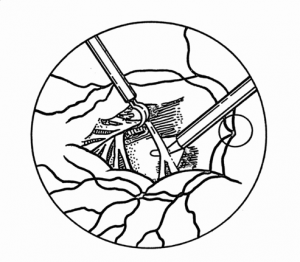
Proliferative Vitreoretinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Proliferative vitreoretinopathy (PVR), a major complication of rhegmatogenous retinal detachment (RRD), is an abnormal process whereby proliferative, contractile cellular membranes form in the vitreous and on both sides of the retina, resulting in tractional retinal detachment with fixed retinal folds. However, it is increasingly being recognised that PVR may be intraretinal also, which causes retinal shortening. Research suggests that membranes form in response to cytokines and inflammatory mediators that arise following anatomic disruption and tissue damage caused by rhegmatogenous retinal detachment (RRD) and resultant inflammation. Treatment is principally surgical and often requires multiple procedures that, in fact, yield a high rate of retinal reattachment; nevertheless, many anatomically successful eyes do not recover good visual function likely due to the long standing macular detachment.
Disease Entity
Proliferative vitreoretinopathy (PVR) is classified using the following International Classification of Disease (ICD) code: H35.20, other non-diabetic proliferative retinopathy, unspecified eye.
Disease
Proliferative vitreoretinopathy
Etiology
Proliferative Vitreoretinopathy (PVR)— formerly named “massive vitreous retraction” and “massive periretinal proliferation” — describes the aberrant process whereby epi/subretinal membranes form following rhematogenous retinal detachment (RRD), ultimately leading to retinal traction and recurrent retinal detachment.[1] [2] [3] Intraretinal PVR is caused by glial tissue that is activated to proliferate within the retina and can cause retinal shortening. PVR arises in an estimated 5-10% of RRD cases, and therefore represents a major complication of retinal detachment.[4]
General Pathology
In general, PVR is comparable to an abnormal wound-healing process following tissue insult, more specifically, retinal detachment. Rhematogenous retinal detachment (RRD) serves as a nidus for membrane formation, in part through induction of ischemia and subsequent cell death that arises from separation of the neuroepithelium from its rich choroidal blood flow. Cell death triggers a break down in the blood-retinal barrier (BRB),[5] [6] thereby facilitating the influx of chemotactic and mitogenic factors that permit cell proliferation, migration, extracellular matrix deposition and contraction. Similarly, the vitreous is suffused with growth factors and inflammatory mediators; thus, it serves as a milieu for cellular proliferation in the case of retinal detachment.[7] Contractile membranes contain fibroblast-like cells in extracellular matrix that have characteristics of RPE and glial cells on the inner surface of the retina and mostly RPE cells on the outer (subretinal) surface of the retina.[8]
Pathophysiology
Although the exact pathophysiology remains disputed, the development of PVR is a complex process involving humoral and cellular factors. Crucial cells in the development of PVR are retinal pigment epithelial (RPE) cells, glial cells, fibroblasts and macrophages.[9] Following retinal detachment and subsequent ischemia, some RPE cells lose their polarity and undergo epithelial-mesenchymal transformation (EMT).[10] EMT is a process whereby epithelial cells lose their typical epithelial morphology and phenotype and acquire a mesenchymal-like morphology and phenotype. Various chemokines and cytokines, most notably TGFbeta, PDGF,[11] VEGF, IL-1, 6, 8, 10 and IFN-gamma,[12] whose presence is permitted via breakdown of the BRB, induce cell proliferation, migration, extracellular matrix deposition and contraction. Transformed cells in membranes become fibroblast-like cells that contain actin and myosin and have the ability to contract. Activation of Muller glia leads to marked gliosis within the retina that leads to retinal stiffness and shortening.[13] Macrophages may play a multifactorial role that involves secretion of enzymes and growth factors (e.g., platelet-derived growth factor, PDGF) and transdifferentiation into fibroblast-like cells.[14] Fibrocytes, circulating cells derived from bone marrow stem cells that transform to fibroblasts in tissue, have also been found in PVR membranes, further evincing the robust cellular buildup in response to circulating growth factors.[15] It is even possible that genetic changes in the cytokine TNF alpha might predispose to PVR in patients with retinal detachment.[16]
Primary Prevention
Risk Factors, Primary Prevention and Medical Therapy:
The best way to prevent PVR is prompt successful repair of primary RRD. However, due to patient, ocular and surgical factors that are not always controllable, PVR continues to occur. A strategy to prevent PVR is to look for risk factors for PVR in eyes with RRD and to try to control or influence those risk factors.
