Best Disease and Bestrophinopathies
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Background
Mutations in the BEST1 gene are causally associated with an increasing number of inherited ophthalmic diseases, which have collectively been termed “bestrophinopathies”[1]. These have initially included inherited retinal degenerative diseases, including Best vitelliform macular dystrophy (BVMD, also known as Best's Disease), one of the most common inherited macular diseases, autosomal recessive bestrophinopathy (ARB), and autosomal dominant vitreoretinochoroidopathy (ADVIRC), among others. However, BEST1 mutations have also been implicated in more complex ophthalmic diseases with anterior segment involvement, namely autosomal dominant microcornea, rod-cone dystrophy, early-onset cataract, posterior staphyloma (MRCS) syndrome.
The increasing body of evidence on the implication of the bestrophin 1 protein (Best1), encoded by the BEST1 gene, in ocular degenerative diseases has made Best1 protein the subjective of intensive research to further understand the physiology of the RPE and for the development of novel therapies.
Definition
Bestrophinopathy is a term enclosing an heterogeneous group of phenotypes of degenerative eye diseases caused by the BEST genes, specifically the BEST1 gene [2] [3]. Diseases involving mutations in BEST1 gene belong thus to a spectrum of diseases characterized by abnormal ocular development which extends beyond the retina. The BEST1 mutation spectrum underlying bestrophinopathies involves over 250 known mutations [4] [5]. In addition, phenotypical differences have been noted between unrelated patients harboring the same mutation and also within families, including age of onset and rate of disease progression [1] [2].
This phenotypic and allelic heterogeneity highlights a significant phenotypic overlap among BEST1-linked disorders, which poses significant diagnostic and prognostic challenges. The pleiotropic effects of BEST1 gene mutations has risen the hypothesis that other unknown factors may play a role in bestrophinopathies, including genetic modifiers, Best1 protein interactors, and environmental components [3] [6].
Interestingly, a decreased electro-oculographic (EOG) Arden ratio (light peak / dark trough) is a hallmark of all bestrophinopathies [7]. This clinical finding has allowed further understanding of the biologic role of Best proteins in the human eye.
The bestrophins
Biology
The bestrophin family of proteins are encoded by four genes in the human genome; two of the Best proteins are known to be expressed in the human eye [8]. The bestrophin genes share a conserved gene structure, but each of the four genes has a unique 3-prime end of variable length [9].
Bestrophins are transmembrane proteins which share a homology region containing a high content of aromatic residues, including an invariant arg-phe-pro (RFP) motif [9].
BEST1 gene
The BEST1 gene (also known as VMD2 gene) is located on the long arm of chromosome 11q12, spanning 11.5 kb of human DNA and containing 11 exons, of which 10 are protein-encoding [10] [11]. BEST1 encodes for Best1 protein, which localizes to the basolateral membrane of the retinal pigment epithelium (RPE) [12]. Best1 protein may also be present intracellularly [13] [14]; the potential implications of this Best1 sub-population in ocular physiology and disease remain to be elucidated [14].
The expression of Best1 is higher in the peripheral than in the macular RPE [15], and this macular-peripheral difference may account for the ocular phenotypes in BVMD and ADVIRC. Missense mutations causing BVMD may cause only macular degeneration because the peripheral RPE may be able to maintain the ionic milieu of the subretinal space even with only one functioning copy of the gene, whereas BEST1-splicing defects cause more severe effects and have been implicated in the more generalized ADVIRC disease [15].
No Best1 protein expression has been found in the neurosensory retina (NSR), ciliary body, iris, cornea, or lens.[1]
Systemically, Best1 has also been detected in the following organs: [1] [11]
- kidney,
- central nervous system (brain and dorsal root ganglion), and
- testis.
Best1 has been detected in astrocytes of hippocampus (dentate gyrus and CA1 regions) and in the cerebellar Punkinje cells, Bergmann glia and lamellar astrocytes [16] [17]. Best1 redistribution within reactive astrocytes in the hyppocampal areas suggests that Best1 may play an important role in the astrocyte physiology as well as in neurologic disease such as Alzheimer’s disease, Parkinson’s disease, stroke, and epilepsy [1].
Best1 protein is a transmembrane protein with a crystal structure comprised of five homologous protomers (homo-pentameric structure) around a central pore [1] . The Best1 ion channel thus includes:
- An ion pore which is a continuous funnel-shaped vestibule with 2 restriction sites;
- A calcium clasp at each protomer.
