Biopsy of Intraocular Tumors and Techniques for Anterior Segment Tumors
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
The accurate diagnosis of most intraocular tumors is feasible through systematic clinical evaluation in conjunction with noninvasive ancillary tests, including ultrasound, fluorescein angiography, radiological imaging, and serology.[1][2][3] However, these non-invasive diagnostic modalities may fail to give accurate diagnosis in rare situations, such as atypical tumor presentation, intraocular metastasis with unknown primary, or uncertainty of a primary lesion versus a metastatic lesion. In these cases, a biopsy, or the surgical removal of a tissue specimen for histologic diagnosis, can be valuable.[4][5][6][7] Additionally, biopsies allow for further genetic and immunocytological analyses that can enhance the characterization of neoplasms for grading, prognosis, and treatment decisions (ex. close surveillance vs. adjuvant therapy vs. surgery).[8][9][10][11][12]
Uveal melanoma is the most common primary intraocular malignancy and is diagnosed clinically without the need for biopsy in most cases. However, biopsies are frequently performed for chromosomal analysis or gene expression profiling to determine the probability of a patient's likelihood to develop metastatic disease. Obtaining this information is important as molecular prognostication status is now included in the National Cancer Care Network guidelines for metastatic surveillance and for inclusion into adjuvant and metastatic treatment clinical trials.
Overview Of Intraocular Biopsy Techniques
While exact details vary according to lesion location, size, suspected diagnosis, and ocular media clarity, general intraocular biopsy techniques include paracentesis, fine-needle aspiration, incisional and excisional biopsies.
- PARACENTESIS: Paracentesis involves the aspiration of intraocular fluid and the accompanying cellular infiltrates if present, a technique applicable to both the anterior segment (aqueous humor tap) and posterior segment (vitreous tap) of the eye.
- FINE-NEEDLE ASPIRATION BIOPSY (FNAB): In FNAB, which is applicable to lesions in both the anterior and posterior segment of the eye, a thin 25-30-gauge needle is inserted into the area of lesion to aspirate (or apply a suction force) to obtain cells for analysis. [10][13][14][15][16] The access of deep lesions requires precise technical skills and thorough knowledge of ocular anatomy. FNAB provides cellular specimen for immunohistochemistry, flow cytometry, chromosome analysis, gene expression profiling, with diagnostic reliability of 88-95% and a false-negative rate of around 3-7%.[6][13][15][17][18][19] While it yields relatively smaller specimen for study as compared to incisional and excisional biopsies, FNAB has comparatively lower risk of complications such as infections.[12][13][15] The drawbacks of FNAB are the potential for hemorrhage, especially in lesions like cavernous hemangiomas, and risk of seeding of malignant cells along the needle tracks (although such reports in literature are rare and incidence decreases with needles of 25-gauge or less).[6][8][20][21][22][23][24][25] Additionally, FNAB is generally contraindicated in suspected cases of retinoblastoma, in which its use may lead to extraocular seeding.[26][27] It should also be avoided in situations where one cannot precisely guide the needle because of limited visibility, such as in retinal detachment or hemorrhage.[18] [28][29][30] Other rare complications of FNAB are listed in Table 1. Finally, FNAB requires that a skilled cytopathologist be readily available to handle and prepare the sample for study immediately after aspiration. See below for specific discussion of FNAB application in intraocular lesions of the anterior segment.
- SURGICAL INCISIONAL BIOPSY: This biopsy technique removes a part of the lesion of interest, providing tissue specimen for diagnostic study rather than mere cells offered by FNAB. Additionally, tissue specimen are fixed and therefore incisional biopsies do not require a cytopathologist to be immediately available. Surgical incisional biopsy is frequently applied to anterior segment lesions but has limited role in posterior segment tumors, and is reserved to few choroidal and ciliary body lesions in the posterior segment.