Clinical factors associated with increased risk of PVR include, but are not limited to:
- existing PVR
- chronic rhematogenous retinal detachment lasting longer than several months,
- previous history of PVR
- aggressive retinitis
- choroidal detachment
- failed RRD surgery, or multiple retinal surgeries
- aphakia
- vitreous hemorrhage
- choroidal detachment
- high vitreous protein levels
- positive smoking history
- preoperative retinal folds
- horseshoe retinal tears exposing three disc diameters or more of RPE
- Giant retinal tear
- failure of previous surgery
- uveitis.[17] [18] [19] [20] [21] [22] [23]
Operative factors associated with increased risk of PVR include
- intra-/postoperative hemorrhage
- vitrectomy
- retinectomy
- cryopexy
- extensive laser
- injection of air.[18] [22] [23]
There is no current pharmacologic agent proven to treat or prevent PVR. A myriad of medical interventions have been considered for PVR and none have been proven conclusively to be superior to surgery alone. However, there is evidence that intravitreal methotrexate may arrest early PVR [24]
- Corticosteroids, for example, have been considered given their mechanism of action and potential to reduce the massive expansion of growth factors contributing to membrane formation. Unfortunately, trials have been variable and have fallen short of expectations. In one randomized, controlled clinical trial of patients at high risk of developing PVR, treatment with systemic corticosteroids led to a decrease in epiretinal membrane formation, but did not improve visual acuity.[25] In another trial, when grade C PVR patients were treated with 4 mg intravitreal triamcinolone acetonide in silicone oil at the time of surgery, as compared to control patients without triamcinolone in silicone oil, treated patients did not exhibit a significant change in visual acuity or PVR recurrence six months post-operatively.[26] Additionally, a more recent prospective trial investigated the use of the slow release dexamethasone intravitreal implant. Patients with grade C PVR underwent vitrectomy with silicon oil tamponade either with or without dexamethasone implant, and the investigators found no difference in surgical outcomes.[27] A meta-analysis of 4 randomized clinical trials and 478 patients found no difference overall unless PVR grades A and B were only considered. Then use of steroids was found to have a reduction in early grades of PVR (grades A and B, see below) and in postoperative macular edema.[28].
- Anti-neoplastic drugs, irrespective of their side effects, have also been considered as a means of treating PVR.
- 5-Fluorouracil (5-FU)has been studied in a variety of circumstances, but its use is limited by toxicity.[29] [30] In eyes with retinal detachments at high risk of developing PVR, intravitreal 5-FU and high molecular weight heparin infused during surgery was shown to potentially reduce the incidence of post-operative PVR.[31] This combination was not effective in improving outcome in patients with existing PVR.[32] Moreso, a large randomized clinical trial investigated 5-FU with low molecular weight heparin and found no improvement in outcomes of macula-off retinal detachments and worse visual outcomes in patients with macula-on detachments.[33]
- Daunorubicin has been used in conjunction with vitrectomy and silicone oil tamponade, although there was no apparent improvement in clinical outcomes.[34]
- Methotrexate, an anti-folate anti-metabolite, has received recent attention as a potential candidate drug following in vitro studies.[35] A retrospective study investigated the outcomes of patients with severe recurrent PVR detachments or severe intraocular inflammation at high risk of PVR who received methotrexate infusion at the time of surgery and found a lower rate of PVR .[36] There is an ongoing randomized study to further investigate the use of methotrexate as a series of postoperative intravitreal injections in eyes with PVR.
- Biologics that interact with implicated growth factors have also shown promise in mitigating PVR. For example, ranibizumab has been shown to have prophylactic effects in a rabbit model of PVR, and decreased bioactivity in the vitreous of patients and experimental animals.[37] However, a prospective trial investigated the use of bevacizumab injected at the end of the case in patients with PVR grade C, who were undergoing vitrectomy and silicone oil placement, and found no difference in outcomes.[38]
- Retinoic acid and colchine which are inhibitors of RPE cell growth, have been investigated with mixed results.[39] [40] A small randomized trial of retinoic acid showed lower rates of redetachment in PVR cases.[41] A larger ongoing study investigated this further.
- Amniotic membranes to reduce risk of PVR have been tested and are being assessed[42].
Diagnosis
Diagnosis of PVR is made via an in-depth patient history, i.e. evidence of longstanding primary RRD or of recent retinal reattachment surgery, and via physical examination, most importantly recognition of retinal detachment with fixed retinal folds.
