Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy (ADNIV) is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
ICD-10
H35.2 Autosomal dominant neovascular inflammatory vitreoretinopathy (ADNIV)
Disease
ADNIV is a rare ocular inflammatory disease that develops slowly over the course of decades to cause significant and devastating blindness. It is characterized by progressive uveitis, retinal pigmentary changes and degeneration, cystoid macular edema (CME), neovascularization of the retina and iris, vitreous hemorrhage (VH) and tractional retinal detachment (TRD).[1][2] There have been three independently reported families with genetically-confirmed ADNIV.[1][3][4][5] There have also been case reports of de novo mutations causing ADNIV in children.
Etiology
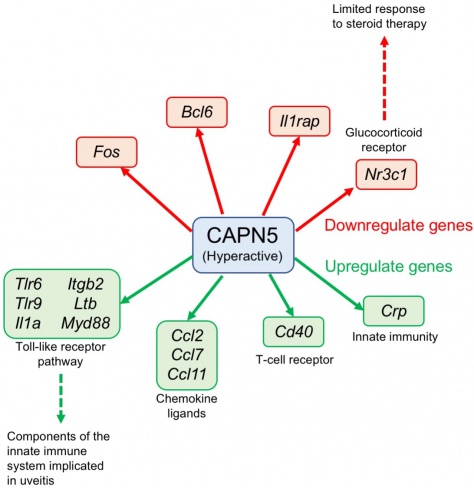
Chromosomal linkage analysis of the original family with the disease (34 of 116 members affected) revealed dysfunction of two genes localized to chromosome 11q13.[1] Analysis of the genome from a second (7 of 16 members affected) and a third family (31 of 79 members affected) confirmed localization to this locus.[4] Two different mutations to the gene encoding calpain-5 (CAPN5), an intracellular calcium-activated cysteine protease, were discovered.[4]These two missense mutations at the catalytic domain (exon 6) of the enzyme (substitution of arginine by leucine at position 243 [arg243leu] or leucine by proline at position 244 [leu244pro]) were found in all affected family members.[4]Since then, three additional mutations of the CAPN5 gene have been described, two affecting the catalytic domain and one affecting the regulatory domain.[6][7]Calpains target intracellular proteins through specific and limited proteolysis that often leads to activation and regulation rather than degradation (Figure 1).[4]
Risk Factors
All three reported families with ADNIV are of northern European ancestry.[1][3][4]There is no sex predilection.[4]Several cases of CAPN5-induced ADNIV have been reported not to be related to the original families.[6]
General Pathology
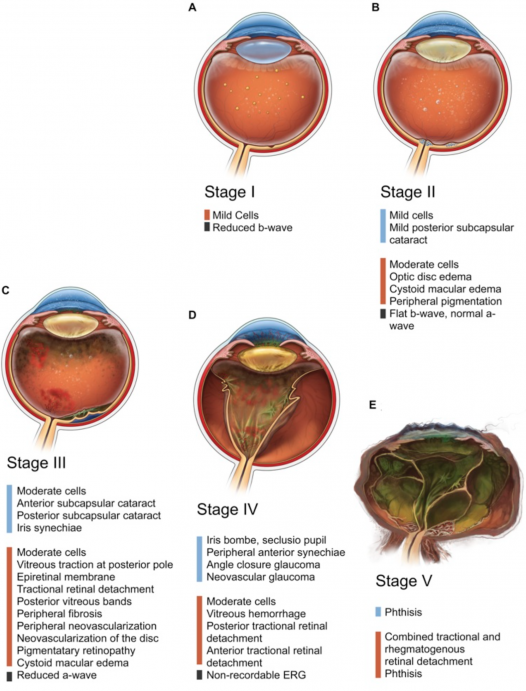
ADNIV was originally described in 1990 after a six-generation family was found to have similar clinical and pathologic findings.[1][3]Subsequent delineation has classified ADNIV into 5 distinct chronologic stages that each last approximately a decade (Figure 1). Disease onset varies between 10 to 30 years of age.[4]The various stages have specific clinical and functional findings described in Table 1. The first stage of the disease is indistinguishable from an autoimmune non-infectious uveitis and may be underdiagnosed. The disease then progresses through a retinal degenerative phase (stage 2) to neovascularization (stage 3) along with fibrotic proliferation leading to TRD and VH (stage 4). Ultimately, the eye degrades to no-light-perception and becomes phthisical (stage 5).[4]Functional analysis with electroretinography (ERG) can be helpful in distinguishing the specific stage of ADNIV.
