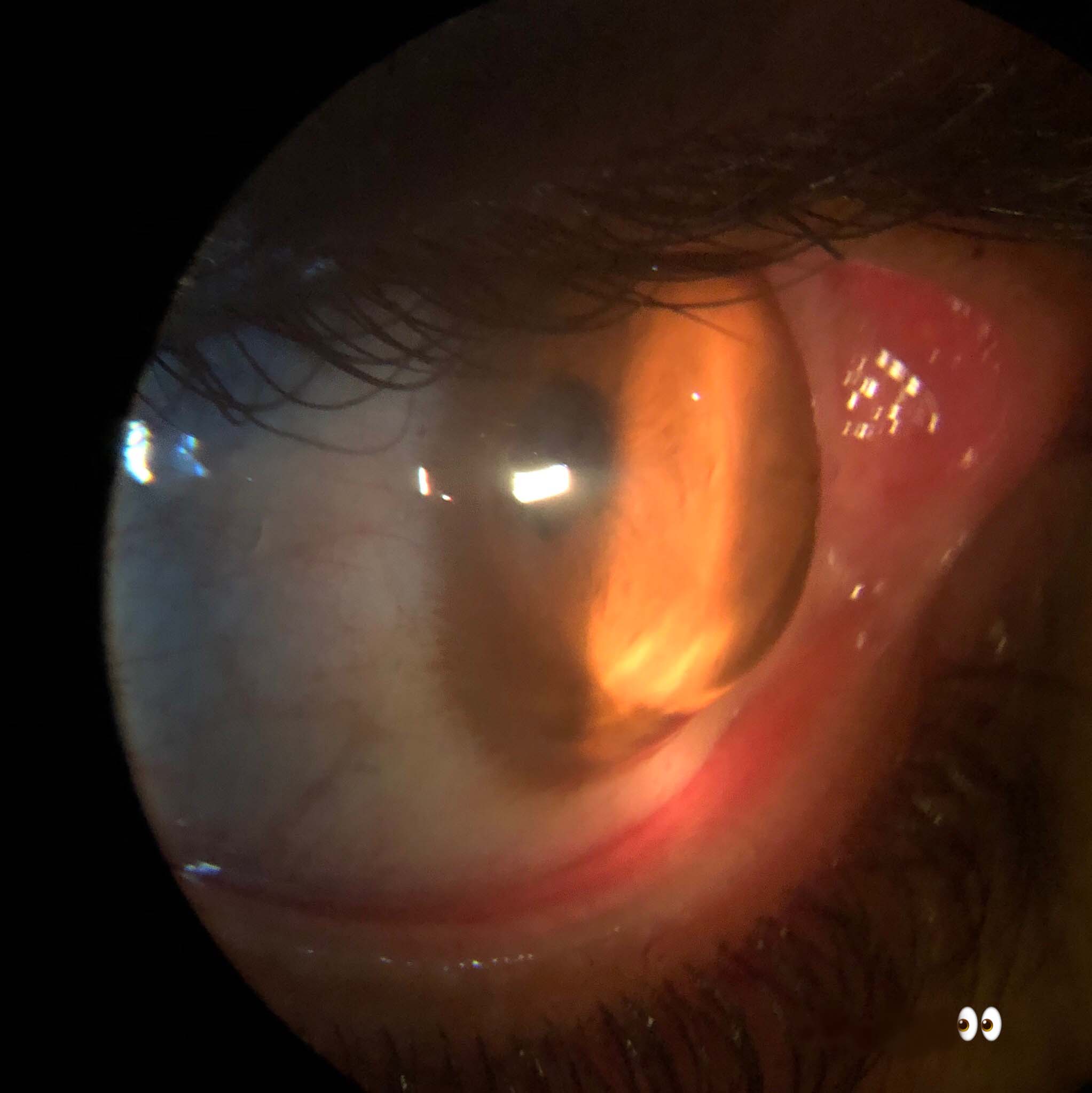
Keratoglobus
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Keratoglobus is a rare, noninflammatory corneal ectasia characterized by diffuse protrusion and thinning of the cornea.[2]
Disease Entity
Disease
Two forms of keratoglobus exist. The congenital form is present at birth and is associated with Ehler-Danlos type VI, Leber congenital amaurosis, and the blue sclera syndrome. The acquired form presents in adulthood and may evolve from preexisting cases of pellucid marginal degeneration or keratoconus.[3][4] It is associated with vernal keratoconjunctivitis, dysthyroid ophthalmopathy, and chronic marginal blepharitis.[5]
Etiology
The etiology is unknown. However, associations between keratoglobus and disorders such as Ehlers-Danlos type VI, Marfan syndrome, and the blue sclera syndrome indicate that the etiology may result from defects in collagen synthesis.[5]
Risk Factors
No risk factors have been identified. Unlike other noninflammatory corneal ectasias such as keratoconus, eye rubbing has not been designated a risk factor.
