Ocular Cicatricial Pemphigoid
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Ocular Cicatricial Pemphigoid is abbreviated OCP.
OCP is considered a subtype of Mucous Membrane Pemphigoid (abbreviated MMP),[1] and these terms are sometimes used interchangeably.
OCP is a type of autoimmune conjunctivitis that leads to cicatrization (i.e. scarring) of the conjunctiva.[2] If OCP is left untreated, it can lead to blindness.
Etiology
The exact pathogenesis of OCP remains to be elucidated but the existing evidence supports a Type II hypersensitivity response caused by an autoantibody to a cell surface antigen in the basement membrane of the conjunctival epithelium and other similar squamous epithelia.[3] [4]
Investigations into the underlying target antigen have led to several possible suspects. The autoantigens responsible for bullous pemphigoid (BP230 (i.e. Bullous pemphigoid antigen I, a desmoplakin) and BP180 (i.e. Bullous pemphigoid antigen II, a transmembrane hemidesmosome)) were studied, and the sera of patients with OCP was shown to bind these antigens.[3][5] [6] [7] However, further investigation supports that the more likely autoantigen is actually the beta-4 subunit of the alpha-6 beta-4 integrin of hemidesmosomes.[5] [6] [7]
Studies of HLA (human leukocyte antigen) typing have found an increased susceptibility to the disease in patients with HLA-DR4.[3] The HLA-DQB1*0301 allele in particular shows a strong association with OCP and other forms of pemphigoid disease.[8] [9] HLA-DQB1*0301 is thought to bind to the beta-4 subunit of the alpha-6 beta-4 integrin (the suspected autoantigen in OCP).[8] [9]
Pathophysiology
Although the exact mechanism remains to be elucidated, the existing evidence supports the production of an autoantibody in susceptible individuals to the beta-4 subunit of the alpha-6 beta-4 integrin of hemidesmosomes in the lamina lucida of the conjunctival basement membrane.[5] [6] [7] [8] [9][10]
Binding of the autoantibody to the autoantigen activates complement, resulting in the cytotoxic destruction of the conjunctival membrane.[3] Disruption of the conjunctival basement membrane subsequently leads to bullae formation.[11]
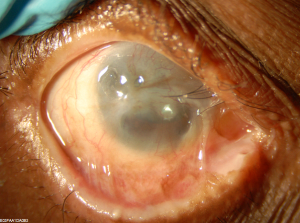
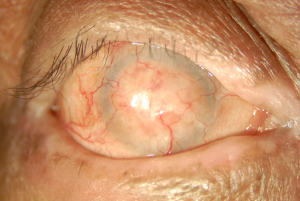
The associated cellular inflammatory infiltrate of the epithelium and substantia propria manifests as the chronic conjunctivitis that is the hallmark of this disease.[10] [11] Eosinophils and neutrophils mediate inflammation in the early and acute phases of the disease, similar to what is observed in the skin.[1][10] Chronic disease has largely lymphocytic infiltration.[1][12] [13] Fibroblast activation leads to subepithelial fibrosis, which in early disease appears as fine white striae most easily seen in the inferior fornix.[14] A scar in the upper palpebral conjunctiva may also be seen. Over time, the fibrotic striae contract, leading to conjunctival shrinkage, symblepharon formation, and forniceal shortening.[1][12][14]In severe cases of conjunctival fibrosis, entropion, trichiasis and symblepharon may develop, leading to associated keratopathy and corneal vascularization, scarring, ulceration, and epidermalization.[3][14]