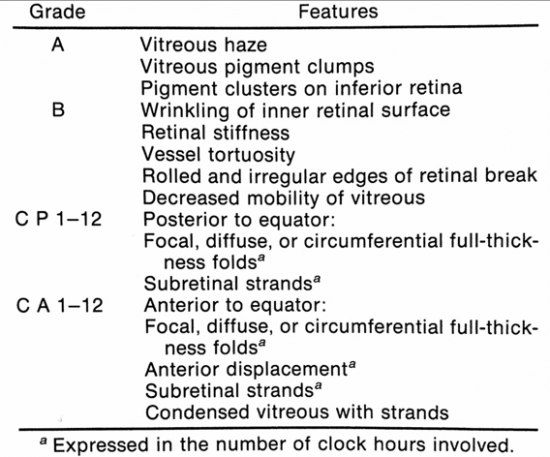
Classification and Staging
Since publishing its seminal classification system in 1983,[43] the Retinal Society Terminology Committee has updated its classification—most recently in 1991—to reflect a greater understanding of the pathogenesis of proliferative vitreoretinopathy.[44] Additional classification systems, specifically the classification criteria utilized in the Silicone Study,[45] have broadened the initial contributions structured in the seminal Retinal Society report by stratifying via: 1) membrane location, 2) clinical severity, and 3) membrane geometry. The updated classification system, as defined by Machemer and his colleagues in 1991,[44] is presented below in adapted forms:
In practice, the revised system, most notably the use of clock hours as opposed to quadrants, in addition to the segregation of membranes into anterior and posterior, allows for better understanding of the pathology of individual cases and comparison of severity of PVR among different clinical studies of PVR.
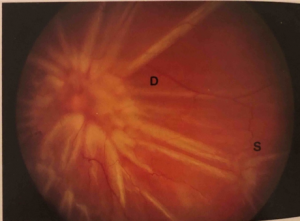
Example case (Figure 1): Patient presents to the clinic eight weeks post-operatively following retinal detachment repair. Patient complains of flashing lights and loss of vision. On indirect ophthalmic exam, the retina is detached with inferior starfolds and diffuse posterior contraction. Using the revised Retinal Society classification system, this patient’s presentation would be classified as CP12.
Clinical Presentation (History/Physical/Signs/Symptoms/Clinical Diagnosis)
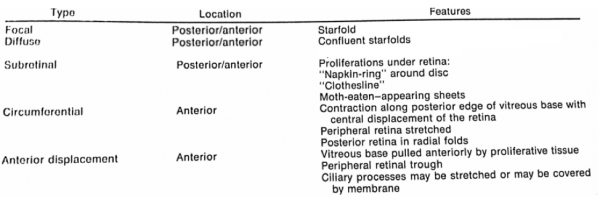
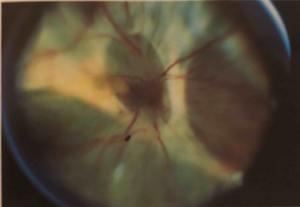
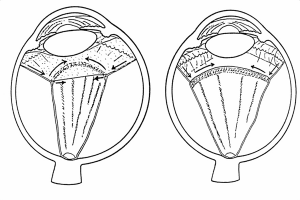
Patients presenting with PVR may be categorized into two groups: 1) those presenting with longstanding PVR arising from primary rhematogenous retinal detachment, and 2) those who have recently undergone surgical repair of RRD. In the case of the former, patients will present with prolonged vision loss often preceded by floaters and/or flashing lights. Patients presenting post-operatively may have initially had vision improvement following surgery, then progressive or rapid onset of vision loss. These patients often present 4-6 weeks post-operatively. The clinical presentation of PVR is defined by fixed folds in the retina on ophthalmic examination, indicative of retinal traction caused by retinal membranes. In the majority of cases, membranes localize to the inferior retina due to gravity.[46] Retinal folds may appear as billows in the retina and often co-present with haze and pigment in the vitreous. In the case of posterior PVR, there may be starfolds with folds radiating from a central area of contracted retina due to epiretinal membranes, more diffuse folds from larger membranes, or subretinal membranes that may look like clotheslines beneath the retina. These folds may even take an annular configuration pulling the retina over the optic disc. Anterior PVR most commonly involves the inferior retina, but in severe cases may extend 360 degrees. Membranes may be circumferential at or posterior to the vitreous base. With contraction at the posterior edge of the vitreous base, the anterior retina may be stretched centrally while the posterior retina is thrown into radial folds extending from the vitreous base posteriorly (Figure 3).