- SURGICAL EXCISIONAL BIOPSY: Typically, surgical excision, or the complete removal, of an intraocular lesion is carried out for therapeutic purposes, after clinical evaluation or FNAB has already established the definitive diagnosis. In such cases, histopathologic analysis of the resected specimen remains helpful in establishing prognosis and deciding follow-up treatment. Yet there are instances where postoperative histological diagnosis is made following resection of an unknown lesion.[31][32] Because of technical challenges, the size of the lesion is an important factor for excision, and excisional biopsy is particularly favored with smaller lesions, especially in the anterior segment of the eye (ex. iridectomy, iridocyclectomy). For posterior segment lesions, two approaches to excisional biopsy are described in literature (trans-scleral and trans-vitreal).[33]
| Advantages | Disadvantages | Complications | Contraindications |
|---|---|---|---|
| 88-95% safety and reliability
Decreased risk of local tumor spread relative to other biopsy techniques Smaller surface wound compared to incisional or excisional biopsy |
Yields comparatively smaller specimen, which may not be adequate for diagnosis
Not always reliable (histopathologic diagnosis) in lesions <2mm thick Requires immediate availability of cytopathologist (unless sending for gene expression profiling of chromosome analysis) Technically challenging in inexperienced hands False-negative rate 5-7%; equivocal results should be followed up with another biopsy modality |
Transient vitreous hemorrhage
Subretinal hemorrhage Retinal detachment Rare tumor seeding along needle track |
In children, due to likelihood of retinoblastoma
In situations of decreased visibility of intraocular anatomy |
Aqueous Tap
Aqueous humour paracentesis, or aqueous tap, is sometimes used to investigate infectious versus autoimmune causes of anterior uveitis. However, there are a number of studies in literature which report the utility of aqueous taps (paired with cytology or flow cytometry analyses) in identifying neoplastic causes of anterior uveitis, which can present as chronic anterior chamber cell infiltration refractory to steroids or anti-infectious therapies (eg. juvenile xanthogranuloma, leukemia, or lymphoma).[34][35][36] A cautionary point is that retinoblastoma can sometimes present as acute anterior uveitis and, as with other forms of intraocular biopsy, aqueous tap is contraindicated in cases of suspected retinoblastoma due to risk of extraocular seeding.[8][37][38]
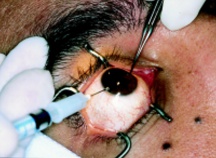
General Technique: Periocular skin is cleaned with povidone-iodine and local anesthesia is applied to the eye (proparacaine 0.5%) with or without sedation. The eyelids are retracted with a speculum and the eye is stabilized with fine tooth forceps (Figure 2). Visualization is achieved with either indirect ophthalmoscopy or a slit-lamp. A 25-30-gauge needle is inserted through clear cornea, over the iris stroma with care to avoid the lens. Aspiration of aqueous (0.1-0.3mL) into a 3mL syringe follows. BSS may be used to reform the chamber. The needle is then withdrawn while placing a sterile cotton-tipped applicator at point of entry. Stable pressure is applied at site of entry for 10-20 seconds. Subsequent studies of specimen follows and may include cytospin, PCR, and/or flow cytometry.[33][35]
There are isolated rare reports of hyphema, corneal tract abscess, endophthalmitis, allergic reaction, lenticular injury and subsequent cataract associated with aqueous taps.[39][40][41][42][43] However, it is a generally safe outpatient diagnostic procedure following adequate aseptic precautions and skilled technique.[37][38][39][40][41][42][43]
FNAB
This section will focus on FNAB for uveal melanoma. These biopsies are tyrpically perfomed in one of three methods: transscleral, transvitreal, and transcorneal. Tumors in the ciliary body or anterior choroid can be biopsied transclerally. A short 25- or 27-gauge needle (30-gauge needles have also been reported) attached to tubing and a syringe is needed. Some surgeons create a scleral flap while others directly penetrate full thickness sclera. The tumor is penetrated with the needle and aspiration applied. One study from the Ocular Oncology Study Consortium in 2020 reported that the transcleral approach was the most common technique for 55% of posterior tumors, 62% of intermediate tumors, and 86% of anterior tumors. .[37][38] For the transvitreal approach, a needle, forceps, or vitreous cutter is passed through the pars plana (3.5 to 4mm posterior to the limbus), through the vitreous, then through the retina directly into the tumor. This approach is performed either using an indirect ophthalmoscope which requires practice as the image of the moving needle is opposite the movement or using a microscope assisted wide-angle viewing lens system. The needle can either be rotated along its long axis or moved in and out of the tumor minimally so as to improve the cellular yield of the aspirate. Suction is applied and without letting go of the plunger, the needle is withdrawn. Cryotherapy in a double freeze thaw fashion can be applied to the entry site. Localized subretinal and/or vitreous hemorrhage is inevitable and is controlled by applying pressure to the globe. If the globe softens, balanced salt solution can be injected into the vitreous cavity.
