Macular Hole
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Macular Hole ICD-9 code: 362.54
Disease
A macular hole (MH) is a retinal break commonly involving the fovea.
Etiology and Risk Factors
Idiopathic macular hole is the most common presentation. Risk factors include age, female gender, myopia, trauma, or ocular inflammation.
General Pathology
Different findings can be observed depending the stage of the MH. Residual cortical vitreous, retinal glial, and retinal pigment epithelial cells are often found on the retinal surface. They are thought to cause tangential traction on the fovea. Cystoid edema in the outer plexiform and inner nuclear layers and thinning of the photoreceptor layer can also be observed.
Pathophysiology
It has been hypothesized that MHs are caused by tangential traction as well as anterior posterior vitreoretinal traction of the posterior hyaloid on the parafovea. MHs are noted to be a complication of a posterior vitreous detachment (PVD) at its earliest stages. MH have been implicated following anterior segment laser procedures which have been thought to be due to vitreoretinal traction.[1]
Primary Prevention
There are no preventative measures for idiopathic MHs. Pars plana vitrectomy has not been clearly demonstrated to be effective in preventing MH formation.
Diagnosis
This is a clinical diagnosis based on history and clinical exam, including slit lamp and dilated fundus examination. In some cases, optical coherence tomography (OCT) is useful in the diagnosis and management of this condition. It is important to distinguish between a full-thickness macular hole versus a lamellar hole (irregular foveal contour with defect in the inner fovea) or pseudohole (an irregular foveal contour with steep edges without true absence of retinal tissue often associated with an epiretinal membrane).
| — |
History
Patients with MHs typically present over the age of 60 and females are more frequently affected. Idiopathic MH occur with an estimated incidence of 8.69 eyes per 100,000 population per year in one study[2]. A careful history should be obtained to investigate for any of the risk factors mentioned above.
Physical Examination
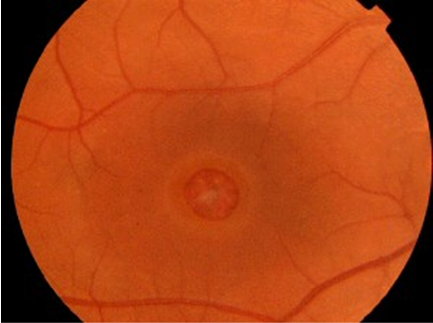
Slit lamp examination with special attention to the macula is important in the evaluation of this disorder (Figure 1). The Watzke-Allen sign can be used as a clinical test in cases of a suspected full thickness macular hole by shining a thin beam of light over the area of interest. The patient would perceive a “break” in the slit beam in cases of a positive test.
Careful examination of the fellow eye is also recommended given that MHs are bilateral in up to 30% of patients.[3] Special attention should be paid to the vitreoretinal interface, involutional macular thinning, and retinal pigment epithelial window defects because these are risk factors for MH development in the fellow eye. Patients without a PVD in the fellow eye have an intermediate risk (up to 28%) of developing a MH[4], whereas patients with a PVD are at low risk for developing a MH.
Signs
Depending on the stage of the MH, a subfoveal lipofuscin-color spot or ring can be noted. In more advanced cases, a partial or full-thickness macular break is observed.
Symptoms
Metamorphopsia (distortion of the central vision), central visual loss, or central scotoma can be reported.
Clinical Diagnosis
There are two main classification schemes for macular holes. Gass first described his clinical observations on the evolution of a macular hole[5]:
- Stage 1 MH, or impending MH, demonstrates a loss of the foveal depression. A stage 1A is a foveolar detachment characterized a loss of the foveal contour and a lipofuscin-colored spot. A stage 1B is a foveal detachment characterized by a lipofuscin-colored ring.
- Stage 2 MH is defined by a full thickness break < 400µm in size. It might be eccentric with an inner layer “roof.” This can occur weeks to months following Stage 1 MHs. A further decline in visual acuity is also noted. In most cases, the posterior hyaloid has been confirmed to be still attached to the fovea on OCT analysis.