The clinical course and severity is variable. Recurrent inflammation causes loss of Goblet cells and obstruction of lacrimal gland ductules, leading to aqueous and mucous tear deficiency.[3][15] The resulting xerosis is severe, and along with progressive subepithelial fibrosis and destruction of limbal stem cells leads to limbal stem cell deficiency and ocular keratinization.[5]
Several pro-inflammatory cytokines are found to be elevated in the conjunctival tissues of patients with OCP. Levels of Interleukin (IL) 1, Tumor Necrosis Factor (TNF) Alpha, migration inhibition factor, and macrophage colony-stimulating factor, and IL-13 have been found to be elevated.[3][11] IL-13 has been found to have a pro-fibrotic and pro-inflammatory effect on conjunctival fibroblasts, and may be implicated in the progressive conjunctival fibrosis that can occur despite clinical quiescence.[11]
Additionally, testing of the tears of patients with OCP found elevated levels of IL-8, Matrix Metalloproteinase (MMP) 8, MMP-9, and myeloperoxidase (MPO), which are thought to result from neutrophilic infiltrate in patients with OCP.[1][16]
Epidemiology
Incidence rates are vary between 1 in 12,000 to 60,000.[12] A study in the UK found that OCP represents 61% of cicatricial conjunctivitis and is estimated to occur with an incidence of 1 in 1 million.[17]
Women are affected more than men by a ratio of 2:1.[3][12] Age on onset is usually age 60 to 80 and rarely younger than 30.[3][17] There is no racial predilection.[18]
Presentation
In patients with MMP, oral involvement is most common (in 90% of cases), followed by ocular involvement (in 61% of cases).[19] Ocular involvement of MMP is considered high risk and carries a poorer prognosis (despite treatment) than when oral mucosa and/or skin alone are affected.[17] Up to one third of patients with oral disease progress to ocular involvement.[19]
Additional sites of involvement include the oropharynx, nasopharynx, esophagus, larynx, genitalia, and anus.[3][17] The skin is involved in approximately 15% of cases.[3] Dysphagia may be a presenting symptom.[3]
There are several clinical scoring systems for OCP, including schema from Foster, Mondino, and Tauber.[17] Clinicians vary in which system they utilize for grading disease clinically and although there are proponents for each system, no consensus exists regarding which system is best to use.[17][20] The existing classification schema are limited by the lack of direct correlation with disease progression and therefore no system can be used to predict need for immunosuppression.[20]
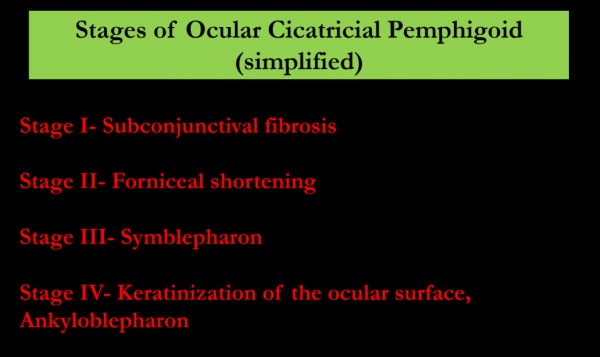
Mondino’s Classification System is based on inferior forniceal depth.[20] A normal inferior forniceal depth is approximately 11 mm.[3][20]
- Stage I: up to 25% inferior forniceal depth loss[20]
- Stage II: 25-50% inferior forniceal depth loss[20]
- Stage III: 50-75% inferior forniceal depth loss[20]
- Stage IV: greater than 75% inferior forniceal depth loss[20]
Foster’s Classification System has four stages as well and is based on specific clinical signs:[3][20]
- Stage I: Early stage[3][20]
- May include nonspecific symptoms and minimal findings which lead to under-recognition of the disease.[3][17] Commonly presents as chronic conjunctivitis, tear dysfunction, and subepithelial fibrosis.[3][12] Subepithelial fibrosis manifests as fine gray-white striae in the inferior fornix.[3] Signs and symptoms are usually bilateral, and may be asymmetric.