Surgeons with vitreoretinal surgery (VRS) training are more likely to perform transvitreal biopsies (41% transvitreal with VRS training, 27% without. Furthermore, when performing transvitreal biopsies, ocular oncologists with VRS training with VRS training were more likely to use wide-angle viewing systems instead of indirect ophthalmoscopy.[37][38]
Surface contact vacuum technique: With the patient at the slit-lamp, a 26-30 gauge needle connected directly to a 1-mL tuberculin syringe is introduced into the anterior chamber (bevel down) at a 45o to 90o angle to the lesion (no tubing). The needle is swept over the surface of lesion in vacuum cleaner fashion while applying gentle aspiration to obtain 0.5mL of fluid. The needle is then withdrawn from anterior chamber and the aspirate is immediately diluted with ethyl alcohol and prepared for analysis. In this study by Grossniklaus, there is no discussion of efficacy of cellular yield per attempt.[44]
Surgical Incisional Biopsy

Incisional biopsy offers tissue samples (rather than mere cytology samples offered by FNAB). Aside from larger specimen for study, the other advantage over FNAB is that there is no need for a cytopathologist to be immediately available in the operating theater as the tissues obtained can be fixed for subsequent study. Several techniques have been described for obtaining tissue specimen of anterior segment lesions for cyto-histopathologic analysis. Of course, this involves skilled surgical technique as well as use of micro-surgical tools that are small enough to allow intraocular access without creating large defects, but big enough to incise and gather the tissue sample. Technology has evolved to enable this, with various designs in microsurgical technology that afford eye surgeons an array of tools from which to choose. Examples include:
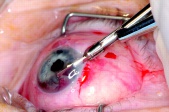

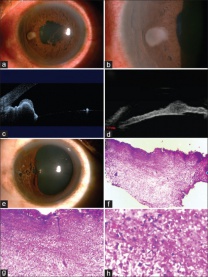
Punch biopsy: Pe’re et al describe a novel technique using a trabeculectomy punch (Kelly Descemet’s membrane punch; Figures 4 & 5) performed on two patients.[45] The punch is inserted through clear cornea incision made with a 3.2 mm keratome into a viscoelastic-filled chamber, where a 0.75mm-deep bite of the lesion is obtained. The punch can traverse a maximum depth of 7mm beneath cornea to reach the lesion (Figure 6). The specimen obtained is then fixed and prepared for immunostaining and the incision is self-sealing. In both cases, this technique yielded sufficient sample for definitive diagnosis and was relatively inexpensive. Both patients experienced self-limiting hyphema and one patient experienced transient postoperative IOP increase; however, authors concluded that the technique is safe and may be useful in situations in which FNAB is not diagnostic.[45]In terms of cost, it is superior to the aspiration cutter technique (see below) which requires vitrectomy equipment. The drawback, as compared to aspiration cutter, is the difficulty of achieving adequate punch with smaller lesions.
