Electroretinogram
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Definition
The electroretinogram (ERG) is a diagnostic test that measures the electrical activity of the retina in response to a light stimulus. The ERG arises from currents generated directly by retinal neurons in combination with contributions from retinal glia. Importantly, the ERG is an objective measure of retinal function that can be recorded non-invasively under physiological conditions. ERGs are often recorded using a thin fiber electrode that is placed in contact with the cornea or an electrode that is embedded within a corneal contact lens. These electrodes permit the electrical activity generated by the retina to be recorded at the corneal surface. The ERG can be elicited by diffuse flashes or patterned stimuli. The International Society for Clinical Electrophysiology of Vision (ISCEV) has introduced standards for the different forms of ERG recordings. The ERG has important clinical utility, in that it provides diagnostic information concerning a variety of inherited and acquired retinal disorders. Moreover, the ERG can be used to monitor disease progression and evaluate retinal toxicity due to various drugs or retained intraocular foreign bodies.
History
The first known ERG was recorded from amphibian retina in 1865 by the Swedish physiologist Alarik Frithiof Holmgren. James Dewar of Scotland subsequently recorded the ERG in humans in 1877. In 1908, Einthoven and Jolly separated the ERG response into three components: a-wave, b-wave, and c-wave, which are further described below. Despite the early discovery of the ERG, widespread application did not occur until 1941, when American psychologist Lorin Riggs introduced a contact-lens electrode for ERG recording. Many of the observations that serve as the basis for our understanding of the ERG were conducted by Ragnar Granit, for which he won the Nobel Prize for Physiology and Medicine in 1967. Granit’s studies were primarily conducted on dark-adapted, rod-dominated cat retina. Using this model he was able to demonstrate the physiology underlying different ERG sources by altering the level of anesthesia and observing the loss of different ERG components. Modern pharmacological manipulations in various animal models have confirmed Granit’s findings and have extended our understanding of the cellular sources of the ERG.
Preparation of the patient
According to ISCEV 2015 full-field ERG guidelines:
- Avoid fundus photography, fundus autofluorescence, fluorescein angiography, and other intense illumination before ERG recording. If this is unavoidable, allow at least 30 min recovery time in ordinary room illumination.
- Maximally dilate the pupils (note pupil size before testing).
- There is no need to correct refractive error.
- Before dark-adapted protocols: 20 min of dark-adaptation.
- Before light-adapted protocols: 10 min of light-adaptation.
- If corneal contact lens electrodes are inserted after dark-adaptation, this should be performed under dim red light. Allow 5 min of extra dark adaptation after insertion of contact lens electrodes.
- Present low strength flashes before stronger flashes to avoid partial light adaptation from strong flashes.
- Request the patient to fixate steadily and not move his/her eyes. Ocular movements introduce large electrical artifacts, change electrode position, and may cause blockage of light by the eyelids/electrode.
- Young children and infants can be placed in the supine position, lying on their parent's legs, with their head under the stimulator.[1]
Types of Recording Electrodes
- Burian-Allen (BA): consists of an annular ring of stainless steel surrounding a polymethylmethacrylate (PMMA) contact-lens core. BA electrodes incorporate a lid speculum, which helps to minimize eye blinks/closure. BA lenses are reusable and are available in sizes ranging from pediatric to adult.
- Dawson-Trick-Litzkow (DTL): Low-mass conductive silver/nylon thread. DTL electrodes are disposable and are typically more comfortable for the patients, as compared to other corneal electrodes.
- Jet: disposable plastic lens with a gold-plated peripheral circumference.
- Skin Electrode: may be used as a replacement for corneal electrodes by placing an electrode on the skin over the infraorbital ridge near lower eyelid. ERG amplitudes tend to be small and noisy, but skin electrodes are better-tolerated in pediatric populations.
- Mylar Electrode: aluminized or gold-coated Mylar (not in common use).
- Cotton-Wick: Burian-Allen electrode shell fitted with a cotton wick, which is useful for minimizing light-induced artifacts (not in common use).