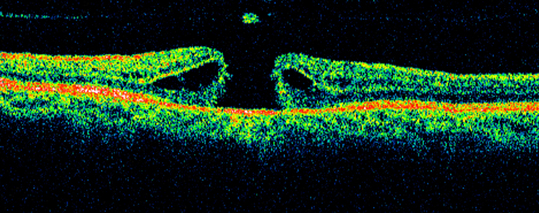
- Stage 3 MH is further progression to a hole ≥400 µm in size. Nearly 100% of stage 2 MHs progress to Stage 3 and the vision further declines. A grayish macular rim often denotes a cuff of subretinal fluid. The posterior hyaloid is noted to be detached over the macula with or without an overlying operculum (Figure 2 and 3).
- Stage 4 MH is characterized by a stage 3 MH with a complete posterior vitreous detachment and Weiss ring.
More recently, the The International Vitreomacular Traction Study (IVTS) Group also formed a classification scheme of vitreomacular traction and macular holes based on OCT findings[6]:
- Vitreomacular adhesion (VMA): No distortion of the foveal contour; size of attachment area between hyaloid and retina defined as focal if </= 1500 microns and broad if >1500 microns
- Vitreomacular traction (VMT): Distortion of foveal contour present or intraretinal structural changes in the absence of a full-thickness macular hole; size of attachment area between hyaloid and retina defined as focal if </= 1500 microns and broad if >1500 microns
- Full-thickness macular hole (FTMH): Full-thickness defect from the internal limiting membrane to the retinal pigment epithelium. Described 3 factors: 1) Size -- horizontal diameter at narrowest point: small (≤ 250 μm), medium (250-400 μm), large (> 400 μm); 2) Cause -- primary or secondary; 3) Presence of absence of VMT
Other features to note on exam include yellow deposits at the base of the hole, retinal pigment epithelial changes at the base of the hole, and epiretinal membrane adjacent to hole.
Figure 1: Clinical photo demonstrating a full thickness macular hole with a grayish macular rim suggestive of subretinal fluid. Note the retinal pigment epithelial changes at the base of the hole.
Figure 2: Optical coherence tomography image of a macular hole with an overlying operculum. The classic “anvil-shaped” deformity of the edges of the retina is noted due to intraretinal edema.
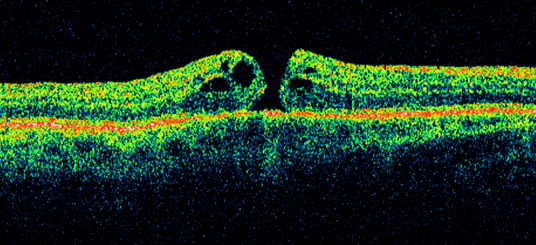
Figure 3: A small full thickness macular hole on optical coherence tomography with intraretinal and subretinal fluid.
Diagnostic procedures
Fluorescein angiography demonstrates a hyperfluorescence pattern consistent with a transmission defect due to loss of xanthophyll at base of the MH. This study is not usually necessary for diagnosis or management.
However, OCT is the gold standard in the diagnosis and management of this disorder. This high-resolution image can allow evaluation of the macula in cross-section and three-dimensionally. OCT can be helpful detecting subtle MHs as well as staging obvious ones.
OCT can also help guide management. OCT can assist in the determination of whether there is an associated epiretinal membrane or if the posterior hyaloid is still attached or not, which can be critical in deciding on the surgical approach. It can also be used to aid in gauging the prognosis of the fellow eye.
Laboratory test
No laboratory tests are indicated in cases of idiopathic MHs.
Differential diagnosis
The clinical appearance of a MH is fairly distinctive. However, epiretinal membrane with a pseudohole, lamellar hole, and vitreomacular traction must also be considered. Cystoid macular edema, subfoveal drusen, central serous chorioretinopathy, or adult vitelliform macular dystrophy are also in the differential diagnosis of a stage 1 MH.
Management
General treatment
The clinical stage and duration of the MH is the most important issue in the management of this entity.
Medical therapy
In general, most Stage 1 MH can be followed conservatively given approximately 50% chance of spontaneous closure[7]. However, if the patient has symptomatic VMT or even a full-thickness macular hole with associated VMT, some may consider one of the following treatment options:
- Intravitreal ocriplasmin. Ocriplasmin is a 27 kilodalton serine protease that essentially performs pharmacolytic vitreolysis, separating the hyaloid from the underlying retina. In the registration MIVI-TRUST clinical trials. At the day 28 post injection time-point, eyes receiving ocriplasmin (injected intravitreally) exhibited greater release of the vitreoretinal attachment (primary endpoint, 26.5% vs. 10.1% in controls who received an intravitreal injection of drug vehicle, p < 0.001) and closure of macular hole (40.6% vs. 10.6%, p < 0.001)[8]. There have been some rare adverse effects reported in association with use of this drug including electroretinography changes, lens subluxation, and dyschromatopsia.