Trans-corneal fine-canula aspiration: Matthews et al describe a novel surgical technique using a minimally invasive aspiration cannula on 12 patients under topical anesthesia.[46] A straight 15o knife is used to create a clear corneal paracentesis in a site opposite to the iris lesion. Viscoelastic is then introduced. A 25-gauge Rycroft cannula, attached to a 2mL syringe is then inserted into the anterior chamber. Negative pressure created with the syringe is used to collect tissue as the cannula is passed repeatedly over and into the lesion to create a “phaco-like” groove within the lesion. Tissue specimen are obtained, which range from 1mm-1.5mm, and are transferred into a fixative and prepared. The corneal incision is self-sealing. In the study of 12 patients, 100% of the samples were adequate upon which definitive diagnoses mwere made. The only complication observed was minimal hemorrhage in the anterior chamber.[46]
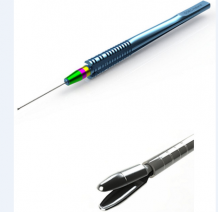
Essen Biopsy Forceps: Chronopoulos et al describes a minimally-invasive technique using the specially-designed Essen-23G biopsy forceps (Figure 7) performed on 7 patients with pigmented iris tumors.[47] The technique requires two corneal incisions in most cases: one for the forceps and a second for use of a 23G scissors for optimal efficacy. Under local anesthesia, a 1mm clear corneal incision is made and the anterior chamber is filled with viscoelastic. The shaft of the forceps is introduced into the anterior chamber and the lesion tissue is grasped and dissected into the cavity of the forceps. Bimanual manipulation with intraocular scissors through a second paracentesis was used for controlled cutting of the tissue when necessary. The forceps cavity (with specimen inside) is then closed and removed from the anterior chamber for subsequent fixation. The anterior chamber is rinsed with BSS and incisions are self-sealing. Tissue obtained measured approximately 1x2mm. Sufficient specimen upon which definitive diagnoses were made was achieved in 6/7 patients (85%). Mild complications reported include temporary punctual bleed (3/7) and postoperative mydriasis treated with pilocarpine (1/7). There were no observations of postoperative ocular hypertension.[47]
Aspiration cutter technique (Finger iridectomy technique): This technique, performed on 55 patients by Petousis et al, can be viewed as a combination of FNAB and incisional biopsy, as it allows for the acquisition of both cells and tissue from iris lesions.[48] Local anesthesia, sedation, and retrobulbar and facial nerve blocks are employed. With an eyelid speculum in place, a 1mm stab-incision through clear juxtalimbal cornea is created with a microvitreoretinal blade on same side of tumor. The anterior chamber is then stabilized with sodium hyaluronate 1%, and a 25-gauge aspiration biopsy cannula (vitrectomy probe) is inserted into the anterior chamber to occlude the tumor with an aspiration port. Aspiration cutting is achieved with suction power (300mmHg) at about 600 cuts/minute. The aspiration cutter is removed and the technique is repeated 2-3 times for adequate retrieval of specimen. Most corneal entry incisions were self-sealing while few required suture. Definitive diagnosis of specimen obtained from this technique was possible in 98.2% of cases. 9.6% of patients experienced transient increase in IOP likely related to retained viscoelastic, 15.3% had postoperative red blood cells in the anterior chamber, and 1.9% developed hyphema. Because this technique is a hybrid of both FNAB and incisional biopsy, it reduces the incidence of inadequate sampling observed in FNAB, yet minimizes the defect that would otherwise be caused by incisional or punch biopsies. The drawback is the cost of equipment.[48]
Surgical Excisional Biopsy
Generally, the indications for excisional removal is where there is threat to sight such as rapid tumor growth, encroachment of tumor over pupillary margin or trabecular meshwork, and for globe-salvage treatment and prognostication in patients with malignant histology as established via FNAB specimen (Ex. uveal melanoma, ciliary body tumors). Resection is classified based on the tissue involved (ex iridectomy, cyclectomy) and repair of resulting defect after resection may be necessary. Below are several approaches to excisional biopsy in the anterior segment.