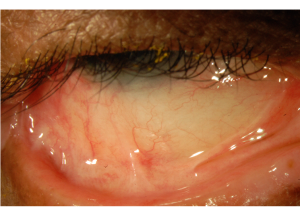
- Stage II: Shortening of the fornices[3][20]
- Stage III: Symblepharon formation[3][20]
- Can be detected by pulling the lower eyelid down while the patient looks up and vice versa.[3]
- Stage IV: Ankyloblepharon[20]
Patients with OCP vary significantly in disease severity and rate of progression, but untreated disease often progresses in up to 75% of patients.[3][10][20] Additionally, the subepithelial fibrosis in OCP can progress even despite clinical quiescence.[14][21] A study conducted in the UK found that 42% of patients had disease progression in the absence of clinical inflammation.[21] Histological analysis of these patients has found significant inflammatory cellular infiltrate despite the white and quiet appearance of the conjunctiva clinically, and this has been termed “white inflammation”.[14] This is particularly important as 30% of patients with advanced conjunctival fibrosis become blind and represents a clinical challenge to treatment of disease.[13] [14]
Diagnosis
Diagnosis is based on clinical signs and positive direct immunofluorescence testing of the conjunctiva.[2] [3][17] Conjunctival biopsy of an actively involved area is needed and the conjunctival tissue must be submitted unfixed for analysis.[2] [3] If involvement is diffuse, biopsy of the inferior conjunctival fornix is recommended.[3] Judicious biopsy is advisable as OCP is an obliterating disease of the conjunctiva and only the minimal amount of tissue necessary should be removed. Alternatively, biopsy of an active oral mucosa lesion can be diagnostic as well.[3]
Immunofluorescence reveals linear staining of the epithelial basement membrane zone.[2][17] The sensitivity of immunofluorescence may be as low as 50%, especially for longstanding/severe cicatrization because of the loss of immunoreactants and the destruction of basement membrane in longstanding disease.[2] [3]
Serological testing is not routinely used in diagnosis. Sequential photographs are useful to monitor clinical progression.[12]
Differential Diagnosis
The differential diagnosis of OCP is broad as it encompasses the differential for cicatricial conjunctivitis. The differential includes infectious etiologies such as trachoma, inflammatory etiologies such as rosacea, autoimmune etiologies such as linear IgA disease, Graft Versus Host Disease (GVHD), and Stevens Johnson Syndrome (SJS), allergic etiologies such as atopy, conjunctival trauma, chemical burns, medicamentosa, radiation, and neoplasia.[3]
A common confounder to clinical diagnosis is medicamentosa, which results in a condition called pseudopemphigoid. Pseudopemphigoid is clinically identical to OCP but is caused by the long-term use of certain offending topical medications. Conjunctival biopsies may show linear staining of the conjunctival basement membrane zone.[3] Differentiation from OCP is difficult. Resolution with discontinuation of the offending agent is diagnostic.[3] Several topical medications have been implicated, including pilocarpine, epinephrine, timolol, idoxuridine, echothiophate iodide, and demecarium bromide.[3]
Treatment
Without treatment, the disease progresses in up to 75% of patients.[10] While systemic treatment stops progression of cicatrization in most patients, it fails in approximately 10% of them.[10] Systemic therapy is necessary in OCP as ocular involvement comprises a high risk subset of MMP and is insufficiently treated with topical therapy alone. Systemic treatment is best managed by a physician trained in the management of anti-inflammatory and immunomodulatory treatment given the significant risk of systemic complications necessitating frequent blood test monitoring.[12][17] Several drugs are effective in treating OCP and a step-wise approach of escalation of therapy when there is insufficient response is recommended.[22]
Topical therapy can be used as an adjunct for surface disease but should not be used in place of systemic therapy. Topical therapy includes optimizing lubrication of the ocular surface with artificial tears and punctual plugging.[22] Topical and subconjunctival steroids can relieve symptoms but are ineffective for treatment of the underlying disease.[22] Topical cyclosporine has been found to be ineffective while topical tacrolimus has been shown to be successful in small case series.[12][17][22] Subconjunctival mitomycin-c has also been investigated in small case series with variable effect.[22]
If the disease remains quiescent following a few years of systemic therapy, many practitioners are often able to discontinue systemic therapy successfully.[22] However, it is important to continue to monitor the patient for recurrence of disease as up to 22% of patients relapse.[22]
