Basal Laminar Drusen
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
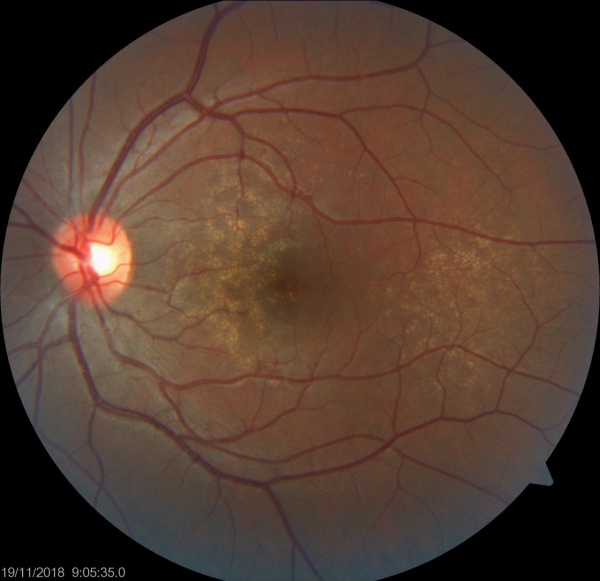
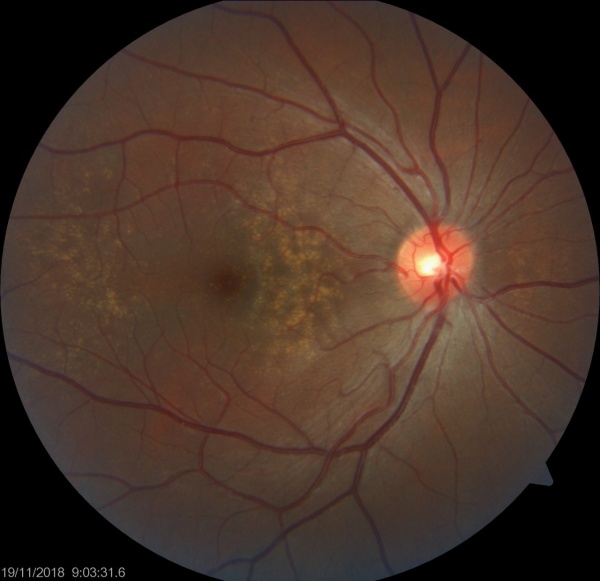
Basal laminar drusen/Cuticular drusen is an uncommon entity. Although originally thought to be distinct from age-related macular degeneration, it is now considered to be in the same spectrum as macular degeneration. Findings include numerous small yellow drusen on examination, which are more obvious on fundus auto-fluorescence. Fluorescein angiography (FA) demonstrates punctuate areas of early staining throughout the macula, with a “stars in the sky" pattern. The development of vitelliform macular lesions, choroidal neovascularization (CNV) and geographic atrophy (GA) may cause vision loss.
Disease Entity
Basal laminar drusen (BLD) are also referred to as “diffuse drusen”, “cuticular drusen” and “early adult onset, grouped drusen” in the literature.
Disease
Basal laminar drusen are considered to be part of a spectrum that includes age-related macular degeneration; they are associated with mutations in the complement factor H (CFH) gene.[1] [2]
Etiology
The etiology is not well understood. It is believed; however, that BLD share a similar biogenesis to drusen associated with age-related macular degeneration (AMD).[2][3]
Risk Factors
Certain risk factors for AMD are also risk factors for basal laminar drusen.
Genotype: Tyr402His allelic variant in the CFH gene has been shown to be associated with BLD vs AMD (odds ratio= 2.0, p<0.05).[4]
Family History In one cohort, 44% of patients with BLD reported a family history of age-related macular degeneration[4] while another cohort reported 53% of patients with a family history of macular degeneration.[5]
Sex Female sex appears to be a risk factor with reports ranging from 60%-92.1% proportion of BLD patients.[6][7][8]
General Pathology
Initially, basal laminar drusen were thought to be distinct from the drusen of age-related macular degeneration based on fundus examination, autofluorescence, fluorescein angiography and histopathology. Dr. Gass and colleagues described basal laminar drusen as areas of focal nodular thickening of the retinal pigment epithelium basement membrane.[9] More recently, however, Russell and colleagues have shown similarities in basal laminar drusen and drusen from age-related macular degeneration in terms of their lipoprotein composition, ultrastructure, and sub-retinal pigment epithelium localization. Since these drusen are in the cuticular layer between the basal lamina of the RPE and inner collagenous layer of Bruch’s membrane, the basilar drusen are more appropriately termed "cuticular drusen". The phenotype of these drusen may be subject to constant remodeling, either regress with time or progress with increasing volume. [10]
Pathophysiology
The pathophysiology is not fully understood. The most well studied cause is a mutation in the CFH gene of the alternative pathway. The Tyr402His allelic variant in the CFH gene is associated with the presence of basal laminar drusen.[4] Because of the association with the CFH Tyr402His variant, it is hypothesized that overactivity of the complement cascade is a contributing factor.[11]
A potential life cycle has been postulated with BLD beginning as “small nodules of hyalinized, protein-rich material external to the RPE” progressing to clusters of drusen coalescing to facilitate a “transport barrier between the RPE and choriocapillaris”. This may act as a locus of inflammation subsequently increasing the risk for certain complications delineated below.[6] small hard drusen in patients with the basal laminar drusen phenotype are subject to a constant dynamic process of drusen remodeling.