Best1 protein has several isoforms [11] and is a multifunctional protein. Known functions of the Best1 protein include:
- Normal ocular development, although the mechanisms remain unclear; the RPE plays a crucial role in the regulation of growth factor signaling to the choroid and sclera, and Best1 protein may be of influence to these mechanisms [1],
- Calcium-activated chloride channel;
- Large anion channel, including bicarbonate anion (HCO3- channel);
- Inhibitor of intracellular voltage-dependent calcium channels (CaV), a process mediated by its intracellular C-terminal domain through the interaction with the β-subunit of these channels [11];
- Transport of γ-amynobutiric acid (GABA) and glutamate, although this has been disputed by protein structure analysis data [1].
The earliest evidence of Best1 as a calcium-activated chloride channel derived from the classical finding of eletro-oculographic decrease in light peak response in Best vitelliform macular dystrophy, has the normal light peak response was presumed to be generated by the activation of calcium-sensitive chloride conductance. However, conflicting evidence in mice has emerged, and in fact, Best1 modulation of intracellular CaV channels may be necessary for the generation of a normal light peak response in mice [1].
BEST2 gene
The BEST2 gene is located on the short arm of chromosome 19 (19p13.2-p13.12) [18], and encodes for Best2 protein. Best2 functions as a calcium-activated anion channel, and has also been shown to mediate bicarbonate transport in colon goblet cells [19] and, possibly, in sweat glands [20]. In addition, it has been detected in nonpigmented epithelium in the ciliary body, and may play a role in intraocular pressure physiology [21].
BEST3 gene
The BEST3 gene is located on the long arm of chromosome 12 (12q14.2-q15) [22], and encodes Best3 protein, the expression of which is broader. In humans, Best3 appears to be highly expressed in skeletal and cardiac muscle, testis, and thymus [23]. Best3 appears to mediate a cGMP-dependent calcium-activated chloride current [24], and it may play cell protective roles against endoplasmic reticulum stress, oxidative stress, and inflammation [25][26][27].
BEST4 gene
The BEST4 gene is located on the short arm of chromosome 1 (1p33-p32.3) [28]. The expression of Best4 protein has not been studied, however Best4 messenger RNA expression has been detected in colon, brain, spinal cord, trachea, and testis [23]. Best4 appears to be a chloride channel activated by calcium in a dose-dependent manner [29], but its physiologic roles remain largely unknown [1].
Pathogenesis of the Bestrophinopathies
BEST1 mutations
The structure analysis of the Best1 protein suggests that there are at least 3 important regions with functional implications [1]:
- The first restriction site at the neck region;
- The calcium-clasp site;
- The second restriction site at the bottom of the ion pore.
A fourth possibly critical region may be the cytosolic aperture of the pore which affects relative anion permeabilites.
Disease-causing mutations in BEST1 gene have been described throughout the entire Best1 protomer, but many mutations associated with bestrophinopathies involve the first restriction site and the calcium-clasp site [1].
Protein mistrafficking
It has been suggested that certain BEST1 gene mutations (Best1T6R, Best1Y227N, Best1V235A, and Best1Q238R) may lead to Best1 protein mistrafficking to the RPE basolateral membrane and intracellular accumulation, as occurs in other known channelopathies [1]. However, laboratory evidence is conflicting in this regard, as protein mislocalization has been found in some RPE cell lines, but not in others. Interestingly, autosomal recessive bestrophinopathy (ARB) mutations have been shown to lead to mislocalized Best1 protein, and to proteasomal degradation of the protein [4].
Anion channel activity
Severe attenuation of anion currents has been demonstrated in Best1 mutants associated with bestrophinopathies (apart from ADVIRC) [30]. Impaired ionic flow across the RPE may lead to altered adhesiveness between the interphotoreceptor matrix and the RPE, or a reduction of the phagocytosis of photoreceptor outer segments by the RPE [3].
Altered permeability to large anions
Certain mutations (the Glu119Cln variant), which has been identified in bull’s eye maculopathy and in age-related macular degeneration, produce a channel with altered relative permeability to large anions [13].
Intracellular calcium signaling
Since Best1 interacts physically and functionally with CaV channels, a process mediated by its intracellular C-terminal domain, it has been hypothesized that certain BEST1 gene mutations uniquely affect Best1 protein’s ability to interact with these voltage-dependent calcium channels [1]. In fact, certain mutations in BEST1 have an inhibitory effect on CaV channels that is lower than that of wild-type bestrophin-1 protein [31].