This presentation differs from primary RRD, in that the latter lacks fixed retinal folds. While eyes with PVR show little if any movement of the retina during eye movements, eyes with primary retinal detachment without PVR will show a “bouncing” movement of the retina with eye movements. Patients with primary retinal detachments often initially notice floaters and/or photopsia followed by loss of visual field in the area of retinal detachment. This may expand to involve central vision, tracking with the growing retinal detachment progressing centrally.
If the circumferential membranes involve the vitreous base, especially following prior vitrectomy, the retina posterior to the vitreous base may be pulled by the contracting membranes anteriorly toward the pars plana, ciliary body, or even the iris (anterior retinal displacement).
Diagnostic procedures
The diagnosis of PVR is by clinical examination in most patients; however, sometimes other diagnostic techniques become necessary. If the ocular media are opaque due to cornea, lens, vitreous or other opacities, the retina cannot be visualized and other techniques must be used. Ultrasound examination can demonstrate the presence of retinal detachment, and there are some characteristics of the retinal detachment on ultrasound that will indicate the presence of PVR. Using dynamic ultrasound (moving the eye while viewing the retinal detachment with ultrasound), compared to RRD without PVR, there is less retinal mobility in a RRD with PVR. In RRD without PVR, the retina attached to the optic nerve usually has a rounded pattern with good mobility as it approaches the disc on ultrasound; with PVR, the leaves of the retina may assume a “V” pattern at the optic disc with the retina straightened with limited mobility as they approach the optic nerve (open funnel retinal detachment). With more severe PVR, the retina may assume a “T” pattern on ultrasound at the optic disc (closed funnel retinal detachment) with the leaves of the retina fused together anterior to the disc, only opening more anterior to the disc with the anterior immobile retina completing the top bar of the “T”. OCT can be helpful if there is a question of whether the macula or other parts of the retina are detached or not.
Differential diagnosis
When assessing a patient for PVR, one should also consider a variety of other proliferative and contractional retinal diseases, in addition to other fibrosing conditions, including:
- Proliferative vascular retinopathy (proliferative diabetic retinopathy, other proliferative ischemic retinopathies and retinopathy of prematurity)
- Retinal detachment following open globe ocular trauma
- Retinal detachment following intraocular foreign body
Rarely, a RRD without PVR can have extensive folds that falsely appear to be fixed. Seen mostly in highly myopic eyes with very thin retinas, the retina in this instance remains normally mobile on eye movement, no membranes are found at surgery and the retina completely flattens following surgery.
Surgery
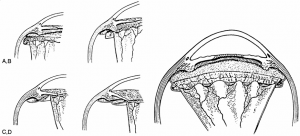
PVR is primarily managed through pars plana vitrectomy and membrane peeling,[1] although these procedures are rarely necessary in the case of grade A or B PVR. While some cases of grade C PVR can be managed with a scleral buckle without vitrectomy,[47] most will require vitrectomy and membrane peeling. If an encircling scleral buckle is present, it is usually left in place. If no scleral buckle is present, an encircling scleral buckle is usually placed in order to help reduce residual peripheral traction sometimes present even after extensive membrane peeling. This procedural combination of vitrectomy, membrane peeling and scleral buckle is done to alleviate the retinal traction in PVR and facilitate retinal flattening. Vitrectomy can be performed with any size vitrectomy instrumentation, although most surgeons currently use 25- or 23-gauge micro-vitrectomy systems. Membrane peeling is usually done with a pick and end-grabbing forceps. Some membranes can be grasped with forceps and removed, while some will require elevation with a pick. Note that mature membranes are often easily removed in a continuous sheet, as opposed to immature membranes that may tear more readily and split. A bimanual approach with the pick in one hand and the forceps in the other hand is the preferred method of membrane peeling (Figure 5), although some surgeons prefer to use two forceps for bimanual membrane peeling.