| Stage | Clinical Findings | ERG Findings |
|---|---|---|
| 1 |
|
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
Pathophysiology
The exact pathologic mechanism for ADNIV is unclear; however, it is associated with a mutation of the CAPN5 gene. Similar to other diseases with an autosomal dominant inheritance pattern, mutations to the CAPN5 gene leads to gain-of-function of the protease.[4][8][9] This mutant protease exhibits a lower calcium threshold for activation, resulting in persistent hyperactivity of CAPN5 that leads to multiple cellular consequences such as apoptosis, migration, and intracellular signaling.[8][9][10] Analysis of abnormal gene regulation in a mouse model of ADNIV provides insight to its possible pathophysiology.[9] Specifically, within the retina, hyperactivity of CAPN5 is linked to abnormal synapse function within the outer plexiform layer.[10]Interestingly, knocking out the CAPN5 gene within mice does not produce pathology.[4]Recently a mutation of the regulatory domain was discovered which lead to a more mild clinical manifestation.[6]The specific downstream signaling pathways that are activated to generate the ADNIV phenotype is currently not well understood and will require further investigation.
Primary Prevention
There are currently no preventative measures for ADNIV.
Diagnosis
Diagnosis of ADNIV is based on ocular exam findings, family history, and functional abnormalities as measured by ERG. Confirmation is made by genetic analysis demonstrating mutation of the CAPN5 gene.[4]
History
A careful and detailed assessment of the patient’s family history is essential for diagnosis as nearly all patients will have an affected first degree relative. Patients with ADNIV characteristically present in their fourth decade or later with vision loss; however, they may have exhibited subtle signs of the earlier stages of the disease that were missed.[1] If presenting prior to their fourth decade of life, the patients are usually asymptomatic other than possible nonspecific vitreous inflammation. The youngest symptomatic patient was found to have VH from peripheral neovascularization at age 16.[1]In one cohort, 9 children less than 14 years of age were examined, and none were found to be symptomatic or have ERG changes.[1] Despite the severe intraocular disease, no systemic inflammatory conditions have been associated.[4]
Clinical Findings
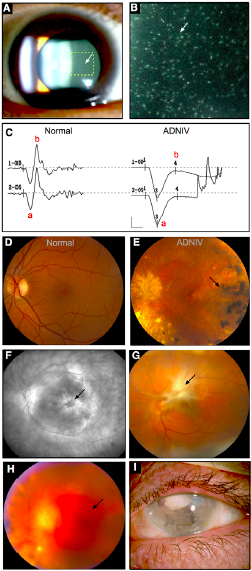
Clinical signs vary depending on the stage of the disease and the age of the patient (Table 1, Figure 2, Figure 3). The earliest manifestation of this disease is non-infectious uveitis typically in the vitreous cavity. Following this, patients may have peripheral pigmentation, increased inflammation in both anterior and posterior segments, and CME. As the disease progresses, the examiner may notice increased inflammation, subcapsular cataracts, iris synechiae, vitreous traction, epiretinal membranes, early TRDs, proliferative vitreoretinopathy, and peripheral or posterior neovascularization. Once the patient reaches the end stages of the disease, he or she can develop the following anterior segment changes: iris bombe, secluisio pupil, peripheral anterior synechiae, angle closure, and neovascular glaucoma. VH, complete TRDs, and worsening vitreous inflammation are also seen. The last stage is phthisis bulbi and total TRD.[1][3][4]
Symptoms
Patients present with vision loss most commonly from CME, cataracts, or vitreous infiltration from inflammation or hemorrhage during the early stages of ADNIV. In a study of 28 patients, visual acuity (VA) was measured to be 20/100 or better in only 43% of patients age 41-59 years old and in only 8% of patients older than 60 years.[1]
Clinical diagnosis
The diagnosis of ADNIV can be made based on history and examination alone in those with a known family history and vitreous inflammation. In patients who do not demonstrate other classic features or those who are younger than 30 years of age, ERG can be performed.