- Hawlina-Konec Electrode: Teflon-insulated thin metal wire (silver, gold, platinum) with three central windows, 3 mm in length, molded to fit into the lower conjunctival sac (not in common use).
Placement of electrodes
Recording electrodes: in contact with cornea, bulbar conjunctiva, or skin below lower eyelid
- Protect corneal surface with non-irritating ionic conductive solution (artificial tears or contact lens solutions containing sodium chloride and no more viscous than 0.5% methyl cellulose). Improper installation of contact lens electrodes can cause corneal abrasions.
- Topical anesthesia is used for contact lens electrodes, but may not be necessary for DTL electrodes.
Reference and ground electrodes
- Electrical activity from the corneal electrode is compared to that of a reference electrode placed at a distant site (ear, forehead, temple are common).
- A differential amplifier is typically used to amplify the difference between two inputs (corneal electrode and reference electrode) and reject signals that are common to both inputs (relative to a ground electrode placed at a third site).
- Reference and ground electrodes are commonly made of a highly conductive material that is fixed to the patient with paste. Gold cup electrodes are common, because they can be reused; disposable adhesive skin electrodes are also available.
- Some corneal electrodes contain a reference, which obviates the need for a reference to be placed elsewhere (e.g. BA bipolar electrodes and some skin electrodes).
Full-field ERG
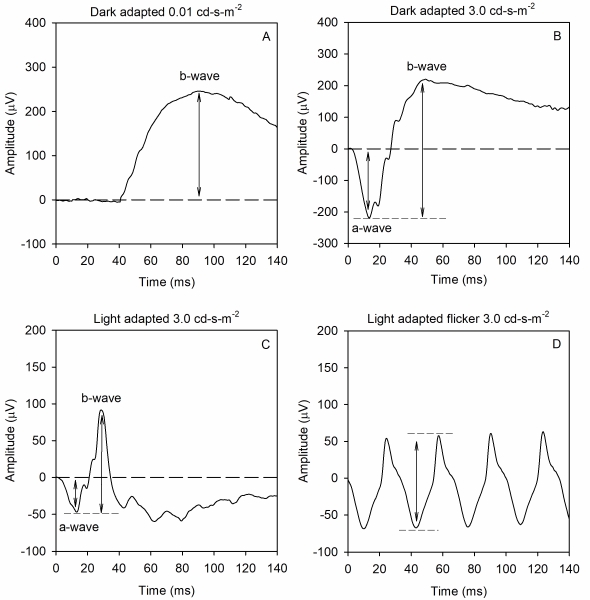
The full-field ERG is a mass response of the retina that has contributions from several retinal sources, summed throughout the retina. This is useful in diseases that have widespread retinal dysfunction: e.g. rod/cone dystrophies, cancer associated retinopathy, and toxic retinopathies. Importantly, the ffERG is not useful for detecting small retinal lesions. The ffERG waveform components and their underlying sources depend on both the strength of the stimulus flash and the state of adaptation. That is, scotopic measurements that target rod-pathway function are made from the dark-adapted eye, whereas photopic measurement that target cone-pathway function are made from the light-adapted eye. A minimum set of responses that should be obtained has been defined by the International Society for Clinical Electrophysiology of Vision (ISCEV) in 1989, which were most recently updated in 2015. Examples of the minimum ISCEV-specified ffERG set of responses under dark- and light-adapted conditions are shown below (See Figure 1).
Panel A shows the ffERG recorded under dark-adapted conditions in response to a weak, diffuse, full-field flash of light. This stimulus elicits a slow cornea-positive potential, termed the b-wave, that is primarily generated by ON-type bipolar cells. The response is quantified by measuring the amplitude of the b-wave from the pre-stimulus baseline voltage (0 µV) to the peak of the response. Timing of the response is also measured: the implicit time of the b-wave is defined as the time between the flash and the peak of the response.