Diagnosis
The diagnosis of basal laminar drusen is clinical, based on dilated fundus examination, fundus autofluorescence and FA.
History
Young adults may be found to have basal laminar drusen on routine examination. Patients may have a family history of age-related macular degeneration. In cases that present with complications such as GA or CNV, visual complaints may also be present.
Physical Examination
Dilated fundus examination reveals numerous, small, yellow drusen distributed throughout the retina.
Signs
Multiple small, hard, subretinal, raised yellow drusen are seen underneath the RPE specifically between the RPE and inner collagenous layer of Bruch’s membrane.[2][6] The mean age of presentation is 57.9±13.4 years.[6]
Symptoms
Most patients are asymptomatic until late in life but should be monitored for complications as discussed below.
Clinical Diagnosis
The classic appearance is multiple, diffusely distributed drusen which are more numerous on fluorescein angiography in a young adult.
Diagnostic procedures
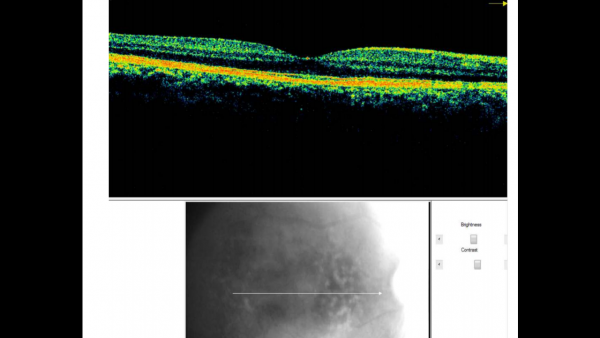
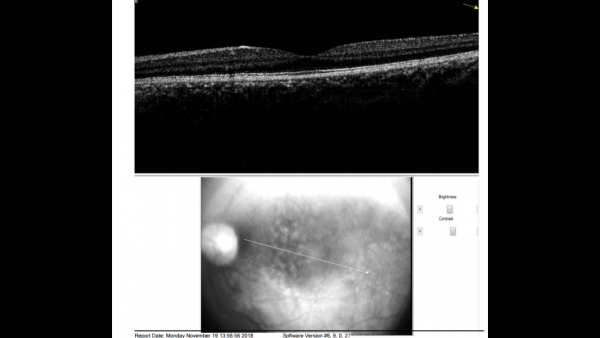
Fundus photos show multiple, yellow, round accumulations sub-RPE. Fundus autofluorescence often demonstrates more numerous hyperautofluorescent drusen than seen ophthalmoscopically. The drusen can demonstrate central hypo-autofluorescence and a rim of hyper-autofluorescence.[6][12] Fluorescein angiography reveals a greater number of small drusen than seen clinically during the early arterio-venous phase. These are spread diffusely throughout the macula, with a classic “stars in the sky” appearance.[6][7] Optical coherence tomography depicts RPE elevations due to drusen beneath the RPE with the most common appearance being a “saw-tooth” appearance as shown in the image below.[6][8]
Differential diagnosis
The differential diagnosis includes other conditions associated with drusen, such as autosomal dominant drusen (Malattia Leventinese), pattern macular dystrophy, North Carolina macular dystrophy, Sorsby macular dystrophy, mitochondrial retinal dystrophy and age-related macular degeneration. In patients with vitelliform pigment epithelial detachment, adult vitelliform foveomacular dystrophy is also a consideration and for those who may present at a younger age, certain juvenile macular dystrophies should be included in the differential such as Best Vitelliform Macular Dystrophy[13] or other lesions with early onset such as Alport Syndrome.[14]
Management
Patients with basal laminar drusen should be monitored for the development of “pseudovitelliform” retinal pigment epithelium lesions/detachment, CNV or GA which may be visually significant.