Pathophysiology
The understanding of the pathophysiology of bestrophinopathies remains incomplete. As explained above, mutations in the BEST1 gene may alter the ionic channel functions of the protein and cause ionic imbalance in the RPE milieu which leads to impaired RPE functions. Most mutations causing BVMD are associated with an absent chloride current, often due to a dominant negative mechanism. Both dominant negative (leading to absent chloride current) BEST1 gene mutations and haploinsufficiency mutations (leading to 10-40% of the wild-type chloride current) have been found in patients with AVMD [11].
BEST1 mutations in BVMD and AVMD show variable expression and incomplete penetration. Whereas in BVMD no clear genotype-phenotype correlation has been found, there seems to be a clear correlation between BEST1 mutations and ADVIRC and MRCS syndrome phenotypes [11]. All implicated mutations in ADVIRC and MRCS affect splicing, leading to in-frame deletions or duplications in ADVIRC, and in-frame deletions in MRCS syndrome. Autosomal recessive bestrophinopathy (ARB) is considered to be a null phenotype caused by homozygous or compound heterozygous nonsense or missense BEST1 mutations [6] [11].
Vitelliform material
For BVMD and AVMD, it has been thought that the accumulation of fluid and vitelliform material is the result of the disruption of ionic transport and fluid homeostasis, leading to the accumulation of fluid in the potential space between the RPE and photoreceptor cells; this would in turn to accumulation of unphagocytosed photoreceptor outer segments, accumulation of toxic fluorophores, and toxic injury to photoreceptors and RPE. However, in light of more recent evidence, this “classical” hypothesis may explain, at best, only partially the pathophysiology of bestrophinopathies [3].
Since lipofuscin deposition is a predominant clinical feature in BVMD and ARB, it has been suggested that lipofuscin deposition could underlie the pathophysiology of bestrophinopathies. However, findings from studies using hyperspectral autofluorescence imaging (HAI) strongly suggest that these RPE fluorophores reflect the premature dysfunction of the affected RPE rather than being involved in the pathophysiology of bestrophinopathies [3] [32].
Cholesterol homeostasis
Cholesterol homeostasis is essential for the maintenance of outer segment structure and function, and dysregulation of this homeostatic process has been detected in bestrophinopathies [3]. Cholesterol homeostasis changes in Best1 mutant retinae include:
- Increased unesterified cholesterol in the RPE;
- abnormal distribution of the esterified cholesterol from the Bruch’s membrane to the photoreceptor outer segments;
- Increased levels of 4-hydroxy-2-nonenal (HNE) adducts (lipid peroxidation by-products) in the retina.
The altered distribution of cholesterol esters and HNE-adducts at the photoreceptor layer may lead to chronic inflammatory stimuli interrelated to impaired calcium signaling and fluid flow, and may contribute to the loss of adhesive forces between RPE, NSR, and interphotoreceptor matrix [3].
Retinal pigment epithelium-photoreceptor interface
The RPE-photoreceptor interaction is affected in bestrophinopathies [3]:
- Best1-mutated RPE cells have retraction of their apical microvilli;
- The normal bilayered extracellular sheath covering the cones, which is responsible for normal apposition of RPE-to cone outer segments, appears to be lost in Best1-mutated retinae.
Spectrum of the ocular phenotypes caused by BEST1 gene mutations
Best vitelliform macular dystrophy (BVMD)
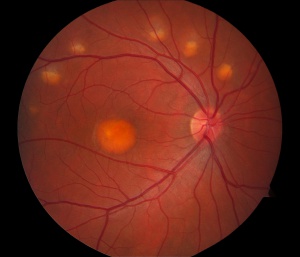
First described by Adams in 1883, but named for Dr. Friedrich Best, who presented a detailed pedigree of the disease in 1905, Best vitelliform macular dystrophy, or Best disease, is a hereditary retinal dystrophy involving the retinal pigment epithelium (RPE), and leads to a characteristic bilateral yellow “egg-yolk” appearance of the macula. This disease tends to present itself in childhood or early adulthood and usually portends a good visual prognosis. BVMD is the most common autosomal dominant macular dystrophy [33] [34]. BVMD is inherited in an autosomal dominant fashion, but shows incomplete penetration and variable expressivity; this variability occurs both between families and within families [1]. The appearance of the vitelliform lesion usually occurs in the ages of 3-15 years old, but can be seen in the later decades of life.