Panel B shows the ffERG recorded under dark-adapted conditions in response to a stronger flash of light. This stimulus elicits a rapid cornea-negative potential, termed the a-wave, and a subsequent positive b-wave. The amplitude of the a-wave is typically measured from the pre-stimulus baseline (0 µV) to the trough of the a-wave. The implicit time of the a-wave is measured from the time of the flash to the trough of the a-wave. The amplitude of the b-wave is measured from the trough of the a-wave to the peak of the b-wave. The implicit time of the b-wave is measured from the time of the flash to the peak of the b-wave. This response is often referred to as the “mixed rod-cone response,” as there are contributions from both rods and cones to the a-wave. However, the rod contribution exceeds the cone contribution, given the rod/cone distribution of the human retina. The b-wave is generated by ON- and OFF-type bipolar cells. Certain conditions including complete congenital stationary night blindness, melanoma-associated retinopathy, and juvenile X-linked retinoschisis produce a characteristic abnormality of this response that has been termed “electronegative.” Specifically, the a-wave has a normal (or nearly normal) amplitude, whereas the b-wave is markedly attenuated. Thus, an electronegative response can have diagnostic value. Of note, a series of wavelets can be seen on the ascending portion of the b-wave. These wavelets are termed oscillatory potentials (OPs) and are thought to be generated primarily by amacrine cells, but details of their source are presently debated. OPs that are reduced in amplitude and/or delayed in time often indicate disorders of the retinal blood supply.
Panel C shows the ffERG recorded under light-adapted conditions in response to a strong flash presented against a light background. The intent of the light background is to suppress the rod response, allowing for assessment of the cone pathway. This stimulus elicits a negative a-wave and a positive b-wave, much like that shown in panel B. The amplitude and implicit times of the a- and b-waves are quantified in the same manner as that for the dark-adapted responses shown in panel B. Given that this response is recorded under photopic conditions, the a-wave is generated by cone photoreceptors, with additional contributions from OFF-type bipolar cells. The b-wave is generated by a combination of ON- and OFF-type bipolar cells.
Panel D shows the ffERG elicited by a 31-Hz flicker train. Rapid flicker is a useful stimulus for assessing cone-pathway function, because rod photoreceptors generally cannot follow rapid flicker. Each stimulus flash of the flicker train generates a response that has a peak and a trough. The amplitude of the flicker ERG is typically defined as the trough-to-peak amplitude, whereas the timing of the flicker response is typically defined as the time between a stimulus flash and the corresponding response peak.
Other waveform components
Photopic negative response (PhNR): The PhNR is a slow negative potential that follows the b-wave recorded under light adapted conditions (panel C, above). The PhNR has gained interest because it is primarily driven by retinal ganglion cells. Thus, it is one of the few ffERG components that provides insight into retinal ganglion cell function. The most effective measure of the PhNR and the optimal recording conditions are debated, but it is often measured from the pre-stimulus baseline to the trough of the response, or at a fixed time following the stimulus flash. In 2018, ISCEV published guidelines for measuring and reporting the PhNR.
c-wave: The c-wave is a slow positive component that follows the b-wave and is generated from the retinal pigment epithelium and photoreceptors. Conventional ISCEV recordings do not provide assessment of the c-wave.
d-wave: The d-wave is a rapid positive potential that follows light offset and is generated by OFF-type bipolar cells. Conventional ISCEV recordings do not provide assessment of the d-wave.
Reporting ffERG according to ISCEV standards
Reports should include:
- At least 20 ms of baseline recording before the stimulus for single flash ERGs
- Stimulus onset time should be marked
- At least 2 responses from each stimulus condition should be obtained to validate consistency/assess variability
- The time-integrated luminance of the stimulus (cd-s-m-2) and background luminance (cd/m2 ) should be reported
- Include reference values and range
- Note deviations from the standard ISCEV protocol
- Time of testing
- Pupil diameter
- Type and position of electrodes
- Any sedation/anesthesia
- Level of compliance
Factors affecting the ffERG
- Duration of stimulus
- Size of retinal area illuminated (amplitude can be reduced if the stimulus is not full-field because the patient is positioned too far from the stimulus source)
- Interval between stimuli
- Size of pupil
- Systemic circulation and drugs
- Development of retina
- Clarity of ocular media (note that mild cataract has minimal effects on the ffERG)
- Age
- ERG amplitude can be reduced in high myopia
- Anesthesia
Other Types of ERG Measurement
Focal ERG (fERG)
The focal ERG (fERG) is used primarily to measure the functional integrity of the central macula and is therefore useful in providing information in diseases limited to the macula. At present, this technique is not in common use, in part due to a lack of commercially-available instruments. In addition, the multifocal ERG (discussed below) can be used to assess macular function. The electrode types and placement discussed for the ffERG can also be applied for fERG measurement. A variety of approaches have been described in the literature for recording fERGs. Differing field sizes varying from 3 degrees to 18 degrees and stimulus temporal frequencies have been used in the various methods. However, each technique must address the challenge of limiting amount of light scattered outside the focal test area. fERG is useful for assessing macular function in conditions such as age-related macular degeneration, however good fixation from the subject is required.
