Pseudophakic Bullous Keratopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Bullous keratopathy - ICD-10-CM H18
Disease
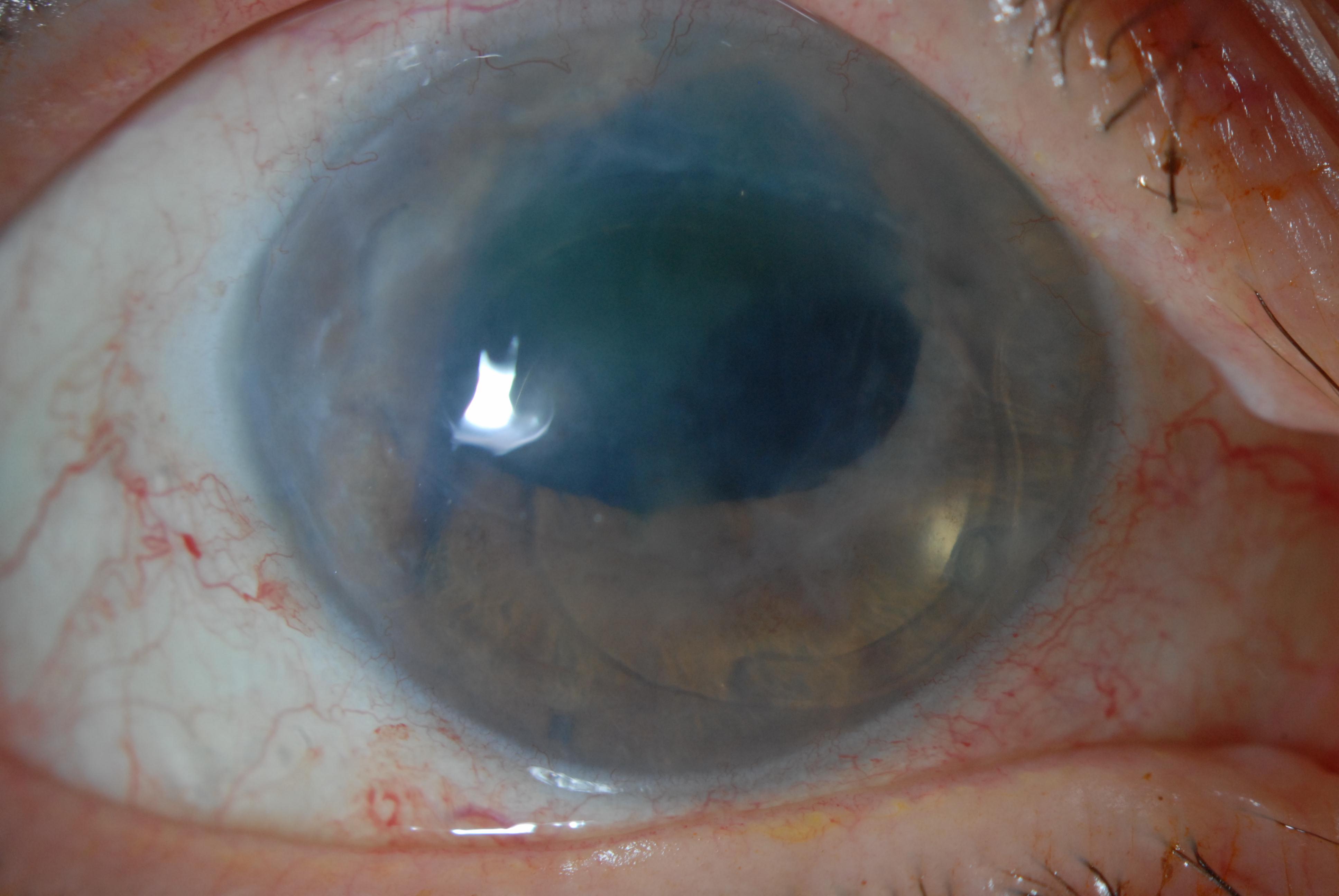
Pseudophakic bullous keratopathy (PBK) or pseudophakic corneal edema (PCE) traditionally refers to the development of irreversible corneal edema after cataract surgery and intraocular lens (IOL) implantation.[1][2] Initially, there is endothelial trauma, which is followed by progressive stromal and epithelial edema. The epithelial edema results in the formation of bullae, hence the name bullous keratopathy.