General Pathology
Histology
Keratoglobus characteristically exhibits diffuse stromal thinning as well as focal disruptions in Bowman's layer which are most severe in the peripheral cornea. Despite this thinning, stromal lamellar organization remains unchanged from its normal configuration.[2][5] Additional findings include central epithelial hyperplasia, neovascularization and scarring of the stroma, disruptions in Descemet's membrane, as well as thickening of Descemet's membrane.[5]
Immunohistochemistry
In an attempt to uncover the mechanism behind the noninflammatory corneal ectasias, specific gene products have been investigated. Various publications have demonstrated decreased levels of alpha1-proteinase inhibitor (a1-PI) within the stroma of keratoconus patients. [6] Sp1, a transcription facter known to suppress a1-PI promoter activity, has been found to be elevated in these same patients. Meghpara et al found similar alterations in expression of Sp1 and a1-PI in patients with keratoglobus. This leads us to believe that the underlying cause of corneal thinning in noninflammatory corneal ectasias may be due to alterations in the stromal degradation.
Pathophysiology
The pathophysiologic mechanism is unknown, but keratoglobus may be associated with a defect in collagen synthesis or degradation. Keratoglobus is not associated with atopic disease.
Primary Prevention
An effective means of prevention has not been discovered.
Diagnosis
History
Patients usually present with a stable or worsening bilateral visual impairment that cannot be properly corrected with spectacles or contact lenses. Rarely, they may present with an episode of severe eye pain due to corneal hydrops or corneal rupture.[7]
Physical Examination
Physical examination begins with an assessment of visual acuity followed by retinoscopy and refraction. Measurements of pupillary reaction, ocular motility and alignment, as well as intraocular pressures should be documented. Gross examination followed by slit lamp biomicroscopy should then be performed to evaluate the anterior segment. Finally, evaluation of the optic disc, macula, retinal vessels, and peripheral retina should be completed after pupillary dilation. Corneal topography and tomography as well as pachymetry can then be utilized to demonstrate diffuse corneal steepening and thinning. Keratometry readings can reach 50-60 D. Fleischer rings, Vogt striae, and anterior stromal scarring are not typical, but Descemet membrane thickening and folds are common.
Signs
- Myopia or high myopia
- Irregular astigmatism
- Irregular retinoscopic reflex
- Globular protrusion of the cornea
- Diffuse corneal thinning, most severe peripherally
- Folds, breaks, or thickening of Descemet's membrane
- Spontaneous rupture/tear of Descemet's membrane
- Acute hydrops
- Scarring or neovascularization of the stroma
Symptoms
- Decreased visual acuity
- Suboptimal visual acuity with spectacle correction
- Poor fit, pain, or suboptimal visual acuity with contact lens use
- A single or multiple episodes of eye pain followed by resolution and decreased visual acuity
Clinical Diagnosis
A clinical diagnosis is made using slit lamp biomicroscopy. Necessary findings include globular protrusion of the cornea and diffuse corneal thinning most severe peripherally.