Multifocal ERG (mfERG)
The multifocal ERG (mfERG) assesses many local ERG responses, typically 61 or 103, within the central 30 degrees. This provides important spatial information that is lacking in the ffERG, allowing dysfunction within the macula that might be missed by ffERG to be assessed. mfERG responses are recorded under light-adapted conditions from the cone-pathway. It is important to note that mfERG is not a replacement for the ffERG: if pan-retinal damage or rod pathway dysfunction is suspected, then the ffERG should also be performed. The mfERG is becoming more commonly used for both research and clinical purposes, and ISCEV provided the first standards for mfERG in 2007 (updated in 2011).
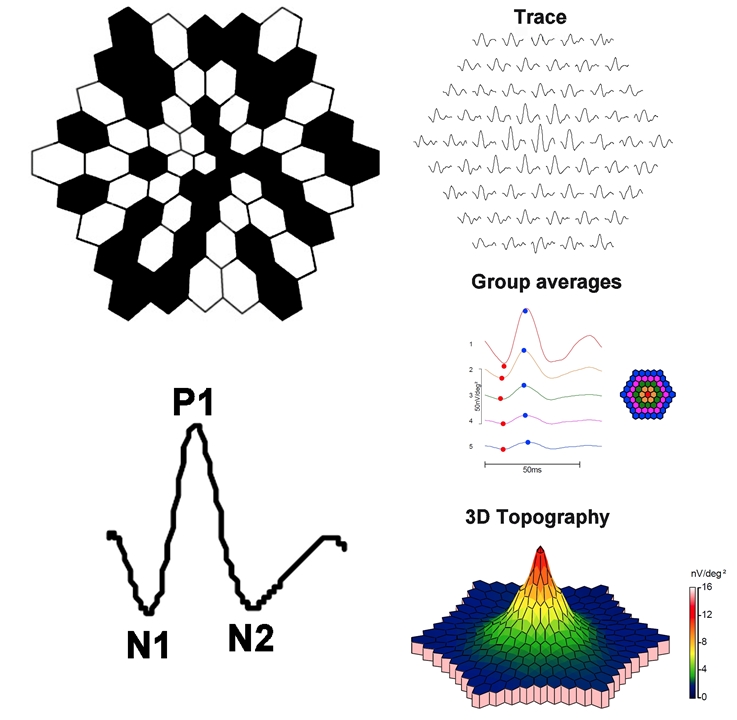
Clarity of the ocular media and proper refraction are important for mfERG measurement. Electrodes and their placement can be the same as those described for the ffERG. A scaled hexagonal pattern, like that shown below, is commonly used to elicit the mfERG. Each of the hexagons in the stimulus has a 50% chance of being illuminated at a given time. Although random in appearance, the same on/off sequence is used for each hexagon (an “m-sequence”). This permits a response to be recovered for each stimulus hexagon. The resulting mfERG waveforms (shown below) are similar in shape to those of the light-adapted ffERG: there is an initial negative deflection (termed N1), followed by a positive deflection (termed P1), and a second negative deflection (termed N2). Research indicates that N1 has generators similar to those of the a-wave of the light-adapted ffERG, whereas the P1 and N2 have generators that are similar to the light-adapted b-wave and OPs. However, the manner in which the mfERG is elicited and processed differs considerably from the ffERG; as such, the mfERG response is not necessarily a miniature ffERG.
