X-linked Retinoschisis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
X-linked Retinoschisis (XLRS) is an inherited retinal disorder that can cause early vision loss in males.
Disease Entity
X-linked Retinoschisis, or X-Linked Juvenile Retinoschisis is a rare congenital disease of the retina caused by mutations in the RS1 gene, which encodes retinoschisin, a protein involved in intercellular adhesion and likely retinal cellular organization. X-linked retinoschisis has also been referred to as: juvenile retinoschisis, congenital retinoschisis, juvenile macular degeneration/dystrophy, degenerative retinoschisis, and vitreous veils of the retina.
Disease
X-linked retinoschisis, with a prevalence of about 1 in 15,000 to 30,000, is one of the main causes of juvenile macular degeneration in males.[2] It is characterized by symmetric bilateral macular involvement beginning in the first decade of life.[3] It is caused by a large variety of mutations in the RS1 gene on Xp22.1-p22.3, which encodes the protein retinoschisin. This protein is involved in intercellular adhesion and likely retinal cellular organization. X-linked retinoschisis is inherited in an X-linked manner with complete penetrance and variable expressivity. Most affected individuals are males, as heterozygous females are rarely affected. However, retinoschisis has been reported in non-consanguinous females.[4][5] The phenotype can be markedly variable even within the same genotype, and can involve the peripheral retina.
Pathophysiology
X-linked retinoschisis is linked to mutations in RS1 on Xp22.1-p22.3. The gene encodes a 224-amino acid protein called retinoschisin, which is secreted by photoreceptors. [6] This protein is found throughout the retina, and is thought to be involved in cell-cell adhesion and intercellular matrix retinal architecture development through interactions with αβ crystallin and β2-laminin. On histopathological examination, the splitting in X-linked retinoschisis occurs predominantly in the nerve fiber layer. [7]
Diagnosis
History
Patients typically present at school age complaining of poor vision or difficulty in school, although they may present in infancy with nystagmus or strabismus. Family history of childhood eye disease consistent with X-linked inheritance should be assessed.
Physical examination
There is large variation in disease severity among patients, even among patients with the same genetic mutation. Visual acuity may range from 20/20 to blindness, and depends on the amount and location of schisis. On first presentation, affected males typically have a visual acuity of 20/60 to 20/120. During the first and second decades of life, visual acuity may deteriorate slightly but then remains relatively stable until the fifth or sixth decade, when slowly progressive macular atrophy can arise. Visual loss may later progress to legal blindness (acuity <20/200).[3]
Peripheral vision may be normal in the unaffected areas. Absolute scotomas may be present in areas of peripheral retinoschisis, which are often inferotemporal. Color vision is often normal.
Patients may also present with strabismus, nystagmus, or amblyopia. Cycloplegic refraction may show hyperopia.
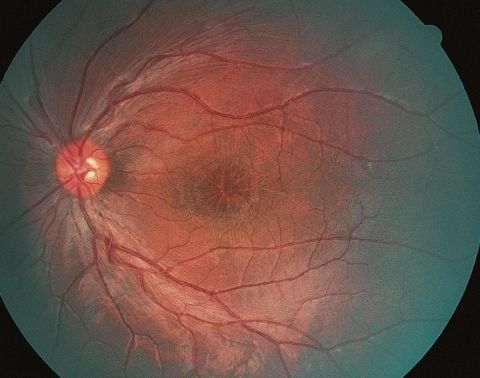
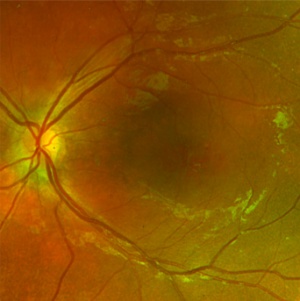
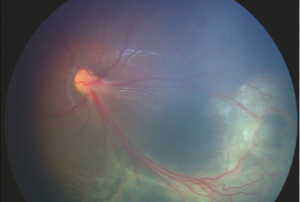
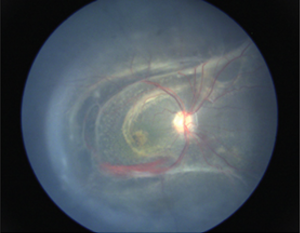
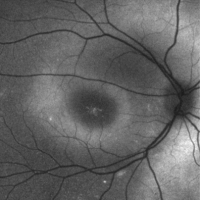
On fundoscopic examination, 98-100% of patients have foveal schisis, noted as a spoke wheel pattern radiating from the fovea and a domelike elevation of a thin layer of retina. It can be more easily identified with ophthalmoscopy using a red-free light. Schisis is most often in the macula, but occurs in the periphery in more than half of patients.[8] The bullous retinoschisis may improve over time. Individuals > 50 years old commonly have pigmentary changes and retinal pigment epithelium atrophy in the macula. The inner retina in areas of retinoschisis can develop large oval gaps.
