Pegcetacoplan
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Intravitreal pegcetacoplan (Syfovre; Apellis Pharmaceuticals, Inc.) was approved in 2023 in the United States as a treatment for geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Background
Geographic atrophy is a late stage of non-neovascular age-related macular degeneration and affects almost one million people in the United States.[1][2] GA significantly reduces a person's quality of life by hindering the ability to read, drive, and recognize faces, among other visual activities that require good central vision.[2][3] Prior to 2023, no treatment was available for this debilitating disease and patients were encouraged to avoid risk factors for progression and were directed to supplements and behaviors that reduce the development of advanced disease.[4] In February of 2023, the FDA approved the first in class treatment for GA with the approval of pegcetacoplan (Syfovre), providing hope that this blinding disease could potentially be slowed.
Disease Entity
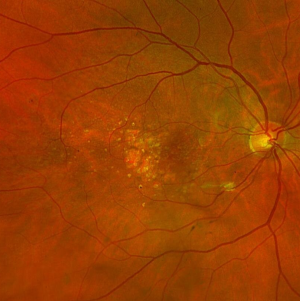
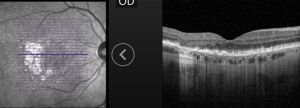
Geographic atrophy is the loss of outer retinal and retinal pigment epithelial tissue due to AMD. This complex and chronic process can be diagnosed through multimodal imaging. On fundus examination, GA lesions are scalloped areas of depigmentation surrounded by areas of drusen and pigmentary changes (Figure 1). On optical coherence tomography (OCT), the areas of atrophy correspond to outer retinal bands loss, loss of the retinal pigment epithelium and choroidal hypertransmission (Figure 2).
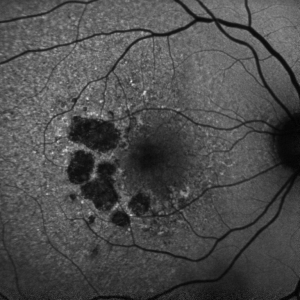
Fundus autofluorescence (FAF) can be helpful in the diagnosis, monitoring and prognosis of GA. Lesions can be readily identified as areas of dense hypoautofluorescence in the macula with variable surrounding autofluorescence (Figure 3). The high contrast between GA and less affected tissue can aid in monitoring disease progression. The degree of hyperautofluorescence surrounding GA lesions heralds further GA progression, and the absence of hyperautofluorescence was an exclusion criteria in major trials due to the slower rate of progression of this subtype.
Other causes of macular atrophy that need to be considered are atrophic lesions from genetic retinal degenerations, prior central serous chorioretinopathy, prior laser treatment and other pathologies. The presence of surrounding drusen or subretinal drusenoid deposits supports the diagnosis of AMD. Other causes of macular atrophy are not currently candidates for treatment with pegcetacoplan.
Mechanism of Action
C3 is a central component to the three complement activation pathways: classical, alternative, and mannose-binding lectin [1][2]. Each of these pathways forms C3 convertase, which catalyzes the hydrolysis of C3 to C3a and C3b, which then activates the common pathway of the complement cascade [5]. Once activated, there is enhanced phagocytosis of foreign and/or damaged materials, inflammation, and activation of the membrane attack complex (MAC) [5].
C3 activation and/or degradation is associated with increasing risks of exudative and atrophic forms of AMD [2]. Components of drusen, lipofuscin, and other oxidative stress products are believed to trigger inflammation via the complement cascade and via the NLRP3 inflammasome [6]. Complement activation products, like C3a and C3b, are often found within drusen of AMD patients and C3 is also elevated in AMD patient’s plasma [7][6]. Studies have found that MAC is elevated in the choriocapillaris in patient eyes with the higher risk CFH allele [6]. There is also higher systemic complement activation in AMD patients compared to non-AMD control patients [5]. Because C3 is involved with all three complement pathways, inhibiting its activation may be a good target in slowing progression of GA [2][3][8].
Pegcetacoplan is a pegylated complement C3 inhibitor peptide that inhibits the C3 convertase, preventing cleavage of C3 into C3a and C3b, which prevent an inflammatory response and opsonization, respectively [1][2]. C3b also supports the enzyme needed to cleave C5 into C5a and C5b, which promote inflammatory reaction and the membrane attack complex responsible to cellular destruction & death [1]. Therefore, inhibiting the complement systems at the level of C3 is suspected to be therapeutically beneficial to manage GA [1].
