Siderosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Ocular siderosis (OS), or siderosis bulbi, is a complication due to a magnetic intraocular or intraorbital foreign body. Generally metals with a low redox potential, such as Fe2+ and Cu2+, have the greatest potential for metallosis. Siderosis may develop within days or as long as long as years after a trauma, but the course is variable depending on the iron content in the foreign body, the size, and its location.[2][3][4] For example, an intraocular foreign body (IOFB) located in vitreous or aqueous causes faster onset of OS compared to an IOFB encapsulated in low metabolism tissue such as lens or cornea. Virtually all ocular structures are involved in the siderotic process, resulting in possible glaucoma, cataract, iris color changes, mydriasis, retinal function destruction, and optic nerve atrophy.[1][5]
Etiology
Ocular siderosis is the result of an intra-ocular foreign body containing significant metallic iron. The most common cause is due to injury from hammering (41.7%), chiseling (16.7%), and lathe turning (16.7%).[5][6][7][8][3] Other causes of metallic intraocular foreign bodies include electric welding, traffic accidents, explosion injuries, injury from nail gun, gunshot, or unknown etiology. The most common cause of ocular siderosis is delayed presentation by the patient or missed diagnosis of IOFB after trauma.
Epidemiology
Males aged 22-25 years old are most common patient demographic seen with metallic IOFB injuries.[3][7][9] Approximately 78% to 86% of all IOFBs are metallic. These are most commonly iron, followed by lead.[7][10][11] The most common entry site for IOFBs is the cornea (82.9%), followed by the sclera (11.4%), and the limbus (5.7%).[11] If lodged in the posterior segment, the most likely location is intravitreal (61%), followed by intraretinal (14%) and subretinal (5%).[3][12][9][2]
Pathophysiology
Ocular damage from siderosis can occur directly from cellular damage, or indirectly from vascular damage distal to the IOFB.[13][14]
Direct siderosis refers to direct cellular damage caused by elemental iron ions producing hydroxyl radicals (OH•). This process occurs through the Haber-Weiss and Fenton reaction (shown below), where trivalent or ferric iron (Fe3+) and molecular oxygen radical (O2•-) produce Fe2+, which then goes on to react with hydrogen peroxide to produce the hydroxyl radical (OH•), which is a strong oxidizing agent. Because F3+ is regenerated by this process, and O2•- and H2O2 are naturally produced during aerobic metabolism, only a small amount of Fe3+ is required as a catalyst to continue to drive the reaction.[15][16][17]
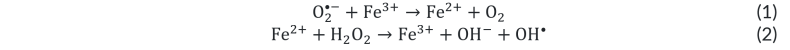
In the retina, OH• can lead to massive release of glutamate, which causes calcium ion (Ca2+) influx, which in turn can trigger subsequent excitotoxicity and apoptosis. The interaction of these three toxic effects is noted in the figure below. Note that this process also likely contributes to toxicity when there is retinal or vitreous hemorrhage as a result of iron in hemoglobin.[19]
Another hypothesis is that intracellular iron accumulation inside lysosomes leads to lysosome dysfunction, causing leakage of ferritin, hemosiderin, and lysosomal contents. This leads to a toxic cascade and eventual cell death.[21][22]
Yet another hypothesis for iron-associated retinal damage associated includes iron-induced activation of the NLRP3 inflammasome, which is an innate immune system signaling complex implicated in pathogenesis of age-related macular degeneration.[14]
Direct siderosis results in pyknosis and degeneration of the photoreceptors and retinal pigment epithelium (RPE), with accompanying inner retinal edema.[23] Note that rods are more susceptible to iron toxicity compared to cones, due to different protective mechanisms against oxidative damage and retinopathic factors, including light-induced degeneration and vitamin A deficiency.[23] In addition, iron can be trafficked by Müller cells cause further damage.[22] Iron ions can even diffuse posterior to anterior across the vitreous to eventually the reach the aqueous. The ions can then access the trabecular meshwork, and then reach the suprachoroidal space, diffuse to the choroid and Bruch’s membrane until finally arriving at the RPE posteriorly.[24][25] Therefore, in the early phase of siderosis, photoreceptors and RPE cells show damage histologically, while Bruch’s membrane, the choroid, and the inner retinal layers are spared.[23]