Subretinal linear fibrosis, pigmentation, white retinal flecks, vascular attenuation, and vascular sheathing may also be present. In extreme cases, the inner layer of the retina is absent, with only the retinal vessels seen to be floating in the vitreous cavity in affected areas; these areas are referred to as vitreous veils. Peripheral dendritiform lesions which consist of sclerosed vessels may also be noted.
Visual function may be severely limited with progression to retinal detachment, which is most often rhegmatogenous and occurs in 5-20% of cases. Vitreous hemorrhage is another common complication, especially with peripheral schisis, and may result in deprivation amblyopia. Hemorrhage may also occur within the schisis cavity. Vitreous hemorrhage and retinal detachment are the main causes of complete vision loss.[9]
Other complications include intraretinal splitting, subretinal exudation, neovascular glaucoma, macular dragging, and optic atrophy.
Clinical diagnosis
Diagnostic procedures
- Digital fundus photography: may help with examination of a child
- Red-free illumination: may help to highlight the area of foveal schisis
- Fundus autofluorescence: increased fundus autofluorescence helps to highlight areas of foveal schisis
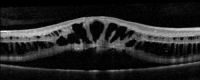
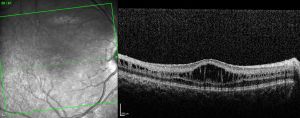
- Optical coherence tomography (OCT): usually demonstrates schisis in the superficial neural retina and thinning of the retina. In most school-aged children, there are often small cyst-like spaces peri-foveally and large cyst-like spaces within the fovea. After adolescence, the cystoid spaces may not be as evident because of flattening of these spaces with increasing age.[3] These cystoid spaces occur predominantly in the nerve fiber layer, although splitting can occur between other retinal layers as well.[10] OCT can reveal areas of schisis that may not be visible on fundus examination.
- Fluorescein angiography: can differentiate foveoschisis (no petaloid leakage, may show pooling of dye in schisis cavities at late phase) from cystoid macular edema (CME), which shows characteristic petaloid macular leakage. In younger individuals it may be normal. In older individuals there may be atrophic changes in the retinal pigment epithelium.
- Full-field electroretinogram (ffERG): electronegative (reduced b-wave with preserved a-wave, "negative waveform"). This is not diagnostic as the differential for electronegative ERG includes several other retinal disorders, the a-wave may be reduced as the disease progresses, and some affected individuals can have a technically normal ERG.
- Genetic testing for mutations in the RS1 gene can confirm the diagnosis. Over 200 disease-causing mutations in the RS1 gene have been identified.
Laboratory test
Genetic testing is available for the RS1 gene that encodes retinoschisin.
Differential diagnosis
- Autosomal dominant and recessive schisis will have a different inheritance pattern and may have a normal ffERG[11]
- Goldmann-Favre (Enhanced S cone syndrome) has associated nyctalopia and pigment clumping
- Degenerative retinoschisis (typically occurs in older individuals)
- Dominantly inherited CME and other causes of CME
- Eales disease
- Wagner syndrome
- Alport’s syndrome
Management
For those <10 years old: Annual evaluation by a pediatric ophthalmologist or retina specialist.[3]
Patient education: Avoid head trauma and high-contact/impact sports due to the increased risk of retinal detachment
Amblyopia: Treat amblyopia, especially in cases of severe retinoschisis, hypermetropia, or following surgery for vitreous hemorrhage or retinal detachment.
Genetic counseling: male patients should be counseled that they will pass the mutation to all daughters (who will most likely be asymptomatic heterozygote carriers), but will not pass the mutation to sons. Female carriers have a 50% chance of passing the mutation - all sons who inherit the mutation will be affected, and daughters who inherit the mutation will most likely be asymptomatic carriers.
For those with low vision: Low vision aids (large-print textbooks), preferential seating in the front of the classroom, handouts with high contrast.[3]
Medical therapy
Carbonic anhydrase inhibitors may help to improve the schisis cavities seen on OCT. Topical dorzolamide or systemic acetazolamide have both been reported to be beneficial in improving the cystic-appearing spaces on OCT.[12][13][14] Clinical improvement can be monitored with visual acuity improvements and reduction in the cystoid fluid on OCT.
Gene therapy with intra-ocular RS1 in knockout mice has restored b-wave function. In 2015, two human XLRS gene therapy trials were initiated (National Eye Institute: ClinicalTrials NCT02317887; AGTC, Inc: ClinicalTrials NCT0241662).
- The NEI trial is evaluating the safety of a gene transfer vector (AVV-RS1) in humans. It found closure of schisis cavities in one individual at higher dosing, which also caused ocular inflammation.[15]
- The AGTC trial is evaluating the safety and efficacy of a recombinant adeno-associated virus vector expressing retinoschisin (rAAV2tYF-CB-hRS1) in patients with X-linked retinoschisis. The study was halted due to inflammation and a lack of positive clinical treatment signs.