- Intravitreal gas or air. More recently, some have found success in treating patients with a small bolus of injected intravitreal gas or air. Research studies are ongoing, but some have reported success rates as high as 83% for the closure of FTMH[9][10].
Medical follow up
Regardless of which treatment is pursued, the patient should be followed regularly. If the patient has an expansile gas placed in the eye, face-down positioning is often recommended for a short period of time, and it is important to remind the patient that he/she cannot fly or be at an elevation >2,500 feet until the gas dissipates.
Surgery
Surgery involves a pars plana vitrectomy procedure with tamponade. This can be done with or without peeling of the internal limiting membrane. A number of different instruments can be used to facilitate removal including intraocular forceps, pick, diamond dusted instruments, as well as other instruments. ICG green, Brilliant Blue, or triamcinolone acetonide are commonly used stains to assist in identifying the internal limiting membrane either by positive or negative staining.
Surgical technique has been debated for many years. While most vitreoretinal surgeons agree that tamponade is important, the type of tamponade and duration of postoperative positioning is debated. The importance of peeling the internal limiting membrane with or without staining has also been debated. More recently, new techniques including placing ILM flaps, neurosensory retinal grafts, amniotic membrane, platelet-rich plasma, autologous serum, perifoveal hydrodissection, or even lens capsule into larger holes have been reported with success[11][12][13][14][15][16].
Surgical follow up
Visual acuity improvement does not occur immediately in some patients. Visual improvement seems to be dependent on preoperative characteristics, duration of the MH, as well as other factors. Few postoperative instructions are as burdensome to our patients as face-down positioning after macular hole surgery. However, evidence from both recent studies and long-term clinical experience challenges this practice. A Cochrane review of 8 randomized controlled trials involving 709 eyes found no significant difference in macular hole closure rates between patients who maintained face-down positioning and those who did not. [17] Among patients with larger macular holes (≥400 μm), the closure rate was 94% in the face-down group compared to 84% in the non-face-down group. For smaller holes (<400 μm), the closure rate was 100% in the face-down group versus 96% in the non-face-down group and they concluded that face-down positioning may have little or no effect on macular hole closure after surgery. Similarly, Paul E. Tornambe, MD, in his 15-year review of macular hole repair without face-down positioning, reported a single-operation success rate of 92% in the no-face-down group compared to 90% in the face-down group. [18]Tornambe’s findings emphasize that the buoyancy of the gas bubble, rather than strict positioning, is key to isolating the macular hole and promoting healing. He noted that patients who avoided face-down positioning experienced similar anatomical success rates, with the added benefit of reduced discomfort and improved quality of life. The impact of this myth extends beyond patient discomfort to affect surgical decision-making and resource allocation. Many elderly or physically limited patients may be denied surgery based on concerns about positioning compliance, while others may require extended stays in skilled nursing facilities solely to ensure adherence. The evidence suggests that modified positioning regimens can achieve equivalent results, reducing the burden on patients and healthcare systems. It’s time to reconsider the necessity of strict face-down positioning and adopt more patient-friendly postoperative protocols.
Complications
The complications are similar to all eyes undergoing pars plana vitrectomy. In particular, these patients are at a higher risk for retinal tear and detachment. The vitreous is the most adherent to the optic nerve, macula, and vitreous base. Patients with macular holes may inherently have an abnormal vitreoretinal interface, and thus, during certain portions of vitrectomy, may have a higher risk for development of retinal tears or retinal detachment. Concurrent rhegmatogenous retinal and choroidal detachments in combination of macular holes are characterized by exceptional hypotony and poor vision.[19]
Prognosis
The visual outcomes following pars plana vitrectomy are very favorable. In general, better the preoperative visual acuity results in better postoperative visual acuity. However, eyes with worse preoperative visual acuity often experience the greatest absolute postoperative improvement. A small number of macular holes can recur after a successful closure with initial surgery[20].