Indirect siderosis, or vascular siderosis refers to damage to vascular tissue causing tissue damage distal to the location of the foreign body.[26] Iron associates with acid mucopolysaccharides, which are a principal component of vascular adventitia. Iron accumulation leads to toxic microvasculopathy and uncontrolled macrophage activity. This damage occurs in the retinal capillaries, which are the only supply to the internal retinal layers.[26][27] Vascular siderosis occurs with chronic ocular siderosis. Therefore, with the concomitant damage from direct siderosis, all the retinal layers are involved in chronic ocular siderosis.[28]
Studies of the pathologic features of eyes with siderosis have revealed iron deposits accumulated in the regions of ocular pumps i.e., corneal endothelium and Descemet's membrane, trabecular meshwork, pupillary constrictor muscles, dilator muscle, non-pigmented ciliary epithelium, lens epithelium, retinal pigment epithelium, and internal limiting membrane.[29][1] Histologically, Prussian blue stains the iron blue and shows it to be present in all ocular epithelial structures and in areas of trabecular meshwork scarring and retinal gliosis.[30] By electron microscopy, intracellular siderosomes (aggregates of hemosiderin and ferritin, possibly derived from lysosomes) are present in the lens epithelium and in corneal keratocytes.[29]
Clinical Manifestations
The earliest clinical signs of ocular siderosis (OS) are mydriasis and iris heterochromia.[31][32][33] Late and end stage symptoms are progressive loss of visual field and nyctalopia.[4]
Other common signs and symptoms include decreased color vision, cataract development, retinal arteriolar narrowing with pigmentary retinal degeneration, and secondary open angle glaucoma.[4][29][34] Unusually, OS can result in uveitis in the anterior or posterior segment, or as panuveitis.[35][36]
In addition, young patients can develop strabismus or amblyopia, so follow-up is required.[37]
Cornea
Iron usually remains within the epithelial cells that take it up, rather than appearing in the basement membrane. Iron granules may deposit in any layer of the cornea but are usually more prominent in the deeper cornea.[31][38]
Trabecular Meshwork
Siderosis from retained iron IOFB can cause open-angle glaucoma. Breakdown products and iron can accumulate in the trabecular meshwork, producing increased intraocular pressure. This may result in trabecular fibrosclerosis. This glaucoma is usually medically uncontrollable and may require drainage surgery or enucleation. However, incidence is low in ocular siderosis (5%).[4][2][38]
The unilaterality may be confusing, particularly when the previous ocular history is unknown and the foreign body is occult. The iron may be seen clinically in the anterior chamber angle as irregular, scattered, black blotches that may resemble malignant melanoma.
Iris & Pupil
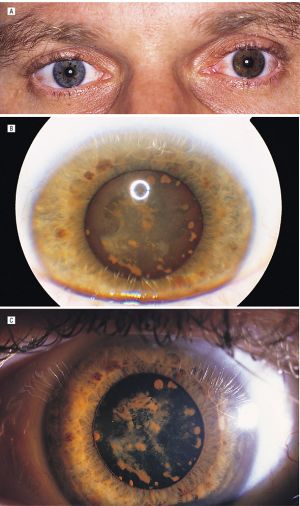
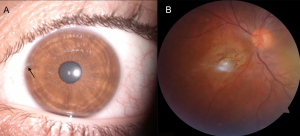
Acquired hyperchromic heterochromia of iris can result from the stromal deposition of iron in siderosis. With siderosis, irides become more brown or rusty, developing after weeks or years.
Anisocoria and abnormal pupillary reaction may be present. Tonic or Adie's pupil has been reported. These patients have been reported to have light-near dissociation, otherwise known as “iron mydriasis.”[32][33][40][41][42][43] This is due to parasympathetic neuropathy resulting from iron deposition in the iris sphincter and dilator muscles. The affected iris may show hypersensitivity to diluted pilocarpine. OS needs to be considered in a patient with tonic or Adie’s pupil, which is thought to be virus or bacterial induced alteration of postganglionic parasympathetic innervation of the sphincter pupillae and ciliary muscle.[33][44] Similarly, these patient’s present with light-near dissociation, or poor light constriction but better and tonic near constriction.