Mild Disease
Dapsone is an effective and commonly used anti-inflammatory treatment in OCP for mild disease and in the absence of rapid progression.[12][17][22][23] Dapsone is started at a dose of 50 mg/day and slowly increased as tolerated by up to 25mg every 7 days to an effective dose, which is usually between 100-200mg/day.[17] If significant improvement is not achieved within 3 months, escalation of therapy is recommended such as to azathioprine or methotrexate.[22]
Systemic complications of dapsone include hemolysis and methemoglobinemia.[17] G6PD (glucose-6-phosphate dehydrogenase) deficiency is a contraindication to dapsone therapy as dapsone can precipitate a hemolytic crisis.[12][17] All patients should be screened for G6PD deficiency before initiation of therapy with dapsone.[17]
Sulphapyridine is also an oral antibiotic and is a well-tolerated alternative in patients with mild disease who are unable to take dapsone.[24] Sulphapyridine’s efficacy (effective in approximately 50% of patients) is lower than dapsone however.[24]
Moderate to Severe Disease
Corticosteroids have a rapid effect and are useful during the acute phase of severe or rapidly progressive disease.[17][22] Adjuvant corticosteroid-sparing immunomodulatory therapy should be initiated simultaneously as it may take weeks to become therapeutic.[17][22] This will allow a quicker taper from steroids and the shortest course of steroid therapy necessary given the significant systemic side effects of long-term steroid therapy.[17][22] Generally, once quiescence is achieved, steroids are tapered slowly.[22] Screening for tuberculosis (TB) is recommended prior to the initiation of steroid therapy.[17]
Azathioprine has been shown to be an effective steroid sparing therapy.[12][22] It takes 8-12 weeks of treatment to achieve maximal effect and thus should be used initially concurrently with steroids.[17] Screening for thiopurine methyltransferase (TPMT) deficiency is recommended prior to initiation of azathioprine as TPMT-deficient patients are at higher risk of developing mvelosuppression.[17] Systemic complications include leukopenia, pancytopenia, infection, malignancy, and drug-induced hypersensitivity syndrome.[22]
Methotrexate has been shown to be an effective monotherapy for OCP with fewer adverse effects when compared to azathioprine, cyclophosphamide, and dapsone.[17][22] The Systemic Immunosuppressive Therapy for Eye Diseases (SITE) trial found that cyclophosphamide was effective in controlling inflammation in 70.7% of patients with OCP at 1 year, with 66.9% patients on less than or equal to 10mg of prednisone.[25] Low dose methotrexate is particularly effective in mild to moderate OCP.[17] Systemic complications include hepatotoxicity, nephrotoxicity, pneumonitis, pulmonary fibrosis, pancytopenia, and malignancy.[22]
Tetracyclines are a well-tolerated anti-inflammatory agent and have been found to be effective for mild to moderate OCP, particularly when combined with nicotinamide.[17]
Mycophenolate mofetil has been shown to be a well tolerated and effective therapy for OCP.[22] Therapeutic dosage is usually 1000-2000mg/day.[22] Systemic complications include leukopenia.[22]
Cyclosporine has only been used in small series of patients and has been reported to have variable levels of effectiveness.[17]
Severe Disease
Cyclophosphamide is first line in patients with severe disease or rapid progression.[12][17][22][26] It should be started in conjunction with steroids and can be dosed orally or IV.[12][17][22][26] A short course of pulsed IV therapy (ex. 3 days) can be particularly effective in achieving rapid control if needed, such as prior to surgery.[22] The SITE trial found that cyclophosphamide was effective in controlling inflammation in 80.8% of patients with OCP at 1 year, with 58.5% of patients on less than or equal to 10mg of prednisone.[27] Systemic complications include myelosuppression, carcinogenesis, and teratogenicity.[22][27]
Intravenous Immunoglobulin (IVIG) is reserved for patients with progressive disease that is unresponsive to systemic steroids and cyclophosphamide and has been found to be an effective therapy.[22][28] Dosing is every 3-4 weeks until quiescence is achieved, usually requiring 4-12 cycles.[22] Systemic complications are severe, and include anaphylaxis, disseminated intravascular coagulation (DIC), aseptic meningitis, and acute renal failure.[22] Therefore, IVIG is which is reserved for refractory disease.