Additional Resources
- Boyd K, Vemulakonda GA. Macular Hole. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/macular-hole-list. Accessed March 18, 2019.
- AAO PPP Retina/Vitreous Panel, Hoskins Center for Quality Eye Care. Idiopathic Macular Hole Preferred Practice Patterns. Idiopathic Macular Hole PPP 2019. American Academy of Ophthalmology. Accessed December 03, 2024.
References
- ↑ Tsui J C, Marks S J (January 10, 2021) Unilateral Stage 1A Macular Hole Secondary to Low-Energy Nd:YAG Peripheral Iridotomy. Cureus 13(1): e12603. doi:10.7759/cureus.12603
- ↑ McCannel CA, Ensminger JL, Diehl NN, Hodge, DN. Population Based Incidence of Macular Holes. Ophthalmology. 2009 Jul; 116(7): 1366–1369.
- ↑ McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol 1982;93(6):777-86.
- ↑ Trempe CL, Weiter JJ, Furukawa H. Fellow eyes in cases of macular hole: biomicroscopic study of the vitreous. 1986. Arch Ophthalmol 104:93–95.
- ↑ Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol 1988;106:629-39.
- ↑ Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole. Ophthalmology. 2013;120:2611-2619.
- ↑ la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002;80:579–587.
- ↑ Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606–615.
- ↑ Chan CK, Mein CE, Crosson JN. Pneumatic Vitreolysis for Management of Symptomatic Focal Vitreomacular Traction. Ophthalmic Vis Res. 2017;12(4):419-23.
- ↑ Jorge R, Costa RA, Cardillo JA, Uno F, Bonomo PP, Farah ME. Optical coherence tomography evaluation of idiopathic macular hole treatment by gas-assisted posterior vitreous detachment. Am J Ophthalmol. 2006 Nov; 142(5):869-71.
- ↑ Nawrocki J, Bonińska K, Michalewska Z. Managing Optic Pit. The Right Stuff! Retina. 2016 Dec;36(12):2430-2432.
- ↑ Liggett PE, Skolik DS, Horio BS, et al. Human autologous serum for the treatment of full-thickness macular holes. A preliminary study. Ophthalmology. 1995;102:1071-6.
- ↑ Konstantinidis A, Hero M, Nanos P, et al. Efficacy of autologous platelets in macular hole surgery. Clin Ophthalmol. 2013;7:745-50.
- ↑ Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36:163-70.
- ↑ Grewal DS, Mahmoud TH. Autologous neurosensory retinal free flap for closure of refractory myopic macular holes. JAMA Ophthalmol. 2016;134:229-30.
- ↑ Meyer CH, Borny R, Horchi N. Subretinal fluid application to close a refractory full thickness macular hole. Int J Retina Vitreous. 2017 Nov 27;3:44. doi: 10.1186/s40942-017-0094-7. PMID: 29209516; PMCID: PMC5702967.
- ↑ Cundy O, Lange CA, Bunce C, Bainbridge JW, Solebo AL. Face-down positioning or posturing after macular hole surgery. Cochrane Database Syst Rev. 2023;11(11):CD008228. Published 2023 Nov 21. doi:10.1002/14651858.CD008228.pub3
- ↑ Tornambe PE. Macular Hole Repair Without Face-down Positioning. Accessed December 29, 2024. https://retinatoday.com/articles/2010-sept/macular-hole-repair-without-face-down-positioning
- ↑ Tsui, Jonathan C MD1; Brucker, Alexander J MD1; Kolomeyer, Anton M MD, PhD1,2,*. Rhegmatogenous Retinal Detachment with concurrent Choroidal Detachment and Macular Hole Formation after Uncomplicated Cataract Extraction and Intraocular Lens Implantation – A Case Report and Review of Literature. Retinal Cases & Brief Reports: October 17, 2022 - Volume - Issue - 10.1097/ICB.0000000000001359
- ↑ Abbey AM, Van Laere L, Shah AR, Hassan TS. RECURRENT MACULAR HOLES IN THE ERA OF SMALL-GAUGE VITRECTOMY: A Review of Incidence, Risk Factors, and Outcomes. Retina. 2017 May;37(5):921-924.