Clinical Stages
Best disease has been extensively described [35]. Essentially, BVMD is considered to have 6 clinical stages:
Stage I (Previtelliform): normal vision, normal or only subtle RPE changes (tiny, central honeycomb structure centrally) with abnormal EOG.
Stage II (Vitelliform): classic “egg-yolk” lesion. 30% have ectopic lesions. Normal vision or mild vision loss.
Stage III (Pseudohypopyon): layering of lipufuscein. Vision similar to stage II.
Stage IV (Vitelleruptive): breakup of material gives “scrambled egg” appearance. Vision may be similar or mildly decreased from stage I/II.
Stage V (Atrophic): Central RPE and retinal atrophy. Vision may range from 20/30 – 20/200.
Stage VI (CNV): This complication occurs in about 20% of patients. Vision often decreased to 20/200 or worse.
Lesions are typically bilateral and relatively symmetric, although sometimes unilateral presentation occurs [33].
Although BVMD usually presents with a single lesion, up to 30% of patients can present with multiple lesions; this can be referred to as multifocal Best disease [33]. In these cases, both small and large lesions are present in foveal and extrafoveal locations, though extrafoveal lesions are usually smaller and tend to locate more superiorly in the macula. Most frequently, vitelliform dystrophy is a bilateral process, although unilateral changes have often been reported. The classically described vitelliform structure resembles an egg yolk in the posterior pole, but at times is more orange, or somewhat elevated with a dark border. The retinal vessels will traverse the edge of these lesions undisturbed. At times, multiple vitelliform structures can be seen at once, but often these lesions never occur.
Visual acuity is minimally affected, especially in early stages. Loss of vision is often asymmetric and is impossible to predict from the visualization of the fundus. Fundus finding is remarkably drastic compared to the good visual acuity. As the disease progresses, patients may experience a slow, bilateral decrease in visual acuity, central scotoma, or metamorphopsia. With secondary CNV, visual decline can be rapid, however. Often the patients will be hyperopic with some degree of astigmatism.
Differential Diagnosis
The differential diagnosis of Best disease includes adult foveomacular vitelliform dystrophy (within the spectrum of pattern dystrophies), age related macular degeneration, dominant drusen, central serous retinopathy, toxoplasmotic retinochoroiditis, solar retinopathy, macular hole, or other causes of central macular atrophy such as toxoplasmosis or myopic degeneration.
Of note, mutations in the VMD2 gene can lead to a wide spectrum of disease, including adult onset foveomacular vitelliform dystrophy, autosomal bestrophinopathy, autosomal dominant vitreoretinochoroidopathy, and the “microcornea, retinal dystrophy, cataract, and posterior staphyloma" syndrome. The EOG is universally abnormal in all of these conditions.
Diagnostic Tests
Although Best disease can usually be diagnosed clinically, several tests may be helpful in confirming the diagnosis.
Electro-oculogram (EOG): Universally abnormal, with an Arden ratio (light:dark) of 1.5 or less.
Electro-retinogram (ERG): Completely normal.
Optical coherence tomography (OCT): Can be used to localize the vitelliform lesion to the subetinal space, demonstrate thickening of the cone outer segments, and can be used to evaluate for fluid associated with CNV.
Fluorescein angiogram (FA): hypofluorescence of typical vitelliform lesion, and as the disease progresses a mixed pattern of hyper and hypo fluorescence eventually gives way to hyperfluorescence of the atrophic stage.
Fundus Autofluorescence (FAF): During the earlier vitelliform stages, hyperautofluorescence predominates. This hyperfluoresecence settles with the pseduohypopyon stage, and becomes mottled with areas of hypoautofluorescence during the vitelleruptive stage, and eventually becomes hypofluorescent during the atrophic stage. Changes seen with FAF may precede or appear more striking than with ophthalmoscopy
Management
There is no medical or surgical management for Best disease. CNV, however, can be a potentially devastating complication. Application of anti-VEGF therapy for CNV in the setting of Best disease has shown potential for improving outcomes, and photodynamic therapy has been attempted as well.
Complications
Late-stage complications of BVMD include [33]:
- Sub-RPE fibrosis,
- RPE atrophy,
- Geographic atrophy,
- Choroidal neovascularization, which some authors consider to be stage VI,
- Subretinal hemorrhage following fairly modest trauma to the head or the eye,
- Macular holes.