Illumination during membrane peeling can be provided by an illuminated pick (a fiberoptic light with an attached pick) or by a “chandelier” (a stationary fiberoptic light placed through another cannula at the pars plana). Peeling is usually started in the posterior retina and progresses anteriorly. In eyes with anterior PVR, membrane peeling can be difficult to adequately relieve traction. After posterior membranes are removed, perfluorocarbon liquid (PFCL), which is is heavier than water or saline, can be used to weigh down and stabilize the posterior retina to ease dissection of the mid peripheral membranes and the retina pulled anteriorly and adherent to membranes at the vitreous base or ciliary body. As a last resort, it is sometimes necessary to perform a relaxing retinectomy to relieve traction. The relaxing retinectomy is a circumferentially cut in the peripheral retina in the area of traction with excision of the devitalized retina anterior to the cut retina. Most relaxing retinectomies are done inferiorly where anterior PVR is most commonly found, but occasionally will extend 360 degrees. The retinectomy should be as anterior as possible to spare as much posterior, functional retina as possible and should almost always be in a circumferential direction. The retinectomy is usually done with the vitrectomy instrument after marking the area and ensuring hemostasis with endodiathermy. The retinectomy should extend beyond the abnormal area on both sides, otherwise retina may not settle and there will be risk of subretinal air or perfluorocarbon liquid. After all traction is relieved by membrane peeling and, if necessary, a peripheral retinectomy, the retina is usually reattached with PFCL. After reattachment, PFCL can be exchanged for a gas or sometimes directly to silicone oil. Laser treatment delivered through a fiberoptic probe (endolaser photocoagulation) is used to treat all retinal breaks and usually to place a laser barrier 360 degrees around the peripheral retina (over the scleral buckle if present) and can be performed through PFCL or a long-acting gas. The benefit of FAX prior to laser is to flatten the anterior detached retina prior to laser. The view can be challenging sometimes through air and then the decision to laser under PFCL is made. However, air can provide a wider angle and improve the visualization of the anterior retina and also be helpful. The PFCL or air is replaced by long-acting gas (usually perfluoropropane [C3F8] gas) or silicone oil. For primary PVR in which the eye has not had a prior vitrectomy, C3F8 gas or silicone oil are equally effective. In eyes with prior vitrectomy for PVR and those with a large peripheral retinectomy or with a giant retinal tear more than 90 degrees in circumference, silicone oil may be more effective.
Surgical follow up and complications
Close surgical follow-up is critical to successful surgery for PVR. Major concerns in the early postoperative period include
- ocular hypertension,
- pupillary block glaucoma, shallowing and closure of the anterior chamber,
- intraocular inflammation,
- Subretinal hemorrhage after retinectomy/inadvertent touch to the choroid
- Subretinal PFCL
- silicone oil in the anterior chamber--that can cause corneal endothelial damage
- corneal erosion.
Any of these complications can cause permanent vision loss in an eye that has had a successful repair of the retinal detachment, so early and frequent surgical follow-up is necessary to identify and treat early complications.
Later complications include
- recurrent retinal detachment,
- glaucoma,
- hypotony-phthisis
- corneal opacification
Recurrent retinal detachment can occur either early or late. Early detachment following surgery usually means that there was inadequate relief of vitreoretinal traction and/or retinal breaks were missed at surgery. Late recurrence of retinal detachment usually indicates that there has been recurrence of proliferative membranes and/or new retinal breaks have formed. Some early retinal detachments can be managed with laser treatment and possibly additional gas injection (in eyes managed with gas at the time of surgery); however, most recurrent retinal detachments will require additional surgery. Glaucoma that cannot be managed medically may require surgical management and severe corneal opacification may need corneal transplant. Hypotony can occur in up to 15-24% of cases following surgery for PVR and is difficult to treat. A search for a treatable cause such as cyclodialysis cleft is warranted and some cases will improve with topical corticosteroids, but most cases will persist, often with vision loss. Ultrasound biomicroscopy may be performed to look for ciliary body atrophy/detachment or cyclitic membrane over the ciliary body.
Prognosis
With current surgical techniques, most eyes with PVR can now be reattached. However, despite recent advances, visual results of surgery for PVR remain poor. For example, Pastor reports that in 40-80% of anatomically successful cases, patients will maintain only ambulatory vision, defined as vision 5/200 or better.[48] Such a prognosis is largely a function of post-operative abnormalities, e.g. macular edema, macular pucker, subretinal membranes, optic atrophy, retinal atrophy, etc.[49] In addition, many eyes will retain silicone oil long after surgery with the possibility of late visual loss associated with chronic retention of silicone oil. Narala et al. recommend a more stringent definition of success following surgery for PVR that includes retinal reattachment, ambulatory vision, and silicone oil removal versus simply anatomical success.[50]
Additional Resources
Idrees S, Sridhar J, Kuriyan AE. Proliferative Vitreoretinopathy: A Review. Int Ophthalmol Clin. 2019;59(1):221-240. doi:10.1097/IIO.0000000000000258
Constable IJ, Nagpal M. Proliferative Vitreoretinopathy. In: Schachat AP, Ed., St. Louis; Ryan's Retina. 6th edition. Elsevier 2018:2031-51 Elsevier, London 2018.