Diagnostic procedures
ERG findings, like clinical findings, vary by the stage of the disease. All of the 9 children less than age 14 had normal ERGs.[1] The first reliable ERG finding is selective loss of the scotopic b-wave, indicating dysfunction of signal transmission from photoreceptors to members of the inner retina such as bipolar cells due to localization of CAPN5 to the photoreceptor synapse.[10]As the disease progresses, there is a demonstrable reduction in scotopic a-wave, indicating progressive dysfunction of photoreceptors within the outer retina. Eventually, the ERG becomes unrecordable, and all electrophysiological function of the eye is lost.[1][3][4]
Laboratory test
Within the appropriate clinical context, laboratory testing is not a requirement for diagnosis. If the patient lacks a family history of ADNIV and presents with vitreous inflammation, a thorough evaluation for infectious and inflammatory etiologies should be performed. Typically, this would include complete blood count (CBC), elevated sedimentation rate (ESR), C-reactive protein (CRP), tuberculin skin test/IGRA, and rapid plasminogen reagent (RPR). Depending on the context, laboratory testing can be expanded to include evaluation of angiotensin-converting enzyme (ACE), sampling aqueous fluid within the anterior chamber for viral etiologies and/or interleukins, and obtaining serologies for toxoplasmosis serology.[11]If further confirmation is required, genetic testing can be completed to evaluate for known mutations to the CAPN5 gene.
Differential diagnosis
The differential diagnosis can vary based upon the stage of the disease:
- Stage 1: All infectious, inflammatory and idiopathic causes of uveitis.
- Stage 2: Retinitis pigmentosa should be considered given similarities in the appearance of the peripheral pigmentation.
- Stage 3: Etiologies leading to neovascularization and CME should be considered such as proliferative diabetic retinopathy. Additionally, a disease known as autosomal dominant vitreoretinochoroidopathy (ADVIRC) demonstrates similar features as ADNIV including inheritance pattern, peripheral retinal pigmentation, retinal neovascularization and vitritis. A key difference between the two diseases is that ADVIRC patients typically have discreet white punctate opacities in the posterior retina, exhibit a normal ERG, and have minimal progression of disease severity.[1]Familial exudate vitreoretinopathy (FEVR) can also present with peripheral neovascularization; however, they do not demonstrate vitreous cell or ERG abnormalities.[1]
Management
General treatment
Treating ADNIV can be difficult and must be tailored to the specific features that are observed. Despite our best efforts, the disease can progress relentlessly and typically results in severe ocular damage and, ultimately, phthisis bulbi.[4]
Medical therapy
Steroids are the cornerstone of treatment, though there have been no prospective studies to assess the optimal use of periocular or intraocular steroids to manage ADNIV. Unfortunately, the majority of ADNIV patients eventually become resistant to conventional steroid-based immunosuppression.[12]
The use of a fluocinolone acetonide (FA) implant (Retisert; Bausch & Lomb, Rochester, NY, USA) has been utilized with success. While it is typically implemented for stage 4 of the disease, there have been reports of successful treatment in stage 2 and 3 eyes.[13][14]A review of 9 eyes treated with the FA implant demonstrated complete resolution of inflammation, regression of neovascularization, and improvement in CME.[13] It did not prevent the decline in VA, and 3 of 7 eyes without prior history of glaucoma required surgical intervention to lower intraocular pressure (IOP).[13]The implant has not been shown to reduce fibrosis and has been associated with fibrotic encapsulation of itself which requires surgical removal (see video).[13][14]