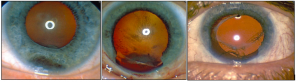
Block excision: This technique, otherwise known as full-thickness eyewall resection (exoresection, partial lamellar sclerouvectomy) is commonly employed in the removal of epithelial tumors in the anterior chamber under general hypotensive anesthesia. It has also been employed in the removal of progressive ciliary body and iris tumors involving the angle, with one study claiming reduced risk of intrascleral tumor recurrence as compared to iridocyclectomy (see below).[49]It involves the removal of all layers of the sclera and cornea in contact with the lesion, as well as removal of adjacent iris and ciliary body in one mass, or en bloc. Anterior vitrectomy follows, and the resulting defect is covered with corneoscleral graft or tectonic corneal graft.[49][50][51] Reports in literature cite intraoperative vitreous hemorrhage and postoperative elevated IOP, cataract, retinal detachment, and decreased visual acuity as common complications of this technique (Figure 8).[49][50][51][52] Recurrence may also occur, necessitating enucleation.[52]
Iridectomy: Performed under local anesthesia, iridectomy is often the first-line treatment for small peripheral iris melanomas that do not involve the angle (Figure 9).[53]It is contraindicated in cases of diffuse and massive tumors. A corneoscleral incision is made and the iris is incised radially with 2mm clearance of the tumor margins, and the sample is removed. The iris may be sutured and the corneoscleral wound is closed.[53] The most common bothersome complication is iris coloboma (especially if the iris is not sutured) which causes photophobia and cosmetic defect, but can be alleviated with a cosmetic contact lens or artificial iris implant.[54][55] Other reported complications from iridectomy include cataract development.[56]Tumor recurrence can occur if the tumor is diffuse, hyphema, and rarely, episcleral seeding of the tumor.[53][54][55]
Iridocyclectomy: Performed under general anesthesia with mild to no systemic hypotension, this technique is indicated for tumors of ciliary body and iris melanomas involving the angle.[53]The conventional approach, which utilizes preoperative mydriasis, is to begin the tumor resection with dissection at the pupil margin going toward the iris root—an antero-posterior dissection with careful conservation of the zonules.[53] A posterior-anterior approach with preoperative miosis has also been described (“cyclo-iridectomy”); authors argue this technique stretches the iris, making dissection easier, and thereby reducing the size of iris coloboma and maximizing conservation of the iris sphincter.[57] However, no randomized control trials have determined the superiority of either approach. While fine details of a conventional iridocyclectomy vary based on tumor location and surgeon preference, the basic steps are to prepare a conjunctival periotomy and subsequent superficial lamellar scleral flap, make deep scleral incisions with blunt scissors, proceed with anteroposterior dissection, and excise the tumor in circumferential fashion.[53][55][57][58]Generally, if tumor circumferential spread exceeds 3 clock hours, iridocyclectomy is contraindicated.[53]In addition to the above complications of iridectomy, iridocyclectomy can also lead to hypotony, lens subluxation, keratopathy, vitreous loss, and high astigmatism.[53][55][57][59] Incomplete excision and tumor recurrence have also been reported, in which cases some argue a full-thickness eyewall resection may have been better; in cases of recurrence post-iridocyclectomy, further globe-salvage is often not possible, necessitating enucleation.[51][60][61]
References
- ↑ Uduma FU, Titalom K. Intra-orbital malignant melanoma: role of MR imaging (a case report and literature review). Global journal of health science. 2011;4(1):253-258.
- ↑ Conway RM, Chew T, Golchet P, Desai K, Lin S, O’Brien J. Ultrasound biomicroscopy: role in diagnosis and management in 130 consecutive patients evaluated for anterior segment tumours. British Journal of Ophthalmology. 2005;89(8):950-955.
- ↑ Accuracy of diagnosis of choroidal melanomas in the Collaborative Ocular Melanoma Study.COMS report no. 1. Archives of ophthalmology (Chicago, Ill : 1960). 1990;108(9):1268-1273.
- ↑ YeaneyGA, Platt S, Singh AD. Primary iris leiomyoma. Survey of Ophthalmology. 2017;62(3):366-370.
- ↑ Johnston RL, Tufail A, Lightman S, et al. Retinal and choroidal biopsies are helpful in unclear uveitis of suspected infectious or malignant origin. Ophthalmology. 2004;111(3):522-528.
- ↑ 6.0 6.1 6.2 Shields JA, Shields CL, Ehya H, Eagle RCJ, De Potter P. Fine-Needle Aspiration Biopsy of Suspected Intraocular Tumors. International Ophthalmology Clinics. 1993;33(3):77-82.
- ↑ Seregard S, All-Ericsson C, Hjelmqvist L, Berglin L, Kvanta A. Diagnostic incisional biopsies in clinically indeterminate choroidal tumours. Eye (London, England). 2013;27(2):115-118.
- ↑ 8.0 8.1 8.2 Foulds WS. The uses and limitations of intraocular biopsy. Eye (London, England). 1992;6 ( Pt 1):11-27.