Lens
In siderosis or hemosiderosis the lens epithelium takes on a yellow-brown or rusty appearance from minute dots of intracellular iron, identifiable by Perls or other iron stains.[45] Focal rusty-brown nodules of subcapsular cataract may develop.[29][39] When the foreign body is in the lens there may be progression to a mature cataract, with diffusion of ionizable iron throughout the lens fibers. The iron appears to bind to enzymes within these cells and becomes insoluble and incorporated in phagolysosomes with eventual cellular degeneration.[46][47]
Retina
The iron concentrates mainly in RPE and inner limiting membrane neurosensory retina.[23][29] Necrosis of the photoreceptor cells occurs over large areas of the retina. Electron-dense material deposits between adjacent saccules in the outer segments of the photoreceptors. Functional damage to the retina occurs at a very early stage, before extensive siderosis is apparent, and before stainable iron is detected in retinal tissues.[34] [48][49] Accumulation of intracellular iron leads to inner retinal and RPE degeneration followed by full-thickness retinal degeneration and secondary gliosis. Vitreous degeneration in association with retinal degeneration may lead to rhegmatogenous retinal detachment. OS causes narrowing of arterioles and sheathing with pigmentary retinal degeneration which can appear similar to retinitis pigmentosa.[50][51] Optic atrophy ensues after longstanding disruption of retinal function. Optic disc swelling/hyperemia and cystoid macular edema has also been observed.[15][44][52][53][54]
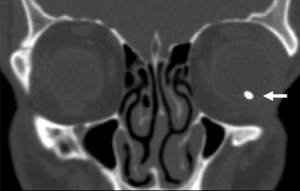
Diagnosis
To avoid ocular siderosis (OS), an IOFB in the cause of penetrating ocular injury must be ruled out, especially where there is a history of possible high-velocity metallic injury. Detailed history of the injury and thorough ophthalmic evaluation of anterior and posterior segments are required. Seidel’s test must be performed for all suspected cases of open globe.[56] With negative Seidel’s test, corneal edema, subconjunctival hemorrhage or iris hole may still indicate an entry wound. Slit lamp exam, gonioscopy, and retinal exam should all be performed to rule out IOFB and evaluate for changes associated with ocular siderosis as enumerated above.
Imaging and Additional Studies
Radiological evaluation is used to rule out an IOFB, as well as assess damage in ocular siderosis. X-ray, computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography (USG), ultrasound biomicroscopy (UBM) and optical coherence tomography (OCT), and electroretinography (ERG) can be used for these purposes. Other tests such as fluorescein angiography (FA), visual field assessment, and electrooculogram can also be used.[57]
USG is both sensitive and specific for detection of both metallic and non-metallic IOFBs, which appear as a hyperechoic lesions with posterior acoustic shadowing on B-scan.[40][58][59][60] USG can also identify lens dislocation, vitreous hemorrhage, retinal or choroidal detachment, and is especially useful when there is obscured view into the eye.[61] However, USG does not provide accurate size measurements, may miss certain IOFBs in the posterior 1/3 of the orbit or within the orbital soft tissues, is operator dependent, and caution must be used when there is possible open-globe.[62][63][64][65]
Non-contrast CT with thin scans (ideally 1–3mm) and coronal reconstruction is the gold-standard for identifying metallic IOFBs, providing accurate location and size of the object. It has high sensitivity for detection (45%-65% ≤ 0.06 mm and 100% > 0.06 mm size), better than orbital x-ray.[40][66][67][68][69][70]
MRI has better sensitivity (95%) than CT for non-metallic IOFB, and allows for visualization of structures without radiation exposure.[71] However, MRI is contraindicated if ferromagnetic foreign bodies are suspected due to possible movement of the IOFB.[72]
Anterior segment OCT, which is requires no contact with the eye and is non-invasive, enables visualization of IOFB in the anterior segment. It can allow for better patient comfort and can be an effective prognostication tool. However, there is poor penetrance beyond the iris, and IOFB behind the iris can be missed.[73][74]
Posterior segment OCT can be useful for accurate localization of an IOFB in the posterior segment or for quantifying amount of cystoid macular edema. It can help also identify encapsulation of a foreign body, which is important in deciding when to proceed with surgery. [4][75]
FA in OS may show hyperfluorescent window defects due to ischemic maculopathy, capillary non-perfusion areas, and RPE changes.[28][57]
Full-field ERG (ffERG) and electrooculogram (EOG) testing is used to study the degree of ocular injury from metallosis and to monitor ocular recovery after metallic foreign body removal, and can be used as an efficient diagnostic and prognosticating tool in both clinical and subclinical ocular siderosis.[76] Initially hypernormal a- and b-waves, followed by a steady decrease in a- and b-wave amplitudes, is seen with iron-containing intraocular foreign bodies in humans. [77][78] The transient increase in a- and b-wave amplitudes in the first week after injury has been attributed to recovery from the ocular trauma before the onset of severe metallic ion injury to the retina and Müller cell responses to ionic changes in the outer retina. Progressive diminution of b wave occurs over 1–2 year period if monitored serially. Other early predictors of OS with ERG include reduction of oscillatory potential amplitude reduction and P1 and N1 wave decreased amplitude with delay in P1-inplict time on multifocal ERG (even in presence of normal ffERG).[79][80][81] In OS, up to 40% of ERG amplitude loss may be reversed with removal of IOFB.[40] EOG changes may not occur in eyes with metallic intraocular foreign bodies presumably because of sparing of the outer segments of the rod and the retinal pigment epithelium. EOG abnormalities indicate severe metallosis and poor visual prognosis.