Biologics, including the anti-TNF agents Etanercept and infliximab, the IL-2 antagonist daclizumab, and the anti-CD20 antibody rituximab have been shown to be efficacious in small studies of patients with refractory OCP.[17][22] The combination of IVIG and rituximab has been shown to be effective as well in refractory OCP.[17]
Complications
Seemingly trivial surgical intervention and conjunctival trauma can lead to serious exacerbation of disease.[3] Surgical intervention, such treatment of trichiasis, entropion and cataract should be deferred if possible until control of active disease is achieved.[12][17][22] In some situations this may not be possible and a multi-disciplinary approach is best.[12][17]
Inferior eyelid retractor plication for trichiasis avoids surgery on the conjunctiva and has been shown to be safe and effective when undertaken in the setting clinically quiescent OCP.[29] Cryotherapy for the treatment of trichiasis has also been shown to be safe and moderately effective when undertaken in the setting clinically quiescent OCP.[30] In a case series of patients with well controlled OCP undergoing entropion repair, successful repair was performed in all patients regardless of type of surgery.[31]
Safe and successful performance of cataract surgery has been shown in several case series of patients with well-controlled OCP.[32] [33] A clear corneal incision is recommended to reduce the risk of exacerbation.[32][33]
Glaucoma is also a possible complication of OCP and is particularly difficult to diagnose and treat. IOP measurements are unreliable, and examination and ancillary testing are limited by ocular surface disease. A case series of 61 patients with severe OCP found that 21% of patients also had glaucoma and an additional 9% developed glaucoma over the course of the follow-up.[34]
Persistent epithelial defect may need amniotic membrane graft or ProkeraⓇ.
OCP has been described in patients with other concurrent rheumatologic illnesses including rheumatoid arthritis, lupus, and HLA-B27 spondyloarthropathies.[35]
Future directions
Membrane array testing of specific tear proteins testing may be a potential method of monitoring response to therapy.[1][16] Using a membrane array kit purchased from RayBiotech Inc, Chan et al tested 43 angiogenic modulators and found elevated levels of IL-8 and MMP-9 in the tears of patients with OCP, and these levels decreased with systemic immune therapy.[16]Arafat et al proposed MPO levels in tears as a sensitive and specific quantitative marker of disease activity.[1]
Cultivated oral mucosal epithelial transplantation has been shown to be effective in the treatment of ocular surface disease secondary to limbal stem cell deficiency (including OCP) and is a promising avenue of treatment.[36]
A keratoprosthesis or osteo-odonto-keratoprosthesis can be used for visual rehabilitation in severe end-stage disease and is an active area of ongoing research.[22] The Boston keratoprosthesis type I has been found to have less favorable clinical outcomes when compared to implantation of the Boston keratoprosthesis type II in OCP.[37]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Arafat SN, Suelves AM, Spurr-Michaud S, et al. Neutrophil Collagenase, Gelatinase and Myeloperoxidase in Tears of Stevens-Johnson Syndrome and Ocular Cicatricial Pemphigoid Patients. Ophthalmology. 2014;121(1):79-87.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Ophthalmic Pathology and Intraocular Tumors. Basic and Clinical Science Course (BCSC). American Academy of Ophthalmology, 2014; pp54-56.
- ↑ Jump up to: 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 3.25 3.26 3.27 3.28 3.29 3.30 3.31 3.32 3.33 3.34 External Disease and Cornea. Basic and Clinical Science Course (BCSC). American Academy of Ophthalmology, 2014; pp344-345.
- ↑ Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR. Ocular cicatricial pemphigoid antigen: Partial sequence and biochemical characterization. Proc. Natl. Acad. Sci. 1996;93(25):14714-14719.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Zakka LR, Reche P, Ahmed AR. Role of MHC Class II Genes in the pathogenesis of pemphigoid. Autoimmunity Reviews. 2011;(11):40–47.