- ↑ Sisley K, Nichols C, Parsons MA, Farr R, Rees RC, Rennie IG. Clinical applications of chromosome analysis, from fine needle aspiration biopsies, of posterior uveal melanomas. Eye (London, England). 1998;12 ( Pt 2):203-207.
- ↑ 10.0 10.1 Midena E, Bonaldi L, Parrozzani R, Radin PP, Boccassini B, Vujosevic S. In vivo monosomy 3 detection of posterior uveal melanoma: 3-year follow-up. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246(4):609-614.
- ↑ Damato B, Duke C, Coupland SE, et al. Cytogenetics of uveal melanoma: a 7-year clinical experience. Ophthalmology. 2007;114(10):1925-1931.
- ↑ 12.0 12.1 Faulkner-Jones BE, Foster WJ, Harbour JW, Smith ME, Davila RM. Fine needle aspiration biopsy with adjunct immunohistochemistry in intraocular tumor management. Acta cytologica. 2005;49(3):297-308.
- ↑ 13.0 13.1 13.2 Char DH, Miller TR, Ljung BM, Howes EL, Jr., Stoloff A. Fine needle aspiration biopsy in uveal melanoma. Acta cytologica. 1989;33(5):599-605.
- ↑ Seregard S, All-Ericsson C, Hjelmqvist L, Berglin L, Kvanta A. Diagnostic incisional biopsies in clinically indeterminate choroidal tumours. Eye (London, England). 2013;27(2):115-118.
- ↑ 15.0 15.1 15.2 Eide N, Syrdalen P, Walaas L, Hagmar B. Fine needle aspiration biopsy in selecting treatment for inconclusive intraocular disease. Acta Ophthalmologica Scandinavica. 1999;77(4):448-452.
- ↑ Shields CL, Materin MA, Teixeira L, Mashayekhi A, Ganguly A, Shields JA. Small Choroidal Melanoma with Chromosome 3 Monosomy on Fine-Needle Aspiration Biopsy. Ophthalmology. 2007;114(10):1919-1924.
- ↑ Char DH, Miller T. Accuracy of presumed uveal melanoma diagnosis before alternative therapy. British Journal of Ophthalmology. 1995;79(7):692-696.
- ↑ 18.0 18.1 Cohen VML, Dinakaran S, Parsons MA, Rennie IG. Transvitreal fine needle aspiration biopsy: The influence of intraocular lesion size on diagnostic biopsy result. Eye. 2001;15(2):143-147.
- ↑ Jensen OA, Prause JU, Scherfig E. Transvitreal retino-choroidal biopsy of suspected malignant lesions of the choroid. Acta Ophthalmologica Scandinavica. 1997;75(4):409-411.
- ↑ Augsburger JJ, Shields JA, Folberg R. Fine needle aspiration biopsy in the diagnosis of intraocular cancer Cytologic-histologic correlations. Ophthalmology. 1985;92(1):39–49.
- ↑ Glasgow BJ, Brown HH, Zargoza AM, Foos RY. Quantitation of tumor seeding from fine needle aspiration of ocular melanomas. Am J Ophthalmol. 1988;105(5):538-546.
- ↑ Shields CL, Ganguly A, Bianciotto CG, Turaka K, Tavallali A, Shields JA. Prognosis of Uveal Melanoma in 500 Cases Using Genetic Testing of Fine-Needle Aspiration Biopsy Specimens. Ophthalmology. 2011;118(2):396-401.
- ↑ McCannel TA, Chang MY, Burgess BL. Multi-Year Follow-up of Fine-Needle Aspiration Biopsy in Choroidal Melanoma. Ophthalmology. 2012;119(3):606-610.
- ↑ Schefler AC, Gologorsky D, Marr BP, Shields CL, Zeolite I, Abramson DH. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmology. 2013;131(9):1220-1224.
- ↑ Sellam A, Desjardins L, Barnhill R, et al. Fine Needle Aspiration Biopsy in Uveal Melanoma: Technique, Complications, and Outcomes. American Journal of Ophthalmology. 2016;162:28-34.e21.
- ↑ Karcioglu ZA. Fine needle aspiration biopsy (FNAB) for retinoblastoma. Retina. 2002;22(6):707-710.