As the complete pathophysiology of BLD has not been elucidated and a CFH mutation has been implicated in the development of membranoproliferative glomerulonephritis type 2 (MPGN type II) (dense deposit disease)[13] and atypical hemolytic uremic syndrome (aHUS), it is recommended that renal function be monitored in those presenting with BLD especially at an early age (between 5 and 30 years). In addition, glomerulonephritis with isolated C3 deposits, a condition that is an intermediate between MPGN and aHUS, also has an association with CFH mutation.[1][15]
There does not appear to be a correlation between severity of disease within the kidney and the eye, though 80% of patients with dense deposit disease present with cuticular drusen.[14][16]
Similarly, genetic analysis for certain mutations may be beneficial to help guide certain patients with known alleles associated with disease progression.[14]
General treatment
Treatment is indicated in patients who develop CNV, which may affect or threaten vision. Vision loss due to geographic atrophy is not treatable, however patients with geographic atrophy should continue to be observed for the development of CNV.
There appear to be differences in the prevalence of complications based on ethnic backgrounds. For example, patients of Korean descent seem to exhibit both a lower rate of macular complications such as vitelliform lesions and differing proportions of complications such as GA and CNV compared to white ethnic groups.[17] And basal laminar drusen show some evidence of earlier age of onset in Japanese patients.[18]
Medical therapy
In cases of isolated basal laminar drusen, no therapy is indicated. Patients should be observed to monitor for the development of CNV or geographic atrophy. CNV should be treated when detected. Photodynamic therapy has been shown to be effective in cases of basal laminar drusen complicated by CNV.[19] There have also been reports of improvement in vision in CNV patients with BLD through the use of bevacizumab injections.[14] Vitelliform pigment epithelial detachments may be observed and have been reported to resolve spontaneously;[9] treatment with photodynamic therapy can worsen vision in these cases.[20] Similarly, vitelliform macular detachments associated with BLD have not been responsive to anti-VEGF intravitreal use.[21] It is unclear whether AREDs supplements, shown to have some benefit for patients with age-related macular degeneration, are of benefit in patients with basal laminar drusen.[22]
Medical follow up
Patients with basal laminar drusen should be followed regularly to monitor for the development of CNV. Patients who develop CNV should be monitored frequently to assess response to treatment. Amsler grid or preferential hyperacuity perimetry are important measures for self-monitoring.
Complications
There appear to be age-related variations in the complications associated with BLD. Pigmentary RPE changes appear to be the most common complication (47.5%), followed by large drusen, vitelliform lesions, GA and CNV. Each of these present with a statistically significant higher proportion in patients older than 60 years of age compared to those 60 years and younger.[6]
Prognosis
Though most patients do not have complications that affect vision, GA has been reported to occur in 11% of eyes with BLD, CNV in 21% of eyes and visual acuity has been reported to be worse than 20/100 in the better seeing eye in 22% of patients.[4]
Future Study
Treatment options focused on prevention or mitigating progression of BLD need to be studied further. Areas of current study include compounds targeting the complement pathway given its role in the pathophysiology of BLD. As affecting the complement pathway systemically will likely lead to a myriad of systemic adverse effects, local administration may prove to be more fruitful. A potential role for recombinant complement factor-H may also show promise.[14]
Additional Resources
- Porter D, Vemulakonda GA. Drusen. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/drusen-list. Accessed March 08, 2019.
References
- ↑ Jump up to: 1.0 1.1 1. Boon, C. J., van de Kar, N. C., Klevering, B. J., Keunen, J. E., Cremers, F. P., Klaver, C. C., Hoyng, C. B., Daha, M. R., & den Hollander, A. I. (2009). The spectrum of phenotypes caused by variants in the CFH gene. Molecular Immunology, 46(8–9), 1573–1594.
- ↑ Jump up to: 2.0 2.1 2.2 2. Russell SR, Mullins RF, Schneider BL, Hageman GS. (2000). Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. American Journal of Ophthalmology, 129:205-14.
- ↑ Mano F, Sprehe N, Olsen TW. Association of Drusen Phenotype in Age-Related Macular Degeneration from Human Eye-Bank Eyes to Disease Stage and Cause of Death. Ophthalmol Retina. 2021 Aug;5(8):743-749. doi: 10.1016/j.oret.2020.11.011. Epub 2020 Nov 20. PMID: 33227563.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Grassi MA, Folk JC, Scheetz TE, Taylor CM, Sheffield VC, Stone EM. (2007). Complement factor H polymorphism p.Tyr402His and cuticular Drusen. Arch Ophthalmology, 125:93-7.