Elevated IOP is a common late finding of ADNIV, typically secondary to steroid response, pupillary block, secondary angle closure from fibrosis, or uveitic glaucoma. Neovascular glaucoma can develop, though use of the fluocinolone implant has essentially eliminated this risk.[13]Treatment of elevated IOP is often refractory to medical therapy and eventually requires surgical intervention. A study of 5 ADNIV patients (9 eyes) with secondary glaucoma found that Ahmed glaucoma valve surgery was required in 5 eyes even after placement of a FA implant. One eye developed angle closure with iris bombe and underwent laser peripheral iridotomy.[15]The use of laser photocoagulation to treat peripheral neovascularization has not been well documented. In one cohort, three patients underwent laser treatment with mixed results in the stabilization of neovascularization.[1]Intravitreal anti-vascular endothelial growth factor agents have been employed to treat CME but showed limited effectivity.[16]
Surgery
As previously indicated, surgical insertion of a FA implant can be used to treat refractory inflammation, CME, or neovascularization. When patients develop TRDs, pars plana vitrectomy (PPV) and retinal detachment repair can be undertaken. Of the first 28 patients reported, 6 patients (7 eyes) underwent PPV for repair of TRD with 5 eyes demonstrating improved VA.[1]Perioperative steroids are required to mitigate the intense inflammatory response.[14]
Development of early cataracts is a hallmark of ADNIV. In the original cohort, all patients older than age 60 underwent bilateral cataract extractions or had mature cataracts. The average age of cataract surgery in the first cohort was 54 years-old.[1]
Complications
The most common complication in treating ADNIV is steroid-response glaucoma. This risk is higher with the use of intraocular or steroid implants.[1][13][14][15]
Prognosis
Untreated or under-treated ADNIV has an extremely poor prognosis. Of those patients who are older than 60 years of age, VA was found to be NLP in 35% while an additional 30% had VA of 20/800 or worse.[1]Phthsis bulbi is the end stage of the disease. Despite aggressive therapy including PPV and peripheral laser or cryotherapy, anterior neovascularization can ultimately occur.[1]
Future Directions
A non-specific calpain inhibitor (SJA6017) has been developed though its use in humans with ADNIV has not been studied.[8] SJA6017 has been shown to reduce retinal damage in hypoxic retinal diseases, though which calpain isoform(s) is inhibited is not known.[8] Calpastatin, an endogenous calpain inhibitor does not bind to CAPN5 which may predict poor binding to SJA6017 as well.[8] Replacement gene therapy has been successfully trialed in loss-of-function recessive retinal diseases; however, dominant gain of function mutations must rely on interference RNA or spliceosome-mediated RNA trans-splicing to remove the toxic mutant gene. There has been recent attempts to repair gain of function mutations in retinitis pigmentosa, which if successful may pave the way for suppression therapy in ADNIV patients.[17]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 Stone EM, Kimura AE, Folk JC, et al. Genetic linkage of autosomal dominant neovascular inflammatory vitreoretinopathy to chromosome 11q13. Hum Mol Genet. 1992;1(9):685-9.
- ↑ Tabbaa T, Mehra AA, Kesav NP, Mahajan VB, Swanson RD, Zubricky R, Sobol WM. Autosomal dominant neovascular inflammatory vitreoretinopathy with CAPN5 c.731T > C gene mutation; clinical management of a family cohort and review of the literature. Ophthalmic Genet. 2023 Dec;44(6):559-567. doi: 10.1080/13816810.2023.2255257.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Bennett SR, Folk JC, Kimura AE, Russell SR, Stone EM, Raphtis EM. Autosomal dominant neovascular inflammatory vitreoretinopathy. Ophthalmology. 1990;97(9):1125-35.