- ↑ Stevenson KE, Hungerford J, Garner A. Local extraocular extension of retinoblastoma following intraocular surgery. British Journal of Ophthalmology. 1989;73(9):739-742.
- ↑ Eide N, Walaas L. Fine-needle aspiration biopsy and other biopsies in suspected intraocular malignant disease: a review. Acta Ophthalmol. 2009;87(6):588-601.
- ↑ Augsburger JJ, Corrêa ZM, Schneider S, et al. Diagnostic transvitreal fine-needle aspiration biopsy of small melanocytic choroidal tumors in nevus versus melanoma category. Trans Am Ophthalmol Soc. 2002;100:225-232; discussion 232-224.
- ↑ Shields CL, Manquez ME, Ehya H, Mashayekhi A, Danzig CJ, Shields JA. Fine-needle aspiration biopsy of iris tumors in 100 consecutive cases: technique and complications. Ophthalmology. 2006;113(11):2080-2086.
- ↑ Krishnan T, Gopal L, Biswas J, Padmanabhan P, Khetan V. Eye wall resections for intraocular tumors: our experience. Indian journal of ophthalmology. 2014;62(4):517-520.
- ↑ Ramasubramanian A, Shields CL, Kytasty C, Mahmood Z, Shah SU, Shields JA. Resection of intraocular tumors (partial lamellar sclerouvectomy) in the pediatric age group. Ophthalmology. 2012;119(12):2507-2513.
- ↑ 33.0 33.1 Rishi P, Dhami A, Biswas J. Biopsy techniques for intraocular tumors. Indian journal of ophthalmology. 2016;64(6):415-421.
- ↑ Casteels I, Olver J, Malone M, Taylor D. Early treatment of juvenile xanthogranuloma of the iris with subconjunctival steroids. The British Journal of Ophthalmology. 1993;77(1):57-60.
- ↑ 35.0 35.1 Finger PT, Papp C, Latkany P, Kurli M, Iacob CE. Anterior chamber paracentesis cytology (cytospin technique) for the diagnosis of intraocular lymphoma. The British journal of ophthalmology. 2006;90(6):690-692.
- ↑ Monsalvo S, Serrano C, Prieto E, et al. Unilateral uveitis masquerade syndrome caused by diffuse large B-cell lymphoma diagnosed using multiparametric flow cytometry of the aqueous humor. Cytometry Part B, Clinical cytometry. 2016.
- ↑ 37.0 37.1 37.2 37.3 Kitazawa K, Sotozono C, Koizumi N, et al. Safety of anterior chamber paracentesis using a 30-gauge needle integrated with a specially designed disposable pipette. The British journal of ophthalmology. 2017.
- ↑ 38.0 38.1 38.2 38.3 Seider M, Mruthyunjaya P. Multi-center analysis of intraocular biopsy technique and outcomes for uveal melanoma: Ocular Oncology Study Consortium report 4. Graefe's Archive for Clinical and Experimental Ophthalmology 2020;258:427-435.
- ↑ 39.0 39.1 39.2 Van der Lelij A, Rothova A. Diagnostic anterior chamber paracentesis in uveitis: a safe procedure? The British journal of ophthalmology. 1997;81(11):976-979.
- ↑ 40.0 40.1 Helbig H, Noske W, Kleineidam M, Kellner U, Foerster MH. Bacterial endophthalmitis after anterior chamber paracentesis. The British journal of ophthalmology. 1995;79(9):866.
- ↑ 41.0 41.1 Cheung CM, Durrani OM, Murray PI. The safety of anterior chamber paracentesis in patients with uveitis. The British journal of ophthalmology. 2004;88(4):582-583.
- ↑ 42.0 42.1 Trivedi D, Denniston AK, Murray PI. Safety profile of anterior chamber paracentesis performed at the slit lamp. Clinical & experimental ophthalmology. 2011;39(8):725-728.
- ↑ 43.0 43.1 Azuara-Blanco A, Katz LJ. Infectious keratitis in a paracentesis tract. Ophthalmic surgery and lasers. 1997;28(4):332-333.