Clinical Trials
Two Phase 3 studies, OAKS and DERBY, were conducted with a total of 1258 patients and evaluated the safety and efficacy of pegcetacoplan in GA due to AMD [1]. (Goldberg et al., 2022;) Patients were either administered pegcetacoplan monthly or bimonthly or administered a sham monthly or bimonthly.
Clinical Efficacy
Both OAKS and DERBY trials found that when pegcetacoplan was administered, there was a reduction in lesion growth rate when compared to the sham administered group at 24 months (Table 1) [1]. The OAKS study showed that by month 24, pegcetacoplan administration reduces GA lesion growth compared to sham by 22% and 19% in monthly and every other month treatment groups, respectively [9][10] The DERBY trial found that by month 24, pegcetacoplan reduces GA lesion growth when compared to sham administration by 18% and 17% in the monthly and every other month groups, respectively [9][10]
Table 1. 24-month efficacy results [9].
| Percent Reduction in GA Growth | ||
| Treatment Arm | OAKS | DERBY |
| Monthly | 22 | 19 |
| Every Other Month | 18 | 16 |
In both studies, when comparing the effect of pegcetacoplan treatment between months 12 to 18 and months 18 to 24, there was an accelerated effect as compared to sham administration.[9] This appeard to be more due to slowed lesion growth rate in treatment groups and not a result of increased lesion growth rate in the sham group.[9] The increased therapeutic benefit that was demonstrated over each sequential six month time period supports the hypothesis that further reduction in disease progression can develop with long-term treatment.
Extrafoveal GA is known to progress at a faster rate than foveal-involving lesions. In the extrafoveal subgroup, treatment with pegcetacoplan reduced lesion growth by 26% and 21% in the monthly and every other months group, respectively. [11] Lower rates of benefit were seen in foveal GA. [11]
Contraindications and Adverse Events
Pegcetacoplan should not be used if there are ocular or periocular infections, nor if there is active intraocular inflammation. The most common adverse effects include: conjunctival hemorrhage, vitreous floaters, choroidal neovascularization, eye pain and/or discomfort, visual impairment, intraocular inflammation, and increased intraocular pressure (Table 2).[8][12][13] Other less common but serious adverse effects include: endophthalmitis, optic ischemic neuropathy, and retinal detachment. [8][12]
Table 2. Side effects data [13].
| Percent of Patients Experiencing Side Effect | |||
| Side Effect | Monthly Treatment Arm | Every Other Month Treatment Arm | Sham Pooled |
| Choroidal Neovascularization | 12 | 7 | 3 |
| Intraocular Inflammation | 4 | 2 | <1 |
| Ischemic Optic Neuropathy | 1.7 | 0.2 | 0 |
| Endophthalmitis | <1 | <1 | <1 |
| Vitreous Floaters | 10 | 7 | 1 |
The side effects of intraocular inflammation, endophthalmitis and retinal detachment are found with all anti-vascular endothelial growth factor (anti-VEGF) agents, which are intravitreal injections used to treated exudative AMD. Two emerging side effects with pegcetacoplan include choroidal neovascularization (CNV) and ischemic optic neuropathy.[13] The risks of choroidal neovascularization was proportional to the frequency of injection, and is considerably higher in the monthly treatment arm (12%) than every other month (7%) and sham groups (3%).[13] The pathogenesis for the increased risk of CNV remains unclear. During the clinical trials, patients who developed CNV continued their treatment arm in addition to receiving anti-VEGF injections.
The rate of ischemic optic neuropathy (ION) was 1.7% in the monthly group and 0.2% in the every other month group, compared to no events in the sham group.[13] Per the trial organizers (unpublished data, verbal communication) only patients with traditional risk factors for nonarteritic anterior ischemic optic neuropathy developed ION. It remains to be seen if this side effect was coincidental or if an association truly exists. It also remains to be clarified if these cases were arteritic or non-arteritic.