Management
Acute IOFB
An acute presentation of metallic IOFB requires the patient vaccination status to be determined and administration of tetanus booster accordingly.[82][83] Topical and systemic broad-spectrum antibiotics, such as 3rd or 4th generation fluoroquinolone-moxifloxacin or levofloxacin are to be administered against the most common pathogens such as coagulase-negative Staphyloccus spp., Streptococcus spp., Bacillus spp. and Clostridium spp.[84][85][86] Once the IOFB is identified on imaging, prompt removal is recommended to avoid development of ocular siderosis and endophthalmitis, with a single-setting surgical procedure associated with better visual outcomes.[87]
An anterior segment IOFB should be removed promptly (within 12 h). It may be removed with forceps or intraocular magnet (IOM) through limbal paracentesis. If it is into the lens, the IOFB can be extracted during cataract surgery.[88][89] For IOFBs in the posterior segment, after proper patient counseling about risks and benefits, pars plana vitrectomy can be performed to remove the IOFB with IOM or forceps through limbus or enlarged sclerotomy.[49]
Cataract may be managed by conventional extracapsular extraction or phacoemulsification with posterior chamber intraocular lens. A metallic IOFB located in the ciliary body may be removed after accurate localization.[56]
For more details on management and surgical removal of IOFBs, see Intraocular Foreign Bodies.
Chronic IOFB
Removal of a chronic foreign body is indicated if there is recurrent uveitis or evidence of progressive retinal damage by serial ERG.[90][49] Ferrous IOFBs should be removed in the case where OS is already diagnosed because visual acuity (VA), ERG, and ocular signs may improve. If the IOFB is located in the subretina or clean lens however, progression of OS is likely to remain delayed, and follow-up can be an option with regular assessment of VA, ophthalmic evaluation, and ERG. ERG is an important tool because reduction in b-wave amplitude occurs earlier than clinical signs.[56]
Role of Deferoxamine
Deferoxamine is a free iron chelating agent used to prevent iron overload in patients who receive multiple blood transfusions.[91] Use of subconjunctival deferoxamine (10–100 mg) has been shown to prevent development of damaged due to ocular siderosis. However, in advanced cases of OS, iron is already bound to tissue, and the tissue damage has already occurred and cannot be reversed.[92] In addition, deferoxamine has many toxic side effects, such as bone dysplasia, sensorineural hearing loss, nyctalopia, color vision impairment, reduction in ERG amplitudes, and RPE alteration.[93] Due to these side effects, deferoxamine use is not recommended for management of OS.[93]
Conclusions
IOFBs are often overlooked, and OS develops mainly due to missed diagnosis or delayed presentation. Providers should maintain a high index of suspicion for presence of an IOFB in all cases of ocular injury, especially in young children who may present without apparent history of trauma. It is important to provide education and encouragement to patients on the use of protective glasses, face shields, or goggles for those engaging in high-risk activities. In addition, warnings should be provided about complications of open-globe trauma and ocular siderosis, and counseling should be provided on final prognosis and importance of regular follow-up visits.
References
- ↑ 1.0 1.1 1.2 Casini G, Sartini F, Loiudice P, Benini G, Menchini M. Ocular siderosis: a misdiagnosed cause of visual loss due to ferrous intraocular foreign bodies—epidemiology, pathogenesis, clinical signs, imaging and available treatment options. Documenta Ophthalmologica. 2021;142(2):133-152. doi:10.1007/s10633-020-09792-x
- ↑ 2.0 2.1 2.2 Kannan NB, Adenuga OO, Rajan RP, Ramasamy K. Management of Ocular Siderosis: Visual Outcome and Electroretinographic Changes. Teoh SCB, ed. Journal of Ophthalmology. 2016;2016:7272465.
- ↑ 3.0 3.1 3.2 3.3 Zhu L, Shen P, Lu H, Du C, Shen J, Gu Y. Ocular Trauma Score in Siderosis Bulbi With Retained Intraocular Foreign Body. Medicine (Baltimore). 2015 Sep;94(39):e1533.
- ↑ 4.0 4.1 4.2 4.3 4.4 Ballantyne JF. Siderosis bulbi. Br J Ophthalmol. 1954 Dec;38(12):727-733.
- ↑ 5.0 5.1 Acharya I, Raut AA. Siderosis Bulbi. [Updated 2022 Feb 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK567781/
- ↑ Jonas JB, Knorr HL, Budde WM. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology. 2000 May;107(5):823-8.
- ↑ 7.0 7.1 7.2 Greven CM, Engelbrecht NE, Slusher MM, Nagy SS. Intraocular foreign bodies: management, prognostic factors, and visual outcomes. Ophthalmology. 2000 Mar;107(3):608-12.