Adult-onset vitelliform macular dystrophy (AVMD) / adult-onset foveomacular vitelliform dystrophy (AFVD)
AVMD, also known as adult-onset foveomacular vitelliform dystrophy (AFVD), has been associated with mutations in BEST1, PRPH2, IMPG1, and IMPG2 genes[34], and belongs to a group of diseases termed “pattern dystrophies” [36]. Only a minority of cases appear to be associated with autosomal dominant mutations of BEST1 [34].
Clinical Manifestations
Classically, AVMD changes include [1] [33] [34]:
- Sporadic, although some familial clustering has been reported,
- Onset typically between 30-50 years of age,
- Autofluorescent, subfoveal yellow vitelliform-like lesion 500-700µm in size,
- No visual symptoms, or-mild-moderate visual acuity decrease,
- Hyperreflective material between the NSR and the RPE.
Complications
Complications of AVMD include:[34]
- Choroidal neovascularization,
- RPE detachment.
Autosomal recessive bestrophinopathy (ARB)
ARB has been hypothesized to represent the human “null phenotype for Best1, as both alleles of BEST1 must be mutated [1]. Most ARB patients are compound heterozygotes, although homozygotes have also been described [6].
Clinical Manifestations
Typical clinical and electrophysiological features of ARB are [2] [11][37]:
- Visual acuity decrease usually starting in the first decade of life, although late presentations have been described as late as in the fifth decade of life;
- Hyperopia,
- Shallow anterior chamber,
- Multifocal punctate, fluorescent, yellows dots/flecks around the vascular arcades,
- Macular lesions with subretinal fibrosis inferior to the fovea,
- Macular edema and subretinal fluid,
- Reduced full-field ERG scotopic and photopic responses;
- Markedly abnormal pattern ERG;
- Reduced multifocal ERG responses,
- Severely reduced light rise in EOG.
- Elevated intraocular pressure and glaucomatous optic neuropathy
Vision decreases over time, but usually very slowly; an important cause of visual loss is the development of choroidal neovascularization [1].
Autosomal dominant vitreoretinochoroidopathy (ADVIRC)
ADVIRC is a rare peripheral chorioretinal pigmentary disorder [33]. To date, four BEST1 gene mutations have been identified as causative of ADVIRC [1]:
- Val86Met
- Tyr236Cys
- Val235Ala
- Val239Met
Clinical Manifestations
Typical features of ADVIRC include [2] [33]:
- A 360-degree, peripheral retinal circumferential hyperpigmented band extending between the equatorial region and the ora serrata;
- vitreous fibillar condensation,
- punctate white opacities in the retina,
- breakdown of the blood-retinal barrier,
- retinal neovascularization.
Patients with ADVIRC may also present with the following features[11]:
- Nystagmus,
- Microcornea,
- Nanophthalmos,
- Hyperopia,
- Narrow anterior chamber angle, with a relatively high incidence of subacute and acute angle-closure glaucoma,
- Retinal arteriolar narrowing,
- Pale optic discs.
Although in earlier stages the post-equatorial region shows no retinal changes, the disease involves the entire retina in later years [2].
The full-field ERG is usually normal but may be subnormal, and throughout life reduced rod and cone responses develop.
Complications
Most patients with ADVIRC retain a good visual acuity during their lifetime. Complications leading to visual loss in ADVIRC patients include:
- Macular edema,
- Chororioretinal atrophy,
- Retinal detachment,
- Vitreous hemorrhage.
Autosomal dominant microcornea, rod-cone dystrophy, early-onset cataract, posterior staphyloma syndrome (MRCS syndrome)
Clinical Manifestations
MRCS syndrome is characterized by [2] [11] [38]:
- Autosomal dominant mode of inheritance,
- Hyperopia,
- Microcornea,
- Early-onset pulverulent cataract,
- Narrow anterior chamber angle,
- Rod-cone dystrophy,
- Posterior staphyloma in eyes with otherwise normal axial lengths;
- Peripheral RPE atrophy and retinal pigmentary abnormalities anterior to the posterior staphyloma in younger patients, which may extend to the posterior pole and staphyloma with advancing age,
- Abnormal EOG,
- Subnormal ERG findings in younger patients, and decreased[1] ERG in older patients.