Following launch of the medication after FDA approval, a handful of cases of retinal vasculitis developed, with visual outcomes ranging from mild impairement to permanent and complete vision loss.[14] While likely extremely rare, the exact cause and frequency of this feared side effect remains elusive. Initially, there was a significant drop in the use of the medication but providers and patients appear increasingly willing to try the treatment again. [15]
Injection: Dosing, Administration, Preparation
Pegcetacoplan is supplied as a sterile, clear solution placed in a single-use vial that should be refrigerated at 2-8 degrees C.[13] The vial comes with enough volume to deliver 0.1 mL of 15 mg/mL with extra medication to aid with syringe and needle loading. The contents of the vial are pulled up through a large-bore needle without a filter. The loading of the medication into a 1 CC syringe is similar to other medications, with the major difference being the high viscosity of Syfovre with extra vacuum needed when pulling up from the vial. To reduce viscosity, it is recommended to allow the vial to warm at room temperature for 15 minutes prior to loading. The medication is typically injected through a thin-walled 29-gauge needle, which is equivalent to the diameter of a typical 27 gauge needle. Before injection, the eye should be anesthetized and prepped in usual intravitreal injection fashion.
A single-dose vial of Pegcetacoplan costs $2190 during wholesale acquisition as of June 2023.[16]
More information on injection preparation and administration can be found on the FDA label: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217171s000lbl.pdf
Patient Selection and Controversy
Some controversy exists about the use of this new medication. As of mid 2023, a spectrum of enthusiasm exists within the retina community, with some providers not planning to use the medication and others who see this is a vital treatment.[17] A slightly higher efficacy with monthly treatment is combined with higher risks of CNV, ION and an increased treatment burden for patients and their families. Thus, while proving clinically beneficial, some providers argue that the risks and downsides of monthly treatment may outweigh the benefits.
The medication slows the progression of GA on FAF, but it remains unclear how this will translate to reduced rates of vision loss and quality of life decline. Analyses of extrafoveal lesions and perlesional sensitivity do point to a functional benefit with treatment.[18] Based on these results, some argue that the medication not only improves structural outcomes but functional outcomes as well.
Numerous clinical and social circumstances will be taken into account when advising patients on treatment. Some of these include baseline vision, size and distribution of GA lesions, documented progression of disease, presence of risks factors for progression, life expectancy, social support to make frequent clinic appointments, enthusiasm and injection tolerance.
In the OAKS phase 3 trials investigating Syfovre in GA, 31% of patients in the monthly group, 21% of patients in every other month group, and 25% of patients assigned to sham groups discontinued treatment prior to month 24 . Similar drop out rates were seen in the DERBY trial.[13] Typically, clinical trials have higher rates of patient retainment than the “real-world” due to additional resources that are available in clinical trials to remind and aid patients to make their appointments and due to patient motivation. The high rate of patient drop out is likely related to a demanding treatment regimen in an older population without any noticeable treatment benefit, only a slowing of visual decline that is not readily apparent to the patient. It wouldn’t be surprising if even higher rates of treatment discontinuation occurred in the real-world application of this treatment.
As with any significant change in therapeutic options, more will be learned about the treatment, it’s tolerability and risks. The GALE phase 3 study is an extension study that aims to evaluate longer-term safety and efficacy of pegcetacoplan injection in patients with GA, as well as any changes in size of GA lesions.[9]
Conclusions
Pegcetacoplan is the first FDA approved drug to treat GA secondary to AMD. It has been found to slow progression of GA lesion growth in patients with dry AMD in two clinical trials. With this benefit being a fairly mild reduction in lesion growth, a nuanced conversation will be needed between provider and patient when formulating a personalized treatment plan.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Khan, H., Aziz, A. A., Sulahria, H., Ahmed, A., Choudhry, N., Narayanan, R., . . . Khanani, A. M. (2023). Emerging Treatment Options for Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration. Clin Ophthalmol, 17, 321-327. https://doi.org/10.2147/opth.s367089
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Liao, D. S., Grossi, F. V., El Mehdi, D., Gerber, M. R., Brown, D. M., Heier, J. S., . . . Francois, C. G. (2020). Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology, 127(2), 186-195. https://doi.org/10.1016/j.ophtha.2019.07.011
- ↑ 3.0 3.1 Desai, D., & Dugel, P. U. (2022). Complement cascade inhibition in geographic atrophy: a review. Eye (Lond), 36(2), 294-302. https://doi.org/10.1038/s41433-021-01765-x
- ↑ Sacconi, R., Corbelli, E., Querques, L., Bandello, F., & Querques, G. (2017). A Review of Current and Future Management of Geographic Atrophy. Ophthalmol Ther, 6(1), 69-77. https://doi.org/10.1007/s40123-017-0086-6
- ↑ 5.0 5.1 5.2 Lin JB, Halawa OA, Miller JW, Vavvas DG. Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results. J Clin Med. 2021;10(13):2890. Published 2021 Jun 29. doi:10.3390/jcm10132890
- ↑ 6.0 6.1 6.2 Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. THE PATHOPHYSIOLOGY OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION AND THE COMPLEMENT PATHWAY AS A THERAPEUTIC TARGET. Retina. May 2017;37(5):819-835. doi:10.1097/iae.0000000000001392
- ↑ Alekseev O, Lad EM, Steinle N. The complement pathway in geographic atrophy explained. Retina Specialist. February 18, 2021. https://www.retina-specialist.com/article/the-complement-pathway-in-geographic-atrophy-explained.