- ↑ Grossniklaus HE. Fine-needle aspiration biopsy of the iris. Archives of ophthalmology (Chicago, Ill : 1960). 1992;110(7):969-976.
- ↑ 45.0 45.1 45.2 45.3 45.4 Pe'er J, Blumenthal EZ, Frenkel S. Punch biopsy of iris lesions: a novel technique for obtaining histology samples. The British journal of ophthalmology. 2007;91(5):660-662.
- ↑ 46.0 46.1 Matthews BJ, Mudhar HS, Rennie IG. Trans-corneal fine cannula aspiration: Rycroft cannula aspiration technique for sampling iris tumours. The British journal of ophthalmology. 2012;96(3):329-331.
- ↑ 47.0 47.1 47.2 Chronopoulos A, Kilic E, Joussen AM, Lipski A. Small incision iris tumour biopsy using a cavernous sampling forceps. The British journal of ophthalmology. 2014;98(11):1539-1542.
- ↑ 48.0 48.1 Petousis V, Finger PT, Milman T. Anterior segment tumor biopsy using an aspiration cutter technique: clinical experience. Am J Ophthalmol. 2011;152(5):771-775.e771.
- ↑ 49.0 49.1 49.2 Naumann GO, Rummelt V. Block excision of tumors of the anterior uvea. Report on 68 consecutive patients. Ophthalmology. 1996;103(12):2017-2027; discussion 2027-2018.
- ↑ 50.0 50.1 Forster RK. Corneoscleral block excision of postoperative anterior chamber cysts. Transactions of the American Ophthalmological Society. 1995;93:83-97; discussion 97-104.
- ↑ 51.0 51.1 51.2 Jonas JB, Groh MJ, Rummelt V, Naumann GO. Rhegmatogenous retinal detachment after block excision of epithelial implantation cysts and tumors of the anterior uvea. Ophthalmology. 1999;106(10):1942-1946.
- ↑ 52.0 52.1 Char DH, Miller T, Crawford JB. Eye-wall resection. Transactions of the American Ophthalmological Society. 2000;98:153-161.
- ↑ 53.0 53.1 53.2 53.3 53.4 53.5 53.6 53.7 Damato B, Groenewald C (2008) Surgical resection of uveal melanoma. In: Albert DM, Miller JW, Azar DT, Blodi BA (eds) Principles and practice of ophthalmology, 3rd Ed, Ch 353, pp 4911–4918. Saunders, Elsevier, Philadelphia.
- ↑ 54.0 54.1 Conway R, Chua W, Qureshi C, Billson F. Primary iris melanoma: diagnostic features and outcome of conservative surgical treatment. The British Journal of Ophthalmology. 2001;85(7):848-854. doi:10.1136/bjo.85.7.848.
- ↑ 55.0 55.1 55.2 55.3 Klauber S, Jensen PK, Prause JU, Kessing SV. Surgical treatment of iris and ciliary body melanoma: follow-up of a 25-year series of patients. Acta Ophthalmologica. 2012;90(2):122-126.
- ↑ Cardine S, Labetoulle M, Kirsch O, et al: Iris melanomas. A retrospective study of 11 patients treated by surgical excision. J Fr Ophtalmol 2003; 26: pp. 31-37
- ↑ 57.0 57.1 57.2 Rospond-Kubiak I, Damato B. The surgical approach to the management of anterior uveal melanomas. Eye. 2014;28(6):741-747.
- ↑ Schmitzer S, Butea-Simionescu C, Gheorghe A, Zemba M, Cioboata M. Iridociliary melanoma – Clinical case. Journal of Medicine and Life. 2016;9(1):88-91.
- ↑ Memmen JE, McLean IW. The Long-term Outcome of Patients Undergoing Iridocyclectomy. Ophthalmology.1990;97(4):429-432.
- ↑ Gupta M, Puri P, Rennie IG. Iris seeding following iridocyclectomy for localised iris melanoma. Eye. 2001;15(6):808-809.
- ↑ Damato BE, Paul J, Foulds WS. Risk factors for residual and recurrent uveal melanoma after trans-scleral local resection. The British Journal of Ophthalmology. 1996;80(2):102-108.

