Retinal Angiomatous Proliferation (RAP)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
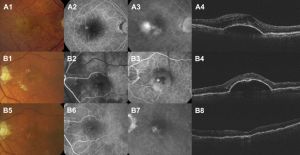
Disease Entity
Retinal Angiomatous Proliferation (RAP) was described by Yannuzzi and co-workers as a distinct form of neovascular Age-Related Degeneration (AMD).
Disease
RAP is a neovascularization that starts at the retina and progresses posteriorly into sub retinal space; eventually the neovascularization reaches the choroidal circulation and forms retinal-choroidal anastomoses. This is distinct from choroidal neovascularization (CNV) in AMD which originates from the choroid and can erode through the retinal pigment epithelium and communicate with the retinal circulation through a different mechanism, resulting in a chorioretinal anastomosis.
There has been some controversy on whether neovascularization arises from the retina or from the choroid. Yannuzzi et al stated in their last review that it is possible that angiogenesis starts in the retina, choroid or both, and they proposed an alternative name for this process: Type 3 neovascularization.[2] This new designation would apply to neovessels within the retina and right underneath and it integrates with the classical neovascularization classification by Gass (Type 1 neovessels being located under the retinal pigment epithelium and Type 2 under the neurosensory retina). RAP remains however the most widely used denomination.
Incidence
Several series have studied the prevalence of RAP, with estimates ranging from 10 to 21% of exudative AMD. In the Comparison of Age- Related Macular Degeneration Treatments Trials (CATT), the prevalence of RAP was 10.7%.[1]
Epidemiology
Patients with RAP tend to be older than patients with other forms of neovascular AMD (mean age of 79 vs 76, respectively). It is most common in white patients, and may also be seen in asian patients. As of 2008, according to Yannuzzi and colleagues, there was no report of RAP in black patients.[2]
Stages
Three stages were originally described by Yannuzzi and co-workers:
Representative images can be accessed at https://www.reviewofophthalmology.com/article/retinal-angiomatous-proliferation-in-amd
Stage I: Intraretinal Neovascularization (IRN)
Vascular proliferation originates from the deep capillary plexus of the retina in the paramacular area and is confined within the retina, as a retinal-retinal anastomosis. Intraretinal haemorrhage and edema are common. Telagiectatic vessels can be seen around the IRN, presumably as a compensatory response to the increased vascular perfusion needed by the IRN.
Stage II: Subretinal Neovascularization (SRN)
Neovascularization invades sub retinal space (above/superficial to the retinal pigment epithelium). Neurosensory and serous pigment epithelial detachment can be found, together with increasing edema of the retina and haemorrhages in the pre-retinal and intraretinal spaces.
Stage III: Choroidal Neovascularization (CNV)
Choroidal neovascularization (subretinal pigment epithelium) is present. It can be associated with vascularized pigment epithelial detachment. A retinal-choroidal anastomosis is formed.
Alternative theories of type 3 neovascularization
An ultimate outcome of type 3 neovascularization may be reached from 3 possible initial circumstances[2]
- IRN alone
- IRN + CNV
- CNV alone
Diagnosis
Clinical Diagnosis
The initial lesion is located extrafoveally, presumably because of lack of capillaries in the foveal avascular zone.
The presence of small macular haemorrhages, sometimes punctiform, often multiple, associated with oedema in an eye with soft drusen, is highly suggestive of RAP in its initial stages. Hemorrhages can be pre, intra or subretinal. Large subretinal hemorrage is rarely seen in stages I and II. As angiogenesis progresses, tortuous, dilated retinal vessels, sometimes showing retino-retinal anastomoses can be found.
Involvement is typically bilateral, with 80% of fellow eyes affected after 1 year and 100% before 3 years.
- older patients in RAP
- intraretinal changes - hemorrhage, exudate and fluid more common
- large subretinal bleed rare
- preretinal hemorrhage can be seen
- usually not at the fovea
- In all cases of pigment epithelial detachment after 50 years of age -polypoidal choroidal vasculopathy (PCV) and RAP needs to be excluded with ICG angiogram preferably digital, scanning laser ophthalmoscope based system (eg., Spectralis, Heidelberg Engineering).