Diagnostic procedures
- Ultrasonic pachymetry of the cornea
- Corneal Topography & Tomography
- Anterior segment optical coherence tomography (OCT)
Differential diagnosis
- Keratoconus
- Pellucid marginal degeneration
- Corneal ectasia following refractive surgery
Management
General treatment
Management begins with spectacle correction. Spectacle correction of high myopia with or without astigmatism may significantly improve visual acuity and is especially important in children to try to prevent ambyopia. In addition, spectacles can provide ocular protection, as these patients' diffusely thin stroma places them at increased greater risk of corneal rupture after minor ocular trauma.[2] Polycarbonate lenses are optimal in these patients because of inherent properties that allow them to flex and deform rather than shatter.[7][8] Opinions are mixed as to whether contact lenses are safe and effective as a second line therapy. Supporters advocate lenses that provide the most ideal combination of visual acuity improvement and fit.[2][9] As in other corneal ectasias, patients should begin with soft contact lenses and move to rigid gas permeable, hybrid, or scleral lenses. Particular attention is required for fitting rigid contact lenses because of complications associated with their use. Rigid lenses that are excessively flat may cause epitheliopathy or thinning at the corneal apex. Overly steep lenses may worsen or complicate the preexisting ectasia.[10] These risks in conjunction with the dramatically increased potential for corneal rupture secondary to minimal trauma lead some experts to discourage contact lens wear altogether.[11]
Medical therapy
No medical therapy has been discovered to treat the underlying pathology. Instead, attention is focused on complications such as acute hydrops. Hypertonic saline, cycloplegia, and bandage soft contact lenses are the mainstay of treatment. Aqueous suppressants have also been proposed to decrease stromal uptake of fluid.[2][12]
Surgery
Surgical management of keratoglobus is challenging. Various techniques have been proposed, but a gold standard has not yet emerged. Traditional penetrating keratoplasty (PK) was one of the first surgical procedures attempted. It has the advantage of being less technically difficult than other surgical options. However, midperipheral suture fixation approximates a normal thickness graft to extremely thin recipient tissue. Significant postoperative astigmatism is likely and results in poor visual outcomes.[11][13] Large diameter PK has also been utilized. Disadvantages include higher risk of graft rejection, delayed re-epithelialization, and postoperative glaucoma due to the proximity of the graft to limbal stem cells, limbal vasculature, as well as the trabecular meshwork. Eccentric PK has been proposed as a way to circumvent the disadvantages of both traditional and large diameter PKs. The initial case report resulted in a visual acuity of 20/50 without the previously described complications.[11]
Lamellar keratoplasty is another viable option. Several variations have been proposed involving trephination of the host cornea followed by lamellar dissection at different depths. The dissection is extended centrifugally posterior to the limbus. The donor tissue is then tucked beneath the limbus within the plane of the lamellar dissection and sutured in place. This technique provides structural support, improves corneal irregularities, and preserves limbal stem cells.[7][13] In cases where there is significantly opacified host tissue or host-donor interface abnormality a two-step procedure can be performed. Six months after the previously described lamellar graft transplantation, a penetrating keratoplasty is undergone. This allows for improved visual potential in selected patients with residual corneal opacification.[14]
Surgical management of acute hydrops resulting from Descemet's membrane tears is another complicated topic. Traditionally, acute hydrops associated with conditions such as keratoconus and keratoglobus was managed medically with hypertonic agents, contact lenses, and patching. Recent research has documented accelerated resolution of corneal edema in keratoconus patients who were treated with intracameral gas. Keratoglobus patients receiving the same therapy have had mixed results.[12][15] [16] This is likely due to the large size of tears upon presentation. A second surgical option involves the use of DSAEK donor tissue to patch the host endothelium.[15] [17]
Surgical Complications
- Graft rejection
- Epithelial nonhealing
- Epithelial downgrowth
- Intrastromal epithelial cyst
- Neurotrophic ulcer
- Interface neovascularization
- Corneal infection involving donor epithelium, donor stroma, or host-donor interface
- Endophthalmitis[7]
Prognosis
The prognosis for keratoglobus is poor. Spectacle correction often results in suboptimal BCVA. In addition, surgical correction is challenging and is associated with complications.