References
- ↑ 1.0 1.1 Pastor, J. C. Proliferative vitreoretinopathy: An overview. Survey of Ophthalmology 43, 3-18, doi:https://doi.org/10.1016/S0039-6257(98)00023-X (1998).
- ↑ Leaver, P. K. Proliferative vitreoretinopathy. The British Journal of Ophthalmology 79, 871-872 (1995).
- ↑ Campochiaro, P. A. Pathogenic mechanisms in proliferative vitreoretinopathy. Archives of Ophthalmology 115, 237-241, doi:10.1001/archopht.1997.01100150239014 (1997).
- ↑ Charteris, D. G., Sethi, C. S., Lewis, G. P. & Fisher, S. K. Proliferative vitreoretinopathy—developments in adjunctive treatment and retinal pathology. Eye 16, 369, doi:10.1038/sj.eye.6700194 (2002).
- ↑ Morescalchi, F., Duse, S., Gambicorti, E., et al. Proliferative vitreoretinopathy after eye injuries: an overexpression of growth factors and cytokines leading to a retinal keloid. Mediators of Inflammation 2013, 269787-269787, doi:10.1155/2013/269787 (2013).
- ↑ Yu, D. Y. & Cringle, S. J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Progress in Retinal Eye Research 20, 175-208 (2001).
- ↑ Elner, S. G., Elner, V. M., Freeman, H. M., Tolentino, F. I. & Albert, D. M. The pathology of anterior (peripheral) proliferative vitreoretinopathy. Transactions of the American Ophthalmological Society 86, 330-353 (1988).
- ↑ Hiscott, P., Morino, I., Alexander, R., Grierson, I. & Gregor, Z. Cellular components of subretinal membranes in proliferative vitreoretinopathy. Eye 3, 606-610, doi:10.1038/eye.1989.94 (1989).
- ↑ Rodrigues, M. M., Newsome, D. A. & Machemer, R. Further characterization of epiretinal membranes in human massive periretinal proliferation. Current Eye Research 1, 311-315, doi:10.3109/02713688108998357 (1981).
- ↑ Xu, K., Chin, EK., Bennett, SR., et al. Predictive factors for proliferative vitreoretinopathy formation after uncomplicated primary retinal detachment repair. Retina 39, 1488-1495, doi:10.1097/iae.0000000000002184 (2019).
- ↑ Lei, H., Rhéaume, M.-A., Velez, G., Mukai, S. & Kazlauskas, A. Expression of PDGFRα is a determinant of the PVR potential of ARPE19 cells. Investigative Ophthalmology & Visual Science 52, 5016-5021, doi:10.1167/iovs.11-7442 (2011).
- ↑ Lei, H., Velez, G., Hovland, T.,et al. Growth factors outside the PDGF family drive experimental PVR. Investigative Ophthalmology & Visual Science 50, 3394-3403, doi:10.1167/iovs.08-3042 (2009).
- ↑ Hiscott, P. & Mudhar, H. Is vasoproliferative tumour (reactive retinal glioangiosis) part of the spectrum of proliferative vitreoretinopathy? Eye 23, 1851-1858, doi:10.1038/eye.2008.351 (2009).
- ↑ Symeonidis, C., Papakonstantinou, E., Souliou, E., et al. Correlation of matrix metalloproteinase levels with the grade of proliferative vitreoretinopathy in the subretinal fluid and vitreous during rhegmatogenous retinal detachment. Acta Ophthalmologica 89, 339-345, doi:10.1111/j.1755-3768.2009.01701.x (2011).
- ↑ Abu El-Asrar, A. M., Struyf, S., Van Damme, J. & Geboes, K. Circulating fibrocytes contribute to the myofibroblast population in proliferative vitreoretinopathy epiretinal membranes. British Journal of Ophthalmology 92, 699-704, doi:10.1136/bjo.2007.134346 (2008).
- ↑ Rojas, J., Fernandez, I, Pastor, JC, et al. A strong genetic association between the tumor necrosis factor locus and proliferative vitreoretinopathy: The Retina 4 Project. Ophthalmology 117, 2417-2423.e2412, doi:https://doi.org/10.1016/j.ophtha.2010.03.059 (2010).
- ↑ Kon, C. H., Asaria, R. H., Occleston, N. L., Khaw, P. T. & Aylward, G. W. Risk factors for proliferative vitreoretinopathy after primary vitrectomy: a prospective study. British Journal of Ophthalmology 84, 506-511 (2000).