- ↑ Barbazetto, I. A., Yannuzzi, N. A., Klais, C. M., Merriam, J. E., Zernant, J., Peiretti, E., Yannuzzi, L. A., & Allikmets, R. (2007). Pseudo-Vitelliform Macular Detachment and Cuticular Drusen: Exclusion of 6 Candidate Genes. Ophthalmic Genetics, 28(4), 192–197.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Balaratnasingam, C, Cherepanoff, S., Dolz-Marco, R., Killingsworth, M.,Chen, F.K., Mendis, R., Mrejen, S. Too, L.K., Gal-Or, O., Curcio, C.A., Freund, K.B., & Yannuzzi, L.A. (2018). Cuticular drusen: Clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology, 125, 100–118.
- ↑ Jump up to: 7.0 7.1 Russell, S. R., Gupta, R. R., Folk, J. C., Mullins, R. F., & Hageman, G. S. (2004). Comparison of color to fluorescein angiographic images from patients with early-adult onset grouped drusen suggests drusen substructure. American Journal of Ophthalmology, 137(5), 924–930.
- ↑ Jump up to: 8.0 8.1 Leng, T., Rosenfeld, P. J., Gregori, G., Puliafito, C. A., & Punjabi, O. S. (2009). Spectral domain optical coherence tomography characteristics of cuticular drusen. Retina, 29(7), 988–993.
- ↑ Jump up to: 9.0 9.1 Gass JD, Jallow S, Davis B. (1985). Adult vitelliform macular detachment occurring in patients with basal laminar drusen. American Journal of Ophthalmology, 99:445-59.
- ↑ Johannes P.H. van de Ven, Camiel J.F. Boon,et al. Short-Term Changes of Basal Laminar Drusen on Spectral-Domain Optical Coherence Tomography. American Journal of Ophthalmology, Volume 154, Issue 3,2012,Pages 560-567.
- ↑ Boon CJ, Klevering BJ, Hoyng CB, Zonneveld-Vrieling MN, Nabuurs SB, Blokland E, Cremers FP, den Hollander AI. (2008). Basal laminar drusen caused by compound heterozygous variants in the CFH gene. American Journal of Human Genetics, 82:516-23.
- ↑ Meyerle CB, Smith RT, Barbazetto IA, Yannuzzi LA. (2007). Auto- fluorescence of basal laminar drusen. Retina, 27:1101- 1106.
- ↑ Jump up to: 13.0 13.1 Altschwager, P., Ambrosio, L., Swanson, E. A., Moskowitz, A., & Fulton, A. B. (2017). Juvenile Macular Degenerations. Seminars in Pediatric Neurology, 24(2), 104–109.
- ↑ Jump up to: 14.0 14.1 14.2 14.3 14.4 Boon, C. J., van de Ven, J. P., Hoyng, C. B., den Hollander, A. I., & Klevering, B. J. (2013). Cuticular drusen: Stars in the sky. Progress in Retinal and Eye Research, 37, 90–113.
- ↑ Van de Ven JP, Boon CJ, Fauser S, Hoefsloot LH, Smailhodzic D, Schoenmaker-Koller F, Klevering J, Klaver CC, den Hollander AI, Hoyng CB. (2012). Clinical evaluation of 3 families with basal laminar drusen caused by novel mutations in the complement factor H gene. Arch Ophthalmology, 130:1038-47.
- ↑ McAvoy, C. E., & Silvestri, G. (2004). Retinal changes associated with type 2 glomerulonephritis. Eye, 19(9), 985–989.
- ↑ Shin, D. H., Kong, M., Han, G., Han, J. C., & Ham, D. I. (2020). Clinical manifestations of cuticular drusen in Korean patients. Scientific Reports, 10(1), 1–8.
- ↑ Sakurada, Y., Tanaka, K., Miki, A., Matsumoto, H., Kawamura, A., Mukai, R., Akiyama, H., Honda, S., Mori, R., & Iijima, H. (2019). Clinical characteristics of cuticular drusen in the Japanese population. Japanese Journal of Ophthalmology, 63(6), 448–456.
- ↑ Guigui B, Martinet V, Leveziel N, Coscas G, Soubrane G, Souied EH. (2009). Photodynamic therapy for choroidal neovascularisation secondary to basal laminar drusen. Eye, 23:2115-8.
- ↑ Ergun E, Costa D, Slakter J, Yannuzzi LA, Stur M. (2004). Photodynamic therapy and vitelliform lesions. Retina, 24:399-406.
- ↑ Chong, D. Y., & Johnson, M. W. (2009). Vitelliform Macular Detachment Associated With Basal Laminar Drusen Is Unresponsive To Vascular Endothelial Growth Factor Blockade. RETINAL Cases & Brief Reports, 3(1), 50–53.
- ↑ Age-Related Eye Disease Study Research Group. (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmology, 119:1417-36.
Acknowledgments
Claudia Krispel, MD, PhD for critical review of this article.