- ↑ 8.0 8.1 8.2 Apellis Pharmaceuticals. SyfovreTM (Pegcetacoplan Injection). Syfovre. 2023. https://syfovre.com/.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 Apellis announces 24-month results showing increased effects over time with pegcetacoplan in phase 3 derby and Oaks Studies in geographic atrophy (GA). Apellis Pharmaceuticals, Inc. August 24, 2022. https://investors.apellis.com/news-releases/news-release-details/apellis-announces-24-month-results-showing-increased-effects.
- ↑ 10.0 10.1 Goldberg, R., Heier, J. S., Wykoff, C. C., Staurenghi, G., Singh, R. P., Steinle, N., . . . Ribeiro, R. (2022). Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies. Investigative Ophthalmology & Visual Science, 63(7), 1500-1500. Hutton, D. (2023, April 24). ARVO 2023: Data outlines Phase 3 functional analyses of pegcetacoplan injection for geographic atrophy. https://www.ophthalmologytimes.com/view/arvo-2023-data-outlines-phase-3-functional-analyses-of-pegcetacoplan-injection-for-geographic-atrophy
- ↑ 11.0 11.1 Apellis announces detailed 18-month results from phase 3 derby and Oaks Studies of Pegcetacoplan for Geographic Atrophy (GA) at arvo Annual meeting. Apellis Pharmaceuticals, Inc. May 2, 2022. https://investors.apellis.com/news-releases/news-release-details/apellis-announces-detailed-18-month-results-phase-3-derby-and.
- ↑ 12.0 12.1 Liao DS, Metlapally R, Joshi P. Pegcetacoplan treatment for geographic atrophy due to age-related macular degeneration: a plain language summary of the FILLY study. Immunotherapy. 2022;14(13):995-1006. doi:10.2217/imt-2022-0078
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Highlights of prescribing information - Apellis. February 2023. https://pi.apellis.com/files/PI_Syfovre.pdf.
- ↑ https://www.healio.com/news/ophthalmology/20230729/asrs-rest-committee-sheds-light-on-timeline-of-syfovre-inflammation-reports
- ↑ https://www.fiercepharma.com/pharma/apellis-stumbling-ga-drug-syfovre-shows-signs-recovery
- ↑ Pegcetacoplan (Syfovre) for geographic atrophy in age-related macular degeneration. Med Lett Drugs Ther. 2023;65(1673):49-50. doi:10.58347/tml.2023.1673a
- ↑ Kaiser PK. Upfront: The Beginning of Geographic Atrophy Treatment. Retinalphysician.com. April 1, 2023. https://www.retinalphysician.com/issues/2023/april-2023/upfront-the-beginning-of-geographic-atrophy-treatm.
- ↑ Hutton, D. (2023, April 24). ARVO 2023: Data outlines Phase 3 functional analyses of pegcetacoplan injection for geographic atrophy. https://www.ophthalmologytimes.com/view/arvo-2023-data-outlines-phase-3-functional-analyses-of-pegcetacoplan-injection-for-geographic-atrophy