This approach produces a wealth of information and there are several ways in which the information can be condensed for display. Example mfERG responses from a normal eye are shown below. The same mfERG data are displayed in three different ways. The array of traces in the top row show the mfERG response obtained from each hexagon. The middle panel of traces shows ‘ring averages.’ These are average mfERG traces within rings of different eccentricity. The red trace, for example, is the mfERG response obtained from the fovea, whereas the orange trace is the average of the ring of hexagons immediately surrounding the fovea. The other traces represent averages of rings of increasing eccentricity, as shown in the schematic to the right. Often, the ratio of amplitudes within rings is compared (i.e. the “ring ratios”). The lower image is a three-dimensional mfERG amplitude plot. This topography plot shows the largest amplitude at the fovea, with a generally uniform decline in amplitude moving towards more eccentric locations. Another useful approach to visualizing the data is to plot the standard deviation of the amplitude (or implicit time) relative to visually-normal controls within each hexagon. Thus, there are a number of ways in which the responses can be summarized for display; the optimal visualization is guided by the question that is being pursued.
Given that mfERGs are useful for detecting localized abnormalities within the macula, a common application has been in assessing retinal dysfunction in hydroxychloroquine toxicity. The mfERG abnormality observed in these patients is often a decrease in the second ring amplitude, relative to the central ring. The mfERG has also been recorded in conditions such as retinitis pigmentosa, branch retinal artery occlusion, and Stargardt disease.
Pattern ERG (pERG)
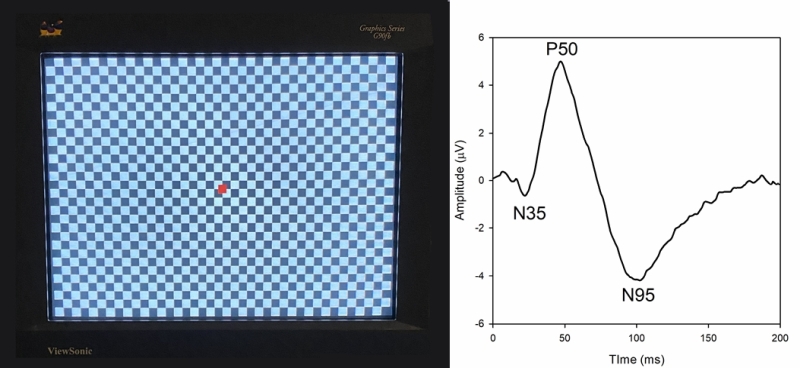
The pattern ERG (pERG) uses contrast reversing pattern stimuli (sinewave gratings or checkerboards) to assess macular retinal ganglion cell (RGC) activity. Electrodes and their placement may be the same as those described for the ffERG. However, contact lens electrodes are often avoided to maintain optimal optical quality of the stimulus. Clarity of the ocular media and proper refraction are important for pERG measurement. The pERG is typically recorded with natural pupils. ISCEV has provided a standard for recording the pERG that has most recently been updated in 2012. An example of a common pERG stimulus is shown below (See Figure 3, left). Over time, the dark checks become light, and the light checks become dark (typically at a rate of 4 reversals per second). It is important that there is no net change in luminance during the dark-to-light transition of the checks (i.e. the average luminance of the screen must be constant over time), or a luminance artifact will be introduced into the response.
Given that the pERG responses have relatively small amplitude, many repetitions are obtained in clinical practice. The trace below (See Figure 3, right) shows the pERG from a visually-normal individual (average of 150 responses). The pERG waveform consists of a small negative deflection near 35 ms, termed the N35 component, a positive deflection near 50 ms, termed the P50 component, and a negative deflection near 95 ms, termed the N95 component. The amplitude and implicit time of each of these components can be measured. Of note, this waveform is characteristic of the “transient pERG” obtained with a stimulus that reverses 4 times per second, so that the response is essentially complete before the next contrast reversal begins. For higher reversal rates (e.g. 16 reversals per second) a “steady-state” pERG is produced, which has different characteristics.