- ↑ Jump up to: 6.0 6.1 6.2 Bhol KC, Dans MJ, Simmons RK, et al. The autoantibodies to alpha 6 beta 4 integrin of patients affected by ocular cicatricial pemphigoid recognize predominantly epitopes within the large cytoplasmic domain of human beta 4. J Immunol. 2000 Sep 1;165(5):2824-9.
- ↑ Jump up to: 7.0 7.1 7.2 Kumari S, Bhol KC, Simmons RK, et al. Identification of ocular cicatricial pemphigoid antibody binding site(s) in human beta4 integrin. Invest Ophthalmol Vis Sci. 2001 Feb;42(2):379-85.
- ↑ Jump up to: 8.0 8.1 8.2 Ahmed AR, Foster S, Zaltas M, et al. Association of DQw7 (DQB1*0301) with ocular cicatricial pemphigoid. Proc. Natl. Acad. Sci. USA. 1991;88(24):11579-11582.
- ↑ Jump up to: 9.0 9.1 9.2 Yunis JJ, Mobini N, Yunis EJ, et al. Common major histocompatibility complex class II markers in clinical variants of cicatricial pemphigoid. Proc. Natl. Acad. Sci. USA. 1994;91:7747-7751.
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 10.5 Heiligenhaus A, Schaller J, Mauss S, et al. Eosinophil granule proteins expressed in ocular cicatricial pemphigoid. Br J Ophthalmol. 1998;82(3):312-317.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Saw VPJ, Offiah I, Dart RJ, et al. Conjunctival Interleukin-13 Expression in Mucous Membrane Pemphigoid and Functional Effects of Interleukin-13 on Conjunctival Fibroblasts in Vitro. Am J Pathol. 2009;175 (6):2406-2415.
- ↑ Jump up to: 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 DaCosta J. Ocular cicatricial pemphigoid masquerading as chronic conjunctivitis: a case report. Clinical Ophthalmology. 2012;6:2093-2095.
- ↑ Jump up to: 13.0 13.1 Lambiase A, Micera A, Mantelli F, et al. T-helper 17 lymphocytes in ocular cicatricial pemphigoid. Molecular Vision. 2009;15:1449-1455.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 14.5 Saw VPJ, Schmidt E, Offiah I, et al. Profibrotic Phenotype of Conjunctival Fibroblasts from Mucous Membrane Pemphigoid. Am J Pathol. 2011;178(1):187-197.
- ↑ Creuzot-Garcher C, H-Xuan T, Bron AM, et al. Blood group related antigens in ocular cicatricial pemphigoid. Br J Ophthalmol. 2004;88:1247–1251.
- ↑ Jump up to: 16.0 16.1 16.2 Chan MF, Sack R, Quigley DA, et al. Membrane Array Analysis of Tear Proteins in Ocular Cicatricial Pemphigoid. Optometry and vision science : official publication of the American Academy of Optometry. 2011;88(8):1005-1009.
- ↑ Jump up to: 17.00 17.01 17.02 17.03 17.04 17.05 17.06 17.07 17.08 17.09 17.10 17.11 17.12 17.13 17.14 17.15 17.16 17.17 17.18 17.19 17.20 17.21 17.22 17.23 17.24 17.25 17.26 17.27 17.28 17.29 17.30 17.31 Xu H-H, Werth VP, Parisi E, Sollecito TP. Mucous Membrane Pemphigoid. Dental clinics of North America. 2013;57(4):611-630.
- ↑ Chan, Roxanne. (1998). Ocular Cicatricial Pemphigoid. Volume III. Retrieved from: http://www.uveitis.org/docs/dm/ocular_cicatricial_pemphigoid.pdf
- ↑ Jump up to: 19.0 19.1 Higgins GT, Allan RB, Hall R, Field EA, Kaye SB. Development of ocular disease in patients with mucous membrane pemphigoid involving the oral mucosa. Br J Ophthalmol. 2006;90(8):964-967.