- ↑ Kong Y, Tang X, Kong B, Jiang H, Chen Y. Six-year clinical study of firework-related eye injuries in North China. Postgrad Med J. 2015 Jan;91(1071):26-9.
- ↑ 9.0 9.1 Khani SC, Mukai S. Posterior segment intraocular foreign bodies. Int Ophthalmol Clin. 1995 Winter;35(1):151-61.
- ↑ Wu TT, Kung YH, Sheu SJ, Yang CA. Lens siderosis resulting from a tiny missed intralenticular foreign body. J Chin Med Assoc. 2009 Jan;72(1):42-4.
- ↑ 11.0 11.1 Demircan N, Soylu M, Yagmur M, Akkaya H, Ozcan AA, Varinli I. Pars plana vitrectomy in ocular injury with intraocular foreign body. J Trauma. 2005 Nov;59(5):1216-8.
- ↑ Williams DF, Mieler WF, Abrams GW, Lewis H. Results and prognostic factors in penetrating ocular injuries with retained intraocular foreign bodies. Ophthalmology. 1988 Jul;95(7):911-6.
- ↑ Richter GW. The iron-loaded cell--the cytopathology of iron storage. A review. Am J Pathol. 1978;91(2):362-404.
- ↑ 14.0 14.1 Gelfand BD, Wright CB, Kim Y, et al. Iron Toxicity in the Retina Requires Alu RNA and the NLRP3 Inflammasome. Cell Reports. 2015;11(11):1686-1693. doi:10.1016/j.celrep.2015.05.023
- ↑ 15.0 15.1 Sandhu HS, Young LH. Ocular Siderosis. International Ophthalmology Clinics. 2013;53(4):177-184.
- ↑ Dziubla T, Butterfield DA. 1.2.4 Fenton/Haber-Weiss Chemistry. In: Oxidative Stress and Biomaterials. Elsevier/Academic Press; 2016:12-14.
- ↑ Casini G, Sartini F, Loiudice P, Benini G, Menchini M. Ocular siderosis: a misdiagnosed cause of visual loss due to ferrous intraocular foreign bodies—epidemiology, pathogenesis, clinical signs, imaging and available treatment options. Documenta Ophthalmologica. 2021;142(2):133-152. doi:10.1007/s10633-020-09792-x
- ↑ Casini G, Sartini F, Loiudice P, Benini G, Menchini M. Ocular siderosis: a misdiagnosed cause of visual loss due to ferrous intraocular foreign bodies—epidemiology, pathogenesis, clinical signs, imaging and available treatment options. Documenta Ophthalmologica. 2021;142(2):133-152. doi:10.1007/s10633-020-09792-x
- ↑ Chao HM, Chen YH, Liu JH, et al. Iron-generated hydroxyl radicals kill retinal cells in vivo: effect of ferulic acid. Hum Exp Toxicol. 2008;27(4):327-339. doi:10.1177/0960327108092294
- ↑ Chao HM, Chen YH, Liu JH, et al. Iron-generated hydroxyl radicals kill retinal cells in vivo: effect of ferulic acid. Hum Exp Toxicol. 2008;27(4):327-339. doi:10.1177/0960327108092294
- ↑ Knave B. Electroretinography in eyes with retained intraocular metallic foreign bodies. Acta Ophthalmol. 1969;100:(suppl):5–63.
- ↑ 22.0 22.1 Tawara A. Transformation and cytotoxicity of iron in siderosis bulbi. Invest Ophthalmol Vis Sci. 1986;27(2):226-236.
- ↑ 23.0 23.1 23.2 23.3 Masciulli L, Anderson DR, Charles S. Experimental Ocular Siderosis in the Squirrel Monkey. American Journal of Ophthalmology. 1972;74(4):638-661. doi:10.1016/0002-9394(72)90826-4
- ↑ Appel I, Barishak YR. Histopathological Changes in Siderosis Bulbi. Ophthalmologica. 1978;176(4):205-210.
- ↑ Gerkowicz K, Prost M. Experimental Investigations on the Penetration into the Eyeball of Iron Administered Intraorbitally. Ophthalmologica. 1984;188(4):239-242.
- ↑ 26.0 26.1 Cibis PA, Yamashita T. Experimental Aspects of Ocular Siderosis and Hemosiderosis*. American Journal of Ophthalmology. 1959;48(5):465-480.
- ↑ Dhoble P, Velis G, Sivakaumar P. Encapsulated metallic intraocular foreign body of long duration presenting with cystoid macular edema and normal full-field electroretinogram. Oman J Ophthalmol. 2019;12(1):50-52.