- ↑ Jump up to: 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 Mahajan VB, Skeie JM, Bassuk AG, et al. Calpain-5 mutations cause autoimmune uveitis, retinal neovascularization, and photoreceptor degeneration. PLoS Genet. 2012;8(10):e1003001.
- ↑ O'Keefe, GA et al. Early Onset Neovascular Inflammatory Vitreoretinopathy Due to a De Novo CAPN5 Mutation: Report of a Case. Ocul Immunol Inflamm. 2019;27(5):706-708. doi: 10.1080/09273948.2019.1582783. Epub 2019 Apr 15.
- ↑ Jump up to: 6.0 6.1 6.2 Randazzo NM, Shanks ME, Clouston P, Maclaren RE. Two Novel CAPN5 Variants Associated with Mild and Severe Autosomal Dominant Neovascular Inflammatory Vitreoretinopathy Phenotypes. Ocul Immunol Inflamm. 2017:1-6.
- ↑ Bassuk AG, Yeh S, Wu S, et al. Structural modeling of a novel CAPN5 mutation that causes uveitis and neovascular retinal detachment. PLoS ONE. 2015;10(4):e0122352.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 Wert KJ, Skeie JM, Bassuk AG, Olivier AK, Tsang SH, Mahajan VB. Functional validation of a human CAPN5 exome variant by lentiviral transduction into mouse retina. Hum Mol Genet. 2014;23(10):2665-77.
- ↑ Jump up to: 9.0 9.1 9.2 Wert KJ, Bassuk AG, Wu WH, et al. CAPN5 mutation in hereditary uveitis: the R243L mutation increases calpain catalytic activity and triggers intraocular inflammation in a mouse model. Hum Mol Genet. 2015;24(16):4584-98.
- ↑ Jump up to: 10.0 10.1 10.2 Schaefer KA, Toral MA, Velez G, et al. Calpain-5 Expression in the Retina Localizes to Photoreceptor Synapses. Invest Ophthalmol Vis Sci. 2016;57(6):2509-21.
- ↑ Sève P, Cacoub P, Bodaghi B, et al. Uveitis: Diagnostic work-up. A literature review and recommendations from an expert committee. Autoimmun Rev. 2017
- ↑ Mahajan VB, Vallone JG, Lin JH, et al. T-cell infiltration in autosomal dominant neovascular inflammatory vitreoretinopathy. Mol Vis. 2010;16:1034-40.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 13.4 13.5 Tlucek PS, Folk JC, Orien JA, Stone EM, Mahajan VB. Inhibition of neovascularization but not fibrosis with the fluocinolone acetonide implant in autosomal dominant
- ↑ Jump up to: 14.0 14.1 14.2 14.3 Tlucek PS, Folk JC, Sobol WM, Mahajan VB. Surgical management of fibrotic encapsulation of the fluocinolone acetonide implant in CAPN5-associated proliferative vitreoretinopathy. Clin Ophthalmol. 2013;7:1093-8.
- ↑ Jump up to: 15.0 15.1 Cham A, Bansal M, Banda HK, et al. Secondary glaucoma in CAPN5-associated neovascular inflammatory vitreoretinopathy. Clin Ophthalmol. 2016;10:1187-97.
- ↑ Rowell HA, Bassuk AG, Mahajan VB. Monozygotic twins with CAPN5 autosomal dominant neovascular inflammatory vitreoretinopathy. Clin Ophthalmol. 2012;6:2037-44.
- ↑ Berger A, Lorain S, Joséphine C, et al. Repair of rhodopsin mRNA by spliceosome-mediated RNA trans-splicing: a new approach for autosomal dominant retinitis pigmentosa. Mol Ther. 2015;23(5):918-930.