- ↑ 18.0 18.1 Nagasaki, H., Shinagawa, K. & Mochizuki, M. Risk factors for proliferative vitreoretinopathy. Progress in Retinal and Eye Research 17, 77-98, doi:https://doi.org/10.1016/S1350-9462(97)00007-4 (1998).
- ↑ Eliott, D., Stryjewski, T. P., Andreoli, M. T. & Andreoli, C. M. Smoking is a risk factor for proliferative vitreoretinopathy after traumatic retinal detachment. Retina 37, 1229-1235, doi:10.1097/iae.0000000000001361 (2017).
- ↑ Chignell, A. H., Fison, L. G., Davies, E. W., Hartley, R. E. & Gundry, M. F. Failure in retinal detachment surgery. British Journal of Ophthalmology 57, 525, doi:10.1136/bjo.57.8.525 (1973).
- ↑ Bonnet, M. [Clinical risk factors of vitreoretinal proliferations in rhegmatogenous retinal detachment]. Journal of French Ophtalmology 17, 530-540 (1994).
- ↑ 22.0 22.1 Bonnet, M. Clinical factors predisposing to massive proliferative vitreoretinopathy in rhegmatogenous retinal detachment. Ophthalmologica 188, 148-152, doi:10.1159/000309357 (1984).
- ↑ 23.0 23.1 Cowley, M., Conway, B. P., Campochiaro, P. A., Kaiser, D. & Gaskin, H. Clinical risk factors for proliferative vitreoretinopathy. Archives of Ophthalmology 107, 1147-1151, doi:10.1001/archopht.1989.01070020213027 (1989).
- ↑ Alabi R, Stryjewski TP, Vora RA, Eliott D, Moussa K. Rescue Intravitreal Methotrexate treatment following early recognition of proliferative vitreoretinopathy. Retin Cases Brief Rep. 2023 Sep 1;17(5):616-619. doi: 10.1097/ICB.0000000000001252. PMID: 36206488.
- ↑ Koerner, F., Koerner-Stiefbold, U. & Garweg, J. G. Systemic corticosteroids reduce the risk of cellophane membranes after retinal detachment surgery: a prospective randomized placebo-controlled double-blind clinical trial. Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 250, 981-987, doi:10.1007/s00417-011-1919-y (2012).
- ↑ Ahmadieh, H., Fegghi, M., Tabatabaei, H., et al. Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: a randomized clinical trial. Ophthalmology 115, 1938-1943, doi:10.1016/j.ophtha.2008.05.016 (2008).
- ↑ Banerjee PJ, Quartilho A, Bunce C, et al. Slow-Release Dexamethasone in Proliferative Vitreoretinopathy: A Prospective, Randomized Controlled Clinical Trial. Ophthalmology. 2017;124(6):757–767. doi:10.1016/j.ophtha.2017.01.021 [PubMed: 28237428]
- ↑ Xu M, Fan X, Huang X, Chen X, Shao Y, Li X. Steroids Drugs as An Adjunct for Reducing the Incidence of Proliferative Vitreoretinopathy After Rhegmatogenous Retinal Detachment Surgery: A Meta-analysis of Randomized Controlled Studies. Ophthalmic Res. 2023 Feb 8;66(1):591–602. doi: 10.1159/000529451. Epub ahead of print. PMID: 36754031; PMCID: PMC9979270.
- ↑ Borhani, H., Peyman, G. A., Rahimy, M. H. & Thompson, H. Suppression of experimental proliferative vitreoretinopathy by sustained intraocular delivery of 5-FU. International ophthalmology 19, 43-49 (1995).
- ↑ Blumenkranz, M., Hernandez, E., Ophir, A. & Norton, E. W. 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology 91, 122-130 (1984).
- ↑ Asaria, R. H. Y., Kon, CH., Bunce, C., et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: Results from a randomized, double-blind, controlled clinical trial. Ophthalmology 108, 1179-1183, doi:https://doi.org/10.1016/S0161-6420(01)00589-9 (2001).
- ↑ Charteris, DG., Aylward, GW., Wong, D., et al. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of established proliferative vitreoretinopathy. Ophthalmology 111, 2240-2245, doi:10.1016/j.ophtha.2004.05.036 (2004).