The N95 component is markedly reduced or eliminated in experimental glaucoma or by blocking action potentials using tetrodotoxin. Thus, the N95 component is likely generated by action potentials from RGCs. The source of the P50 is debated, but there is some evidence suggesting that it is generated by RGCs with additional contributions from more distal sites. The P50 and N95 components are dependent on macular cone function, as the photoreceptors provide input into the RGCs. Macular cone dysfunction can reduce the amplitude of the P50 and delay the response. Selective reduction of the N95 amplitude, with preservation of the P50 component, suggests RGC dysfunction. The pERG can be useful for assessing RGC function in conditions such as glaucoma and ischemic optic neuropathy. The pERG has also been shown to be abnormal in diabetic retinopathy and idiopathic intracranial hypertension.
Abnormalities in various disease states
| Disease entity | Full-field ERG findings | Multifocal ERG findings |
|---|---|---|
| Abusive head trauma | reduction of the b wave; preservation of the a wave[2] | reduction of the b wave; preservation of the a wave[2] |
| Anesthesia | Amplitude of scotopic responses; implicit time prolongation of all components[1] | Abnormal |
| Achromatopsia (rod monochromacy) | Scotopic responses are normal/nearly normal; photopic responses are undetectable | Abnormal |
| Batten disease | Abnormal scotopic responses; strong flash response can be electronegative; photopic responses are abnormal | Abnormal |
| Best vitelliform macular dystrophy | Normal ffERG (abnormal electroocoulogram) | Possible mfERG abnormalities that localize to lesion location |
| Birdshot chorioretinopathy | Variable depending on disease state; photopic flicker response is commonly delayed; responses may be super-normal in early stages and reduced/delayed in late stages | Can be reduced/delayed; few reports are available in the literature |
| Cancer associated retinopathy (CAR) | Often severely abnormal or undetectable; photopic responses often more abnormal than scotopic | Often significantly abnormal |
| Central retinal artery and vein occlusions | Often significantly abnormal; reduced scotopic b-wave amplitude; OP abnormalities | Variable |
| Chloroquine/Hydroxychloroquine | Scotopic and photopic responses are variable in mild cases; more likely to be abnormal in severe | Parafoveal abnormality in early stages with later fovea/central involvement |
| Choroideremia | Often severely abnormal; scotopic responses often worse than photopic | Typically abnormal, particularly with late macular involvement |
| Cone dystrophy | Abnormal photopic responses with normal/nearly normal scotopic responses | Often shows early and severe abnormalities |
| Congenital red-green color deficiency | Normal | Normal |
| Cone-rod dystrophy | Cone and rod abnormalities; photopic responses are more affected than scotopic responses | Often shows early and severe abnormalities |
| Congenital stationary night blindness (Complete; Schubert-Bornschein type) | Dark adapted weak flash response is absent; strong flash response is electronegative; photopic responses are usually abnormal | Abnormal |
| Congenital stationary night blindness (Incomplete; Schubert-Bornschein type) | Dark adapted weak flash response is abnormal; strong flash response is electronegative; photopic responses are substantially abnormal | Abnormal |
| Congenital stationary night blindness (Riggs type) | Scotopic responses are absent; photopic responses are typically normal | Normal |
| Diabetic retinopathy | Variable depending on disease stage; oscillatory potentials can be abnormal in early stages; flicker responses can be reduced and delayed; PhNR can be reduced | Patchy abnormalities; location of timing delays may correlate with present/future microaneurisms |
| Enhanced S-cone syndrome | Undetectable/significantly abnormal scotopic responses; significantly abnormal photopic responses | Abnormal |
| Fundus albipunctatus | Abnormal scotopic responses; variable photopic responses; scotopic responses improve after prolonged dark adaption | Variable |