The earliest visual symptom is typically nyctalopia during teenage years. Progressive decrease in visual acuity worsens after the age of 30 years, often leading to cataract surgery in the second of third decades; final visual acuity usually ranges from 20/100 to absence of light perception [11].
Although posterior staphyloma in an eye with normal axial length is the most common finding, some patients with a Val239Met BEST1 mutation had nanophthalmos instead of staphyloma, largely overlapping with the ADVIRC phenotype [11].
Full-field ERG shows subnormal scotopic and photopic responses with more abnormal scotopic responses (rod-cone dystrophy) in the first two decades of life. With time, the ERG becomes extinguished [11]. The EOG is abnormal in all patients with MRCS syndrome.
It has been suggested that MRCS syndrome may be a variant of AD VIRC, and that these syndromes represent a spectrum of ocular abnormal development and retinal dysfunction caused by Best1 mutations [1] [11].
Retinitis Pigmentosa (RP)
Some patients with a concentric RP-like retinal dystrophy, have been found to carry missense mutations in BEST1, three of which appeared autosomal dominant while the other appeared autosomal recessive [39].
Whether these patients were misdiagnosed cases of ADVIRC is disputed, and recent reports suggest that Best1-related RP may be multigenic [1].
A BEST1 variant (Glu119Gln) has been identified in some patients with bull’s eye maculopathy and age-related macular degeneration (AMD); although the chloride currents were normal, large anion permeability was detected [13]. Although two other BEST1 variants have been identified in patients with AMD, these mutations did not affect the anion channel function of the protein [11] .
BEST2, BEST3, and BEST4 genes in ocular health and disease
Among the bestrophin proteins, only Best1 mutations have been linked to human eye disease. Interestingly, evidence from Best2 knockout mice suggests that Best2 protein plays a role in aqueous humor dynamics, as an antagonist of aqueous humor production, and possibly as a modulator of the outflow pathway [40][41].
Potential therapies for the bestrophinopathies
At present, no definitive therapies or treatments exist for patients with bestrophinopathies. The increasing understanding of the role of Best1 in the ocular physiology and pathophysiology has allowed the research into novel potential therapies, including novel drug treatments, gene therapy, and RPE transplantation.
Pharmacological therapies
Proteasome inhibitors
In vitro studies have found that in ARB mutants with mislocalized Best1 protein and proteasomal degradation, treatment with two proteasome inhibitors, 4-phenylbutyrate and bortezomib, rescued the location of Best1 to the basolateral plasma membrane in MDCK-II cells, and restored chloride conductance [4].
Restoration of photoreceptor outer segment degradation
It has been shown that some BVMD mutations are associated with reduced rates of photoreceptor outer segment (OS) degradation. In vitro studies using RPE stem-cells derived from patients with BVMD suggested that valproic acid therapy with or without combined rapamycin could increase the rate of photoreceptor OS degradation [42].
Gene therapies
Administration of gene therapy in retinal disease has been shown to provide clinical benefits in RPE65 gene-related inherited retinal dystrophies (RP and Leber’s congenital amaurosis) [43], and a number of gene therapy trials for IRDs are currently under investigation [44] .
The BEST1 gene appears to be a good target for gene therapy [7], particularly in autosomal recessive diseases, namely ARB. In a canine model of ARB, called canine multifocal retinopathy, adenoviral vector-mediated (AAV2) BEST1 transfer into retinal pigment epithelium led to reversal of retinal lesions for at least 23 months[45]. In addition, AAV2-mediated BEST1 gene transfer led to reversal of vitelliform lesions and light-modulated microdetachments, and at the immunochemistry level correction of structural alterations at the RPE-photoreceptor interface was observed [46].
Stem cell-based retinal pigment epithelium transplantation
Stem cell-based treatments using induced pluripotent stem cells (iPSC) are under intense research for the treatment of retinal degenerative diseases, including IRDs and AMD. There is an ongoing clinical trial for allograft transplantation of iPSC-derived RPE to assess safety of this procedure. The potential use of autologous iPSC-RPE is being considered for treatment of retinal degenerative diseases, and may be a potential therapy to alleviate or entirely cure BVMD, AVMD, ARB, ADVIRC, and BEST-related RP. [1]
Conclusion
The pathologic mechanisms of bestrophin-related diseases are challenging to explore. Animal models of bestrophinopathies are scarce, and evidence of definitive association between animal/in vitro findings and human disease is limited at best. However, the increasing understanding of the biologic roles of Best proteins and the implication of BEST1 mutations in ocular diseases has opened a window of opportunity for research into novel forms of treatment, including gene therapy and stem cell-based therapies, which may lead to alleviation or cure of bestrophinopathies.