- ↑ Jump up to: 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 20.12 20.13 20.14 Elder MJ, Bernauer W, Leonard J, Dart JK. Progression of disease in ocular cicatricial pemphigoid. Br J Ophthalmol. 1996;80(4):292-296.
- ↑ Jump up to: 21.0 21.1 Williams GP, Radford C, Nightingale P, Dart JKG, Rauz S. Evaluation of early and late presentation of patients with ocular mucous membrane pemphigoid to two major tertiary referral hospitals in the United Kingdom. Eye. 2011;25(9):1207-1218.
- ↑ Jump up to: 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 22.13 22.14 22.15 22.16 22.17 22.18 22.19 22.20 22.21 22.22 22.23 22.24 22.25 22.26 22.27 22.28 22.29 Neff AG, Turner M, Mutasim DF. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag. 2008;4(3):617-626.
- ↑ Fern AI, Jay JL, Young H, MacKie R. Dapsone therapy for the acute inflammatory phase of ocular pemphigoid. Br J Ophthalmol. 1992;76(6):332-335.
- ↑ Jump up to: 24.0 24.1 Elder MJ, Leonard J, Dart JK. Sulphapyridine--a new agent for the treatment of ocular cicatricial pemphigoid. Br J Ophthalmol. 1996;80(6):549-552.
- ↑ Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for Ocular Inflammatory Diseases. Ophthalmology. 2009;116(11):2188-98.e1.
- ↑ Jump up to: 26.0 26.1 Elder MJ, Lightman S, Dart JK. Role of cyclophosphamide and high dose steroid in ocular cicatricial pemphigoid. Br J Ophthalmol. 1995;79(3):264-266.
- ↑ Jump up to: 27.0 27.1 Pujari SS, Kempen JH, Newcomb CW, et al. Cyclophosphamide for ocular inflammatory diseases. Ophthalmology. 2010;117(2):356.
- ↑ Sami N, Bhol KC, Ahmed AR. Treatment of oral pemphigoid with intravenous immunoglobulin as monotherapy. Long-term follow-up: influence of treatment on antibody titres to human α6 integrin. Clin Exp Immunol. 2002;129(3):533-540.
- ↑ Elder MJ, Dart JK, Collin R. Inferior retractor plication surgery for lower lid entropion with trichiasis in ocular cicatricial pemphigoid. Br J Ophthalmol. 1995;79(11):1003-1006.
- ↑ Elder MJ, Bernauer W. Cryotherapy for trichiasis in ocular cicatricial pemphigoid. Br J Ophthalmol. 1994;78(10):769-771.
- ↑ Gibbons A, Johnson TE, Wester ST, et al. Management of Patients with Confirmed and Presumed Mucous Membrane Pemphigoid Undergoing Entropion Repair. Am J Ophthalmol. 2015;159(5):846–852.
- ↑ Jump up to: 32.0 32.1 Puranik CJ, Murthy SI, Taneja M, Sangwan VS. Outcomes of cataract surgery in ocular cicatricial pemphigoid. Ocul Immunol Inflamm. 2013;21(6):449-54.
- ↑ Jump up to: 33.0 33.1 Geerling G, Dart JK. Management and outcome of cataract surgery in ocular cicatricial pemphigoid. Graefes Arch Clin Exp Ophthalmol. 2000;238(2):112-8.
- ↑ Miserocchi E1, Baltatzis S, Roque MR, et al. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology. 2002;109(1):111-8.
- ↑ Kaushik P, Ghate K, Nourkeyhani H, et al. Pure ocular mucous membrane pemphigoid in a patient with axial spondyloarthritis (HLA-B27 positive). Rheumatology. 2013;52:2097-2099.
- ↑ Sotozono C, Inatomi T, Nakamura T, et al. Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmologica. 2014;92(6):e447-e45.
- ↑ Palioura S, Kim B, Dohlman CH, et al. The Boston Keratoprosthesis Type I in Mucous Membrane Pemphigoid. Cornea. 2013;32:956–961.