- ↑ 28.0 28.1 Shaikh S, Blumenkranz MS. Fluorescein angiographic findings in ocular siderosis. American Journal of Ophthalmology. 2001;131(1):136-138.
- ↑ 29.0 29.1 29.2 29.3 29.4 Hope-Ross M, Mahon GJ, Johnston PB. Ocular siderosis. Eye (Lond). 1993;7 (Pt 3):419-425.
- ↑ Yang Z,Yang XL,Xu LS,Dai L,Yi MC. Application of Prussian blue staining in the diagnosis of ocular siderosis. Int J Ophthalmol 2014;7(5):790-794.
- ↑ 31.0 31.1 Barr CC, Vine AK, Martonyi CL. Unexplained heterochromia. Intraocular foreign body demonstrated by computed tomography. Survey of Ophthalmology. 1984;28(5):409-411.
- ↑ 32.0 32.1 Welch RB. Two remarkable events in the field of intraocular foreign body: (1) The reversal of siderosis bulbi. (2) The spontaneous extrusion of an intraocular copper foreign body. Trans Am Ophthalmol Soc. 1975;73:187-203.
- ↑ 33.0 33.1 33.2 Monteiro ML, Ulrich RF, Imes RK, Fung WE, Hoyt WF. Iron mydriasis. Am J Ophthalmol. 1984 Jun;97(6):794-796
- ↑ 34.0 34.1 Kuhn F, Kovacs B. Management of postequatorial magnetic intraretinal foreign bodies. Int Ophthalmol. 1989 Sep;13(5):321-325.
- ↑ Politis M, Rosin B, Amer R. Ocular Siderosis Subsequent to a Missed Pars Plana Metallic Foreign Body that Masqueraded as Refractory Intermediate Uveitis. Ocul Immunol Inflamm. 2018;26(4):598-600.
- ↑ Yeh S, Ralle M, Phan IT, Francis PJ, Rosenbaum JT, Flaxel CJ. Occult intraocular foreign body masquerading as panuveitis: inductively coupled mass spectrometry and electrophysiologic analysis. J Ophthalmic Inflamm Infect. 2012 Jun;2(2):99-103
- ↑ Meier P. Combined anterior and posterior segment injuries in children: a review. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2010;248(9):1207-1219.
- ↑ 38.0 38.1 Kearns M, McDonald R. Generalised siderosis from an iris foreign body. Aust J Ophthalmol. 1980 Nov;8(4):311-313.
- ↑ 39.0 39.1 Pollack A, Oliver M. Reversal of Siderosis. Archives of Ophthalmology. 1998;116(5):678-679.
- ↑ 40.0 40.1 40.2 40.3 Weiss MJ, Hofeldt AJ, Behrens M, Fisher K. Ocular siderosis. Diagnosis and management. Retina. 1997;17(2):105-108.
- ↑ Verhoeff FH. SIDEROSIS BULBI. Br J Ophthalmol. 1918 Nov;2(11):571-573.
- ↑ Black NM. Siderosis Bulbi with Dilated Inactive Pupil. Recovery of Pupillary Activity After Removal of Foreign Body. Trans Am Ophthalmol Soc. 1923;21:171-182.
- ↑ Wilhelm H. Neuro-ophthalmology of pupillary function--practical guidelines. J Neurol. 1998 Sep;245(9):573-83.
- ↑ 44.0 44.1 Dowlut MS, Curragh DS, Napier M, et al. The varied presentations of siderosis from retained intraocular foreign body. Clinical and Experimental Optometry. 2019;102(1):86-88.
- ↑ Wolkow N, Song Y, Wu TD, Qian J, Guerquin-Kern JL, Dunaief JL. Aceruloplasminemia: retinal histopathologic manifestations and iron-mediated melanosome degradation. Arch Ophthalmol. 2011;129(11):1466-1474.
- ↑ Siantar RG, Agrawal R, Heng LW, Ho BC. Histopathologically proven siderotic cataract with disintegrated intralenticular foreign body. Indian J Ophthalmol. 2013 Jan-Feb;61(1):30-32
- ↑ Rychener RO. Siderosis following intralenticular foreign body. Am J Ophthalmol. 1946 Mar;29:346
- ↑ Knave B. Long-term changes in retinal function induced by short, high intensity flashes. Experientia. 1969;25(4):379-380.
- ↑ 49.0 49.1 49.2 Sneed SR, Weingeist TA. Management of Siderosis Bulbi due to a Retained Ironcontaining Intraocular Foreign Body. Ophthalmology. 1990;97(3):375-379.
- ↑ Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Progress in Retinal and Eye Research. 2016;51:125-155.