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 Johnson AA et al. Bestrophin 1 and Retinal Disease. Prog Retin Eye Res. 2017 May ;58: 45–69. doi:10.1016/j.preteyeres.2017.01.006.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 Pasquay C. et al. Bestrophin I – phenotypes and functional aspects in Bestrophinopathies. Ophthalmic Genetics, Early Online, 1–20, 2013. DOI:10.3109/13816810.2013.863945.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Guziewicz K.E. et al. Bestrophinopathy: An RPE-Photoreceptor Interface Disease . Prog Retin Eye Res. 2017 May ; 58: 70–88. doi:10.1016/j.preteyeres.2017.01.005
- ↑ Jump up to: 4.0 4.1 4.2 Uggendi C. et al. Restoration of mutant bestrophin-1 expression, localisation and function in a polarised epithelial cell model Disease Models & Mechanisms (2016) 9, 1317-1328 doi:10.1242/dmm.024216.
- ↑ http://www.retina-international.com/sci-news/best-1-mutation/
- ↑ Jump up to: 6.0 6.1 6.2 Nguyen T.T. et al. Next generation sequencing identifies novel disease-associated BEST1 mutations in Bestrophinopathy patients. 2018. SCIeNTIFIC RePorTS | (2018) 8:10176 | DOI:10.1038/s41598-018-27951-8
- ↑ Jump up to: 7.0 7.1 Yang T. et al. BEST1: the best target for gene and cell therapies. Mol Ther. 2015;23(12):1805–9.
- ↑ Marmorstein A.D; Cross H.E. Functional Roles of Bestrophins in Ocular Epithelia. Prog Retin Eye Res. 2009 May ; 28(3): 206–226. doi:10.1016/j.preteyeres.2009.04.004.]
- ↑ Jump up to: 9.0 9.1 https://www.omim.org/entry/607854
- ↑ Petrukhin K. et al. Identification of the gene responsible for Best macular dystrophy. 1998 Nat. Genet. 19, 241–247;
- ↑ Jump up to: 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 Boon C.J.F. et al. The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog Retin Eye Res. 28 (2009) 187–205doi:10.1016/j.preteyeres.2009.04.002
- ↑ Marmorstein A.D. et al. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Nat. Acad. Sci. 97: 12758-12763, 2000.
- ↑ Jump up to: 13.0 13.1 13.2 Yu K. et al. 2007. Chloride channel activity of bestrophin mutants associated with mild or late-onset macular degeneration. Invest.Ophthalmol. Vis. Sci. 48, 4694–4705.
- ↑ Jump up to: 14.0 14.1 Strauss O. et al. The role of bestrophin-1 in intracellular Ca(2+) signaling. Advances in experimental medicine and biology. 2014; 801:113–119.
- ↑ Jump up to: 15.0 15.1 Mullins et al. 2007. Differential Macular and Peripheral Expression of Bestrophin in Human Eyes and Its Implication for Best Disease. IOVS, July 2007, Vol. 48, No. 7
- ↑ Woo D.H. et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012; 151:25–40.
- ↑ Park H. et al. Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009; 29:13063–13073
- ↑ https://www.omim.org/entry/607335?search=best2&highlight=best2
- ↑ Yu K. et al. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. The Journal of clinical investigation. 2010; 120:1722–1735.
- ↑ Cui C.Y. et al. Forkhead transcription factor FoxA1 regulates sweat secretion through Bestrophin 2 anion channel and Na-K-Cl cotransporter 1. Proceedings of the National Academy of Sciences of the United States of America. 2012; 109:1199–1203.
- ↑ Bakall B. et al. Bestrophin-2 is involved in the generation of intraocular pressure. Investigative ophthalmology & visual science. 2008; 49:1563–1570.
- ↑ https://www.omim.org/entry/607337?search=best3&highlight=best3
- ↑ Jump up to: 23.0 23.1 Stohr H. et al. Three novel human VMD2-like genes are members of the evolutionary highly conserved RFP-TM family. Eur J Hum Genet 2002;10: 281–284
- ↑ Matchkov VV et al. Bestrophin-3 (vitelliform macular dystrophy 2-like 3 protein) is essential for the cGMP-dependent calcium-activated chloride conductance in vascular smooth muscle cells. Circ. Res. 103: 864-872, 2008.