Diagnostic procedures
Fluorescein angiography (FA)
Fluorescein angiography can help in the diagnosis of early stage RAP lesions, but in later stages the dye stains the whole vascular-exudation complex and can be confused with occult CNV.
- Features on FA:[1]
- Required: Focal area of intense intraretinal hyperfluorescence (hot spot) in the early phase of FA
- + 1 or more of the following signs:
- Focal intraretinal superficial hemorrhages
- lipid
- serous or fibrovascular pigment epithelial detachments
- retinal vascular abnormalities, such as anastomoses between retinal vessels, anastomoses between retinal and choroidal vessels, or anastomoses between retinal vessels and the underlying CNV complex.
Indocyanine green angiography (ICG)
- Shows hot spot in mid and/or late frames
- hairpin loop (linked perfusing retinal arteriole and draining retinal venule)
- RAP shows typically late-phase leakage of the ICG molecule into intraretinal spaces or cystoid edema. The ICG molecule has an affinity for fibrin, which is present in the cystic spaces.
- ICG can also reveal the retinochoroidal anastomosis clearly.
Optical coherence tomography (OCT)
- OCT is useful in the initial stage of RAP to image the vessels and the cystic spaces within the retina. As the disease progresses and a serous or vascularized PED occurs, changes beneath the pigment epithelium become difficult to assess accurately. Sub–pigment epithelium blood, exudates, and neovascularization produce photoreflectance that can be indistinguishable.
- New angio-OCT systems have the potential of becoming a very useful diagnostic tool for this disease.
Representative images can be accessed at https://amdbook.org/content/neovascular-phenotypesrap-retinal-angiomatous-proliferation
Differential diagnosis
- Idiopathic parafoveal telangiectasia is a condition involving dilation of retinal capillaries located near the fovea, in one or both eyes. Telangiectasias are not associated with serous PED, the RPE is healthier and choroidal neovascularization associated with parafoveal telangiectasias occurs less frequently.
- Other forms of choroidal neovascularization (CNV) including polypoidal choroidal vasculopathy (PCV). Retinal haemorrhages in PCV are normally larger, with round reddish-orange macular lesions being observed in the eye fundus. Polyps can be seen in OCT as abrupt protrusions from the REP/Bruch’s membrane band, often associated with neurosensory detachment.
- macular branch retinal venous occlusion
Management
Medical therapy
Retinal angiomatous proliferation demonstrate good response to anti-vascular endothelial growth factor agents (Anti-VEGF). In the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT), when treated with intravitreal ranibizumab or bevacizumab, eyes with RAP were less likely to have fluid, FA leakage, scar, and subretinal hyperreflective material were compared with all other eyes with neovascular AMD but without RAP.[1] Visual acuity outcomes at 1 year were better in the RAP group than in the non-RAP group, however, these differences did not persist at year 2. In addition, eyes with RAP were also found to be more likely to have geographic atrophy than eyes without RAP.
Other therapies include
- laser photocoagulation of feeder vessels
- intravitreal triamcinolone to reduce exudation (intraretinal/subretinal) followed 10 days later by PDT
References
- ↑ 1.0 1.1 1.2 1.3 Daniel, E., Shaffer, J., Ying, G.-shuang, Grunwald, J. E., Martin, D. F., Jaffe, G. J., & Maguire, M. G. (2016). Outcomes in eyes with retinal angiomatous proliferation in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology, 123(3), 609–616. https://doi.org/10.1016/j.ophtha.2015.10.034
- ↑ 2.0 2.1 2.2 Yannuzzi LA, Freund KB, Takahashi BS. Review of retinal angiomatous proliferation or type 3 neovascularization. Retina. 2008 Mar;28(3):375-84. doi: 10.1097/IAE.0b013e3181619c55. Review. PubMed PMID: 18327130.