- ↑ Yamaguchi K, Tamai M. Siderosis bulbi Induced by Intraocular Lens Implantation (With 1 color plate). Ophthalmologica. 1989;198(3):113-115.
- ↑ Slusher MM, Sarin LK, Federman JL. Management of intraretinal foreign bodies. Ophthalmology. 1982 Apr;89(4):369-373.
- ↑ Elgin U, Eranil S, Simsek T, Batman A, Ozdamar Y, Ozkan SS. Siderosis Bulbi Resulting From an Unknown Intraocular Foreign Body. Journal of Trauma and Acute Care Surgery. 2008;65(4).
- ↑ Xie H, Chen S. Ocular siderosis. Eye Sci. 2013;28(2):108-112.
- ↑ Pinto A, Brunese L, Daniele S, et al. Role of Computed Tomography in the Assessment of Intraorbital Foreign Bodies. Seminars in Ultrasound, CT and MRI. 2012;33(5):392-395. doi:10.1053/j.sult.2012.06.004
- ↑ 56.0 56.1 56.2 Katz SE. Ocular trauma: principles and practice. Optom Vis Sci. 2003;80(3):196.
- ↑ 57.0 57.1 Schocket SS, Lakhanpal V, Varma SD. Siderosis from a retained intraocular stone. Retina. 1981;1(3):201-207.
- ↑ Laroche D, Ishikawa H, Greenfield D, Liebmann JM, Ritch R. Ultrasound biomicroscopic localization and evaluation of intraocular foreign bodies. Acta Ophthalmol Scand. 1998;76(4):491–495.
- ↑ Costa MA, Garcia PN, Barroso LF, Ferreira MA, Okuda EA, Allemann N. Composition of intraocular foreign bodies: experimental study of ultrasonographic presentation. Arquivos brasileiros de oftalmologia. 2013;76(1):13–17.
- ↑ Thickman DI, Ziskin MC, Goldenberg NJ, Linder BE. Clinical manifestations of the comet tail artifact. Journal of Ultrasound in Medicine. 1983;2(5):225-230.
- ↑ Deramo VA, Shah GK, Baumal CR, Fineman MS, Correa ZM, Benson WE, Rapuano CJ, Cohen EJ, Augsburger JJ. The role of ultrasound biomicroscopy in ocular trauma. Trans Am Ophthalmol Soc. 1998;96:355–365
- ↑ Cascone G, Filippello M, Ferri R, Scimone G, Zagami A. B-scan echographic measurement of endobulbar foreign bodies. Ophthalmologica. 1994;208(4):192-194.
- ↑ McNicholas MM, Brophy DP, Power WJ, Griffin JF. Ocular trauma: evaluation with US. Radiology. 1995 May;195(2):423-427.
- ↑ Weisman RA, Savino PJ, Schut L, Schatz NJ. Computed tomography in penetrating wounds of the orbit with retained foreign bodies. Arch Otolaryngol. 1983 Apr;109(4):265-268.
- ↑ Wang K, Liu J, Chen M. Role of B-scan ultrasonography in the localization of intraocular foreign bodies in the anterior segment: a report of three cases. BMC Ophthalmol. 2015 Aug 14;15:102.
- ↑ Saeed A, Cassidy L, Malone DE, Beatty S. Plain X-ray and computed tomography of the orbit in cases and suspected cases of intraocular foreign body. Eye (Lond). 2008 Nov;22(11):1373-1377.
- ↑ Chacko JG, Figueroa RE, Johnson MH, Marcus DM, Brooks SE. Detection and localization of steel intraocular foreign bodies using computed tomography. A comparison of helical and conventional axial scanning. Ophthalmology. 1997 Feb;104(2):319-323.
- ↑ Lit ES, Young LH. Anterior and posterior segment intraocular foreign bodies. Int Ophthalmol Clin. 2002 Summer;42(3):107-120.
- ↑ Otto PM, Otto RA, Virapongse C, Friedman SM, Emerson S, Li KC, Malot R, Kaude JV, Staab EV. Screening test for detection of metallic foreign objects in the orbit before magnetic resonance imaging. Invest Radiol. 1992 Apr;27(4):308-311.
- ↑ Lakits A, Prokesch R, Scholda C, Bankier A. Orbital helical computed tomography in the diagnosis and management of eye trauma. Ophthalmology. 1999;106(12):2330–2335.
- ↑ Weber AL, Caruso P, Sabates NR. The optic nerve: radiologic, clinical, and pathologic evaluation. Neuroimaging Clin N Am. 2005 Feb;15(1):175-201.
- ↑ Ta CN, Bowman RW. Hyphema caused by a metallic intraocular foreign body during magnetic resonance imaging. Am J Ophthalmol. 2000 Apr;129(4):533-534.