- ↑ Lee W.K. et al.. ERK1/2-dependent bestrophin-3 expression prevents ER-stress-induced cell death in renal epithelial cells by reducing CHOP. Biochimica et biophysica acta. 2012; 1823:1864–1876
- ↑ Svenningsen P. Stressed podocytes - Bestrophin-3 is not just Bestrophin-3. Acta physiologica. 2015; 214:430–431.
- ↑ Golubinskaya, V. et al. 2015. Bestrophin‐3 is differently expressed in normal and injured mouse glomerular podocytes. Acta Physiol (Oxf) doi:10.1111/apha.12516.
- ↑ https://www.omim.org/entry/607336?search=best4&highlight=best4
- ↑ Tsunenari T. et al. 2006. Ca(2+)-activated Cl(-) current from human bestrophin-4 in excised membrane patches. J. Gen. Physiol. 127: 749-754, 2006.]
- ↑ Xiao Q., Hartzell H.C.,Yu K. Bestrophins and retinopathies. Pflugers Archiv: European journal of physiology. 2010; 460:559–569.
- ↑ Yu K et al. The Best Disease-Linked Cl Channel hBest1 Regulates Cav1 (Ltype) Ca2+ Channels Via SH3-binding Domains J Neurosci. 2008 May 28; 28(22): 5660–5670. doi:10.1523/JNEUROSCI.0065-08.2008.
- ↑ Singh R et al. iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013a; 23:593–607.
- ↑ Jump up to: 33.0 33.1 33.2 33.3 33.4 33.5 33.6 Schachat A.P., Wilisoson CP., Hinton DR., Sadda SR., Wiedemann P. Ryan’s Retina 6th edition, Elsevier (2018).
- ↑ Jump up to: 34.0 34.1 34.2 34.3 34.4 Chowers I. et al. Adult-onset foveomacular vitelliform dystrophy: A fresh perspective. Prog Retin Eye Res. 2015 Jul;47:64-85. doi: 10.1016/j.preteyeres.2015.02.001
- ↑ https://eyewiki.aao.org/Best_Disease
- ↑ https://eyewiki.aao.org/Pattern_dystrophies
- ↑ Crowley C, Paterson R, Lamey T, McLaren T, De Roach J, Chelva E, Khan J. Autosomal recessive bestrophinopathy associated with angle-closure glaucoma. Doc Ophthalmol. 2014 Aug;129(1):57-63.
- ↑ Reddy, M.A. et al. 2003 .A clinical and molecular genetic study of a rare dominantly inherited syndrome (MRCS) comprising of microcornea, rod-cone dystrophy, cataract, and posterior staphyloma. Br. J. Ophthalmol. 87, 197–202.
- ↑ Davidson A.E. et al. Missense mutations in a retinal pigment epithelium protein, bestrophin-1, cause retinitis pigmentosa. American journal of human genetics. 2009; 85:581–592.
- ↑ Zhang Y. et al. Enhanced inflow and outflow rates despite lower IOP in bestrophin-2-deficient mice. Investigative ophthalmology & visual science. 2009; 50:765–770.
- ↑ Hafler BP. Clinical Progress in Inherited Retinal Degenerations: Gene Therapy Clinical Trials and Advances in Genetic Sequencing. Retina. 2017;37(3):417–423.
- ↑ Singh R. et al. Pharmacological Modulation of Photoreceptor Outer Segment Degradation in a Human iPS Cell Model of Inherited Macular Degeneration. Molecular therapy: the journal of the American Society of Gene Therapy. 2015 23:1700–1711.
- ↑ https://eyewiki.aao.org/Voretigene_neparvovec-rzyl_(Luxturna%E2%84%A2)
- ↑ Hafler BP. Clinical Progress in Inherited Retinal Degenerations: Gene Therapy Clinical Trials and Advances in Genetic Sequencing. Retina. 2017;37(3):417–423.
- ↑ Guziewicz KE et al. Recombinant AAV-mediated BEST1 transfer to the retinal pigment epithelium: analysis of serotype-dependent retinal effects. PloS one. 2013; 8:e75666.
- ↑ Guziewicz KE et al. BEST1 gene therapy corrects a diffuse retina-wide microdetachment modulated by light exposure. PNAS March 20, 2018 115 (12) E2839-W2848.