- ↑ Wickham L, Bunce C, Wong D, McGurn D, Charteris DG. Randomized controlled trial of combined 5-Fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachments undergoing primary vitrectomy. Ophthalmology. 2007;114(4):698–704. doi:10.1016/j.ophtha.2006.08.042 [PubMed: 17398320]
- ↑ Sadaka, A. & Giuliari, GP Proliferative vitreoretinopathy: current and emerging treatments. Clinical Ophthalmology (Auckland, N.Z.) 6, 1325-1333, doi:10.2147/OPTH.S27896 (2012).
- ↑ Amarnani, D., Machuca-Parra, AI., Wong, L., et al. Effect of methotrexate on an in vitro patient-derived model ofproliferative vitreoretinopathy. Investigave Ophthalmology and Visual Sciences 58, 3940-3949, doi:10.1167/iovs.16-20912 (2017).
- ↑ Sadaka A, Sisk RA, Osher JM, Toygar O, Duncan MK, Riemann CD. Intravitreal methotrexate infusion for proliferative vitreoretinopathy. Clin Ophthalmol Auckl NZ. 2016;10:1811–1817. doi: 10.2147/OPTH.S111893
- ↑ Pennock, S., Kim, D., Mukai, S., et al. Ranibizumab is a potential prophylaxis for proliferative vitreoretinopathy, a nonangiogenic blinding disease. The American Journal of Pathology 182, 1659-1670, doi:10.1016/j.ajpath.2013.01.052 (2013).
- ↑ Ghasemi Falavarjani K, Hashemi M, Modarres M, Hadavand Khani A. Intrasilicone oil injection of bevacizumab at the end of retinal reattachment surgery for severe proliferative vitreoretinopathy. Eye Lond Engl. 2014;28(5):576–580. doi:10.1038/eye.2014.21
- ↑ Lemor, M., Yeo, J. H. & Glaser, B. M. Oral colchicine for the treatment of experimental traction retinal detachment. Archives of Ophthalmology 104, 1226-1229, doi:10.1001/archopht.1986.01050200132067 (1986).
- ↑ Fekrat, S., de Juan, E., Jr. & Campochiaro, P. A. The effect of oral 13-cis-retinoic acid on retinal redetachment after surgical repair in eyes with proliferative vitreoretinopathy. Ophthalmology 102, 412-418 (1995).
- ↑ 47 Ghasemi Falavarjani K, Hashemi M, Modarres M, Hadavand Khani A. Intrasilicone oil injection of bevacizumab at the end of retinal reattachment surgery for severe proliferative vitreoretinopathy. Eye Lond Engl. 2014;28(5):576–580. doi:10.1038/eye.2014.21
- ↑ Caporossi T, Molle A, Carlà MM, Picardi SM, Gambini G, Scampoli A, Governatori L, Bernardinelli P, Rizzo S. Applications of Human Amniotic Membrane Patching Assisted Vitrectomy in the Management of Postoperative PVR in Complex Retinal Detachments. J Clin Med. 2023 Feb 1;12(3):1137. doi: 10.3390/jcm12031137. PMID: 36769785; PMCID: PMC9918292.
- ↑ The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 90, 121-125 (1983).
- ↑ 44.0 44.1 Machemer, R., Aaberg, TM., Freeman, HM., et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. American Journal of Ophthalmology 112, 159-165 (1991).
- ↑ Lean, J. S., Stern, W. H., Irvine, A. R. & Azen, S. P. Classification of proliferative vitreoretinopathy used in the silicone study. The Silicone Study Group. Ophthalmology 96, 765-771 (1989).
- ↑ Thompson, J. T. in Retina (Fourth Edition) (eds Stephen J. Ryan, David R. Hinton, Andrew P. Schachat, & C. P. Wilkinson) 2283-2309 (Mosby, 2006).
- ↑ Freeman, W. R. Practical Atlas of Retinal Disease and Therapy. (Raven Press, 1993).
- ↑ Pastor, J. C., de la Rúa, E. R. g. & Martı́n, F. Proliferative vitreoretinopathy: risk factors and pathobiology. Progress in Retinal and Eye Research 21, 127-144, doi:https://doi.org/10.1016/S1350-9462(01)00023-4 (2002).
- ↑ Bonnet, M. Macular changes and fluorescein angiographic findings after repair of proliferative vitreoretinopathy. Retina 14, 404-410 (1994).
- ↑ Narala, R., Nassiri, N., Kim, C., et al. Outcomes of repeat pars plana vitrectomy after failed surgery for proliferative vitreoretinopathy. Retina 38 Suppl 1, S49-s59, doi:10.1097/iae.0000000000002000 (2018).