Surgery
- Complications such as retinal detachment and vitreous hemorrhage may require surgical intervention such as vitrectomy or scleral buckling.
- Laser photocoagulation may prevent retinal detachment. However, it may also induce detachment by creating iatrogenic retinal breaks. Thus, prophylactic laser is controversial.[10]
- External drainage has also been attempted.[16]
Prognosis
Onset usually occurs in the first decade of life, and visual acuity (VA) at first presentation is usually 20/60 to 20/120. VA often deteriorates in the first and second decades, but then is typically stable until the fifth or sixth decade (there may be some reduction in VA due to macular atrophy). By the sixth or seventh decade, VA may drop to legal blindness (i.e. VA <20/200).[3]
References
- ↑ American Academy of Ophthalmology. X-linked juvenile retinoschisis. https://www.aao.org/image/x-linked-juvenile-retinoschisis Accessed May 20, 2020.
- ↑ Sikkink SK, Biswas S, Parry NRA, Stanga PE, Trump D. X-linked retinoschisis: an update. Journal of Medical Genetics. 2007 Apr 1;44(4):225–32.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Sieving PA, MacDonald IM, Hoang S. X-Linked Congenital Retinoschisis. 2003 Oct 24 [Updated 2020 Nov 5]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1222/
- ↑ Saleheen D, Ali A, Khanum S, Ozair MZ, Zaidi M, Sethi MJ, et al. Molecular analysis of the XLRS1 gene in 4 females affected with X-linked juvenile retinoschisis. Can J Ophthalmol. 2008 Oct;43(5):596–9.
- ↑ Rodríguez FJ, Rodríguez A, Mendoza-Londoño R, Tamayo ML. X-linked retinoschisis in three females from the same family: a phenotype-genotype correlation. Retina (Philadelphia, Pa). 2005 Jan;25(1):69–74.
- ↑ Grayson C, Reid SNM, Ellis JA, Rutherford A, Sowden JC, Yates JRW, et al. Retinoschisin, the X-linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri–Rb1 cells. Hum Mol Genet. 2000 Jul 22;9(12):1873–9.
- ↑ Yassur Y, Nissenkorn I, Ben-Sira I, Kaffe S, Goodman RM. Autosomal Dominant Inheritance of Retinoschisis. American Journal of Ophthalmology. 1982 Sep 1;94(3):338–43.
- ↑ George NDL, Yates JRW, Moore AT. Clinical Features in Affected Males With X-Linked Retinoschisis. Arch Ophthalmol. 1996 Mar 1;114(3):274–80.
- ↑ George NDL, Yates JRW, Moore AT. Clinical Features in Affected Males With X-Linked Retinoschisis. Arch Ophthalmol. 1996 Mar 1;114(3):274–80.
- ↑ 10.0 10.1 Rao P, Vaidehi D, Drenser K. Congenital X-Linked Retinoschisis: An Updated Clinical Review. Asia-Pacific Journal of Ophthalmology: May 2018 - Volume 7 - Issue 3 - p 169-175. doi: 10.22608/APO.201803
- ↑ Yassur Y, Nissenkorn I, Ben-Sira I, Kaffe S, Goodman RM. Autosomal Dominant Inheritance of Retinoschisis. American Journal of Ophthalmology. 1982 Sep 1;94(3):338–43.
- ↑ Zhang L, Reyes R, Lee W, Chen C-L, Chan L, Sujirakul T, et al. Rapid resolution of retinoschisis with acetazolamide. Doc Ophthalmol. 2015 Aug;131(1):63–70.
- ↑ Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinoschisis. Retina (Philadelphia, Pa). 2006 Sep;26(7):741–5.
- ↑ Gupta K, Das D, Bhattacharjee H, Deka H, Magdalene D, Deshmukh S. Juvenile X-Linked retinoschisis: Response to topical dorzolamide therapy. TNOA J Ophthalmic Sci Res [serial online] 2018 [cited 2018 Sep 2];56:35-7. Available from: http://www.tnoajosr.com/text.asp?2018/56/1/35/233726
- ↑ Cukras CA, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y, Vijayasarathy C, Marangoni D, Ziccardi L, Kjellstrom S, Park TK, Hiriyanna S, Wright JF, Colosi P, Wu X, Bush RA, Wei LL, Sieving PA. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a Phase I/IIa trial by intravitreal delivery. Mol Ther. 2018;26:2282–94.
- ↑ Rishi E, Gopal L, Rishi P, Deshmukh H. Congenital x-linked retinoschisis: a novel approach for management of a large schitic cavity overhanging the macula. Retin Cases Brief Rep. 2009;3(1):105–7.