| Leber congenital amaurosis | Severely abnormal or undetectable scotopic and photopic responses; abnormalities often present in infancy | Abnormal |
| Melanoma-associated retinopathy (MAR) | Dark adapted weak flash response is absent; strong flash response is electronegative; photopic responses are variable, but can be abnormal | Abnormal |
| Multiple evanescent white dot syndrome (MEWDS) | Scotopic/photopic abnormalities that resolve following the acute phase | Variable; abnormalities can be observed that resolve following the acute phase |
| North Carolina Macular Dystrophy | Typically normal | Abnormal in central macula |
| Oguchi disease | Dark adapted weak flash response is absent; strong flash response is electronegative; photopic responses are normal; scotopic responses improve after prolonged dark adaption | Normal |
| Pattern dystrophy | Normal | Normal |
| Quinine toxicity | Abnormal scotopic responses; strong flash response can be electronegative; abnormal photopic responses | Abnormal |
| Retinitis pigmentosa | Severely abnormal or undetectable scotopic responses; photopic responses are variable, but usually abnormal; scotopic/photopic are undetectable in late-stage | Variable |
| Retinopathy of prematurity | Lower sensitivity of rods, which corresponds with severity of ROP[3] | May be abnormal |
| Siderosis | Usually abnormal; scotopic responses are usually more affected than photopic; initially can produce supernormal responses followed by amplitude loss over time | Can be abnormal |
| Stargardt disease | Variable: can find normal scotopic and photopic responses; normal scotopic and abnormal photopic; abnormal scotopic and photopic | Abnormal |
| Vitamin A deficiency | Abnormal scotopic responses; normal photopic responses (but can vary) | Normal |
| X-linked retinoschisis | Dark adapted weak flash response is significantly reduced/absent; strong flash response is often electronegative; photopic responses are abnormal | Abnormal |
References
- McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015). ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
- Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM (2012). ISCEV Standard for clinical multifocal electroretinography (2011 edition). Doc Ophthalmol 124:1–13
- Bach M, Brigell MG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S (2013). ISCEV standard for clinical pattern electroretinography (PERG) – 2012 update. Doc Ophthalmol 126:1–7
- Frishman L, Sustar M, Kremers J, McAnany JJ, Sarossy M, Tzekov R, Viswanathan S. (2018). Protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Oph. 136:207-211.
- Brigell M, Bach M, Barber C, Moskowitz A, Robson J (2003). Guidelines for calibration of stimulus and recording parameters used in clinical electrophysiology of vision. Doc Ophthalmol 107:185–193
- Robson AG, Nilsson J, Li S, Jalali S, Fulton AB, Tormene AP, Holder GE, Brodie SE (2018). ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol 136:1–26.
- Marmor MF, Cabael L. (2018). Clinical display of mfERG data. Doc Ophthalmol. 137:63-70.
- Electrophysiologic Testing in Disorders of the Retina, Optic Nerve, and Visual Pathway (Pearls Series) by Gerald Allen Fishman M.D. Publication Date: January 2, 2001 | ISBN-10: 1560551984 | ISBN-13: 978-1560551980 | Edition: 2
- Principles and Practice of Clinical Electrophysiology of Vision. Heckenlively JR, Arden G. (eds). Cambridge, MA, MIT Press; 2006.
- Tzekov R, Arden GB (1999) The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 44(1):53-60.
- Bearse MA Jr, Ozawa GY (2014). Multifocal electroretinography in diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 14:526.
- Vincent A, Robson AG, Holder GE. (2013). Pathognomonic (Diagnostic) ERGs a Review and Update. Retina, the Journal of Retinal and Vitreous Diseases. 33: 5-12.
- ↑ 1.0 1.1 Parness-Yossifon, R. & Mets, M. B. (2008). The electroretinogram in children. Current Opinion in Ophthalmology, 19 (5), 398-402. doi: 10.1097/ICU.0b013e32830abf11.
- ↑ 2.0 2.1 Greenwald MJ, Weiss A, Oesterle CS, Friendly DS. Traumatic retinoschisis in battered babies. Ophthalmology. 1986 May;93(5):618-25. doi: 10.1016/s0161-6420(86)33688-1. PMID: 3725321.
- ↑ Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol 2001; 119:499–505.