References
- ↑ Keratoglobus. Ophthalmic Images in Diverse Patient Populations. American Academy of Ophthalmology. https://eyewiki.org/Ophthalmic_Images_in_Diverse_Patient_Populations Accessed December 5, 2024.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 External Disease and Cornea. Basic and Clinical Science Course, Section 8. American Academy of Ophthalmology; 2011:302-3.
- ↑ Karabatsas CH, Cook SD. Topographic Analysis in Pellucid Marginal Corneal Degeneration and Keratoglobus. Eye(Lond). 1996;10(Pt4):451-5.
- ↑ Rumelt S, Rehany U. Surgically induced keratoglobus in pellucid marginal degeneration. Eye(Lond). 1998;12(Pt1):156-8.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Meghpara B, Nakamura H, Vemuganti G, et al. Histopathologic and Immunohistochemical Studies of Keratoglobus. Arch Ophthalmol. 2009 Aug; 127(8): 1029-35.
- ↑ Shen X, Park JS, Qiu Y, et al. Effects of Sp1 overexpression on cultured human corneal stromal cells. Genes Cells. 2009 Oct;14(10):1133-9.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Javadi MA, Kanavi MR, Ahmadi M, Yazdani S. Outcomes of epikeratoplasty for advanced keratoglobus. Cornea. 2007 Feb;26(2):154-7.
- ↑ Diallo ML, Simonet P, Frenette B, Sanschagrin B. Resistance of plastic ophthalmic lenses: the effect of base curve on different materials during static load testing. Optom Vis Sci. 2001 Jul;78(7):518-24.
- ↑ Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984 Jan-Feb;28(4):293-322.
- ↑ McMonnies CW. The biomechanics of keratoconus and rigid contact lenses. Eye Contact Lens. 2005 Mar;31(2):80-92.
- ↑ Jump up to: 11.0 11.1 11.2 Kodjikian L, Baillif S, Burillon C, Grange JD, Garweg JG. Keratoglobus surgery: penetrating keratoplasty redux. Acta Ophthalmol Scand. 2004 Oct;82(5):625-7.
- ↑ Jump up to: 12.0 12.1 Basu S, Vaddavalli PK, Ramappa M, Shah S, Murthy S, Sangwan VS. Intracameral Perfluoropropane Gas in the Treatment of Acute Corneal Hydrops. Ophthalmology. 2011 May;118(5):934-9.
- ↑ Jump up to: 13.0 13.1 Vajpayee RB, Bhartiya P, Sharma N. Central Lamellar Keratoplasty With Peripheral Intralamellar Tuck. Cornea. 2002 Oct;21(7):657-60.
- ↑ Jones DH, Kirkness CM. A New Surgical Technique for Keratoglobus-Tectonic Lamellar Keratoplasty Followed by Secondary Penetrating Keratoplasty. Cornea. 2001 Nov;20(8):885-7.
- ↑ Jump up to: 15.0 15.1 Palioura S, Chodosh J, Pineda R. A Novel Approach to the Management of a Progressive Descemet Membrane Tear in a Patient With Keratoglobus and Acute Hydrops. Cornea. 2013 March;32(3):355-8.
- ↑ Mohebbi M, Pilafkan H, Nabavi A, Mirghorbani M, Naderan M. Treatment of Acute Corneal Hydrops with Combined Intracameral Gas and Approximation Sutures in Patients with Corneal Ectasia. Cornea. 2020 Feb;39(2):258-262.
- ↑ Sharma S, Fernandes M. Descemet stripping automated endothelial keratoplasty: An alternate surgical modality for Descemet's membrane detachment following hydrops in keratoglobus. Indian J Ophthalmol. 2020 March;68(3)513-514.