Etiology
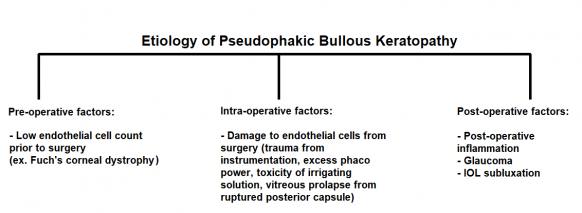
The corneal endothelium is important in maintaining corneal transparency. Loss of corneal endothelial cells pre-operatively, intra-operatively, and post-operatively are all thought to contribute to the development of irreversible corneal edema in PBK. [1]
Pre-operatively, low endothelial cell counts increase the risk of PBK in these eyes. Intra-operatively, surgical trauma is an important cause of endothelial loss. This can include direct damage of the corneal endothelium from instrumentation and the IOL, excess use of phaco power, toxicity of irrigating solutions (toxic anterior segment syndrome or TASS), and posterior capsular rupture with vitreous prolapse into the anterior chamber. Post-operatively, various entities can increase risk of developing post-operative corneal edema including but not limited to inflammation and glaucoma.
The average time to the development of PBK after cataract surgery ranges from 8 months to 7 years. [1]
Risk Factors
In some cases, PBK can occur after an uneventful cataract surgery without any apparent predisposition. Eyes that are at risk for having lower pre-operative endothelial cell counts are at increased risk of developing PBK.
- Advanced age
- Pre-existing Fuchs' corneal dystrophy or other endothelial diseases
- Insertion of anterior chamber intraocular lens (ACIOL)
- Previous intraocular surgery
- Shallow anterior chamber
- Glaucoma
- Previous ocular trauma
- Intraoperative manipulations
- Systemic conditions (diabetes, chronic obstructive pulmonary disease)
- Toxic anterior segment syndrome
Prevention
In eyes with increased risk of developing PBK, it is important to modify the surgical technique to lower the risk. Dispersive viscoelastic should be applied generously and frequently during the case. Ultrasound sparing techniques including use of pre-chop techniques should be considered. In high-risk cases, zero-ultrasound techniques like extracapsular cataract extraction or small incision cataract surgery (SICS) can also be considered.
Pathophysiology
The endothelial cells play an important role in maintaining corneal transparency by continuously pumping fluid out of the corneal stroma. Damage to the endothelial cells will adversely affect corneal transparency when aqueous entering the cornea outweighs the pumping capacity of the remaining viable endothelial cells. Roughly, 700 cells/mm2 are needed to maintain corneal transparency. [1]
Epidemiology
It is estimated that 1-2 % of people undergoing cataract surgery will develop persistent post-operative edema. Old surgical techniques and intraocular lens designs were associated with higher numbers of post-operative corneal edema. Currently, pre-operative endothelial cell count, and surgical trauma are the most important factors for the development of PBK. No race or sexual predilection has been found in cases of PBK. [2][3]
Diagnosis
The diagnosis of PBK is primarily clinical, based on history, careful evaluation of risk factors including a detailed history of previous intraocular surgery, and slit lamp examination.
History
Patients with PBK will typically be older with a previous history of intraocular surgery in the affected eye. They will report gradual worsening of their vision, particularly in the morning. Patients may also indicate that their previous intraocular surgery was not straightforward, with intra-operative and/or post-operative complications.
Physical examination
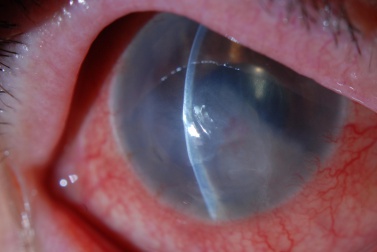
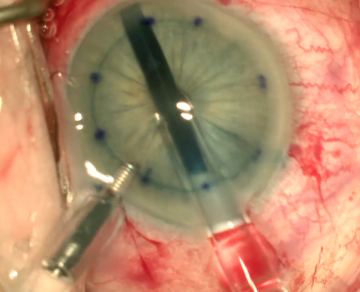
Vision will be decreased in the affected eye. Slit lamp examination may demonstrate central corneal edema with associated corneal thickening and presence of deep corneal folds. Signs of previous intra-operative surgical complications including iris trans-illumination defects, pupil irregularity, ruptured posterior capsule, and presence of vitreous in the anterior chamber may be present. The location and type of intraocular lens, if present, must be noted. Careful examination of the corneal endothelium should be performed. In more advanced stages of PBK, bullae and vesicles may be observed on the corneal surface. The contra-lateral eye should be examined to look for an underlying endothelial corneal dystrophy.
Signs
- Corneal edema (epithelial, stromal, and endothelial)
- Endothelial folds
- Epithelial microcysts
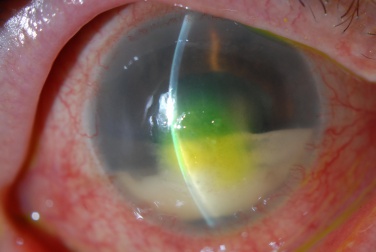
- Epithelial bullae
- Stromal haze and scar formation
- Epithelial defect
- Corneal neovascularization
- Increased corneal thickness
Symptoms
Patients may be asymptomatic at presentation. In the early stage, patients may note mild reduction in visual acuity and contrast sensitivity. In more advanced stages, patient may note a significant decline in vision corresponding to presence of stromal or epithelial edema. They may also endorse discomfort and pain, due to the swelling of the epithelium with resultant stretching of corneal nerves or from a ruptured corneal bullae.