- ↑ Mansouri K, Sommerhalder J, Shaarawy T. Prospective comparison of ultrasound biomicroscopy and anterior segment optical coherence tomography for evaluation of anterior chamber dimensions in European eyes with primary angle closure. Eye (Lond). 2010 Feb;24(2):233-239.
- ↑ Wylegala E, Dobrowolski D, Nowińska A, Tarnawska D. Anterior segment optical coherence tomography in eye injuries. Graefes Arch Clin Exp Ophthalmol. 2009 Apr;247(4):451-455.
- ↑ Chao HM, Chen SJ, Hsu WM, Lee FL, Chen KH. Siderosis oculi: visual dysfunctions even after iron removal: a role of OCT. Cutan Ocul Toxicol. 2006;25(2):131-140.
- ↑ Karpe G. Early diagnosis of siderosis retina by the use of electroretinography. Doc Ophthalmol. 1948;2(1 vol.):277-96.
- ↑ Declercq SS, Meredith PC, Rosenthal AR. Experimental siderosis in the rabbit: correlation between electroretinography and histopathology. Arch Ophthalmol. 1977 Jun;95(6):1051-8.
- ↑ Imaizumi M, Matsumoto CS, Yamada K, Nanba Y, Takaki Y, Nakatsuka K. Electroretinographic assessment of early changes in ocular siderosis. Ophthalmologica. 2000 Sep-Oct;214(5):354-359.
- ↑ Tanabe J, Shirao Y, Oda N, Kawasaki K. Evaluation of retinal integrity in eyes with retained intraocular metallic foreign body by ERG and EOG. Doc Ophthalmol. 1992;79(1):71-78.
- ↑ Gupta S, Midha N, Gogia V, Sahay P, Pandey V, Venkatesh P. Sensitivity of multifocal electroretinography (mfERG) in detecting siderosis. Can J Ophthalmol. 2015 Dec;50(6):485-490.
- ↑ Sahay P, Kumawat D, Gupta S, Tripathy K, Vohra R, Chandra M, Venkatesh P. Detection and monitoring of subclinical ocular siderosis using multifocal electroretinogram. Eye (Lond). 2019 Oct;33(10):1547-1555.
- ↑ Liang JL, Tiwari T, Moro P, et al. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018; 67:1.
- ↑ Havers FP, Moro PL, Hunter P, et al. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the Advisory Committee on Immunization Practices - United States, 2019. MMWR Morb Mortal Wkly Rep 2020; 69:77.
- ↑ Yeh S, Colyer MH, Weichel ED. Current trends in the management of intraocular foreign bodies. Curr Opin Ophthalmol. 2008;19(3):225–233.
- ↑ Bhagat N, Nagori S, Zarbin M. Post-traumatic infectious endophthalmitis. Surv Ophthalmol. 2011;56(3):214–251.
- ↑ Cornut PL, Youssef el B, Bron A, Thuret G, Gain P, Burillon C, Romanet JP, Vandenesch F, Maurin M, Creuzot-Garcher C, Chiquet C, French Institutional Endophthalmitis Study G. A multicentre prospective study of post-traumatic endophthalmitis. Acta Ophthalmol. 2013;91(5):475–482.
- ↑ Falavarjani KG, Hashemi M, Modarres M, Parvaresh MM, Naseripour M, Nazari H, Fazel AJ. Vitrectomy for posterior segment intraocular foreign bodies, visual and anatomical outcomes. Middle East Afr J Ophthalmol. 2013;20(3):244–247.
- ↑ Foss AJ, Forbes JE, Morgan J. An intralenticular foreign body and a clear lens. Brit J Ophthalmol. 1993;77(12):828.
- ↑ Pieramici DJ, Capone A Jr, Rubsamen PE, Roseman RL. Lens preservation after intraocular foreign body injuries. Ophthalmology. 1996;103(10):1563–1567.
- ↑ Kuhn F, Witherspoon CD, Skalka H, Morris R. Improvement of siderotic ERG. Eur J Ophthalmol. 1992 Jan-Mar;2(1):44-45.
- ↑ Kwiatkowski JL. Current recommendations for chelation for transfusion-dependent thalassemia. Ann N Y Acad Sci. 2016 Mar;1368(1):107-114.
- ↑ Wise JB. Treatment of experimental siderosis bulbi, vitreous hemorrhage, and corneal blood staining with deferoxamine. Arch Ophthalmol. 1966 May;75(5):698-707.
- ↑ 93.0 93.1 Baath JS, Lam WC, Kirby M, Chun A. Deferoxamine-related ocular toxicity: incidence and outcome in a pediatric population. Retina. 2008 Jun;28(6):894-899.