Diagnostic procedures
In addition to slit lamp examination, a number of procedures can be utilized to confirm the diagnosis:
- Corneal pachymetry can reliably obtain central corneal thickness (CCT) over the area of corneal edema and confirm the presence of increased corneal thickness. Preoperative corneal pachymetry may be useful as pre-operative CCT values over 640 microns predispose a patient to developing irreversible corneal edema after cataract surgery. In such cases, it may be prudent to plan cataract surgery along with endothelial keratoplasty.
- Specular microscopy can confirm endothelial dysfunction, as evidenced by a low endothelial cell count or variability in endothelial cell size and shape.
Differential diagnosis
- Fuchs' endothelial corneal dystrophy
- Posterior polymorphous corneal dystrophy (PPMD)
- Congenital hereditary endothelial dystrophy (CHED)
- Iridocorneal endothelial syndrome (ICE)
- Herpetic stromal keratitis (HSK)
- Recurrent corneal erosion syndrome (RCES)
Management
Management of PBK is usually medically managed first. However, if medical management fails, surgery should be considered.
Medical therapy
Medical treatment should be instituted if the patient is symptomatic from the corneal edema. Hypertonic saline drops (Sodium Chloride 5%) and ointment (Sodium Chloride 6%) are the first line treatment. These drops create a hypertonic tear film, drawing water out of the cornea. Ruptured epithelial bullae should be managed as similar to a corneal abrasion, including treatment with antibiotic and lubricating drops, and use of bandage contact lens for symptomatic relief. Infected epithelial bullae should be managed as an infectious keratitis with antibiotic drops. Corneal cultures should be performed if the keratitis is vision threatening.
Surgery
Corneal transplantation is the definitive treatment for PBK as it restores the normal structure and function of endothelial cells. It can be done in the form of penetrating keratoplasty (PK), Descemet membrane endothelial keratoplasty (DMEK) or Descemet stripping automated endothelial keratoplasty (DSAEK). [4][5] DMEK has shown to have better graft survival and lower rejection rates for cases of PBK when compared to PK or DSAEK. [5] Other surgical options such as corneal collagen cross linking (CXL), amniotic membrane transplant (AMT), anterior stromal puncture (ASP) and phototherapeutic keratectomy (PTK), all can be used to relieve pain and/or improve corneal edema temporarily while awaiting corneal transplantation. [6][7] CXL with riboflavin and ultraviolet A is thought to induce stromal compaction with thinning and less water influx into the cornea and consequently less fluid in the subepithelial space (bullae formation). Therefore, it helps in both improving vision and in managing pain. [6] AMT on the other hand helps in pain control by its composition of various growth factors and protease inhibitors which promote epithelial cells migration and adhesion to the underlying basement membrane. [7] ASP is thought to work similarly by increasing the formation of various extracellular matrix proteins that increase the adhesion of the epithelial cells to the underlying stroma. It also induces subepithelial fibrosis which acts as physical barrier limiting fluid migration to the subepithelial space. Lastly, PTK can help in decreasing pain perception by ablations the subepithelial nerve plexus. It also strengthens the adhesions between the epithelial cells and underlying stroma.
References
- ↑ 1.0 1.1 1.2 1.3 Narayanan R, Gaster RN, Kenney MC. Pseudophakic corneal edema: a review of mechanisms and treatments. Cornea. 2006 Oct 1;25(9):993-1004
- ↑ 2.0 2.1 Pricopie S, Istrate S, Voinea L, Leasu C, Paun V, Radu C. Pseudophakic bullous keratopathy. Romanian journal of ophthalmology. 2017 Apr;61(2):90.
- ↑ Waring GO. The 50-year epidemic of pseudophakic corneal edema. Archives of Ophthalmology. 1989 May 1;107(5):657-9.
- ↑ Kang PC, Klintworth GK, Kim T, Carlson AN, Adelman R, Stinnett S, Afshari NA. Trends in the indications for penetrating keratoplasty, 1980-2001. Cornea. 2005 Oct 1;24(7):801-3.
- ↑ 5.0 5.1 Woo JH, Ang M, Htoon HM, Tan D. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. American journal of ophthalmology. 2019 Nov 1;207:288-303.
- ↑ 6.0 6.1 Khan MS, Basit I, Ishaq M, Shakoor T, Yaqub A, Intisar R. Corneal collagen cross linking (CXL) in treatment of pseudophakic bullous keratopathy. Pakistan journal of medical sciences. 2016 Jul;32(4):965.
- ↑ 7.0 7.1 Pires RT, Tseng SC, Prabhasawat P, Puangsricharern V, Maskin SL, Kim JC, et al. Amniotic membrane transplantation for symptomatic bullous keratopathy. Arch Ophthalmol. 1999;117(10):1291-7.