Prostaglandin Associated Periorbitopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Prostaglandin Associated Periorbitopathy (PAP) is the general term given to describe the constellation of eyelid and orbital changes that accompany the administration of topical prostaglandin analogue eye drops.
History
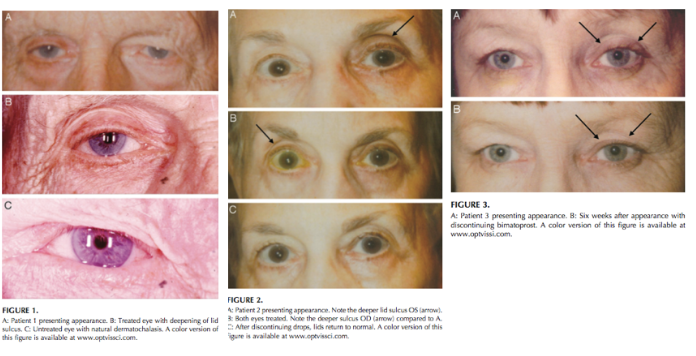
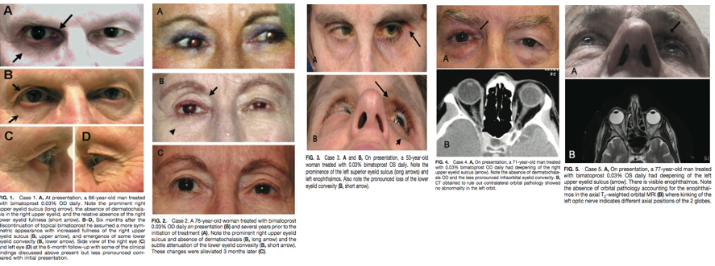
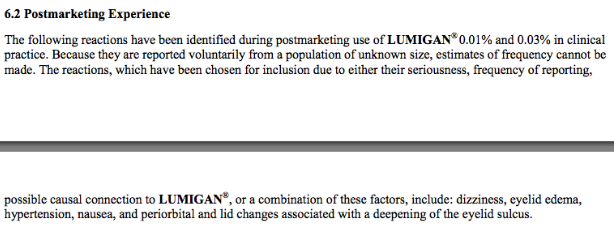
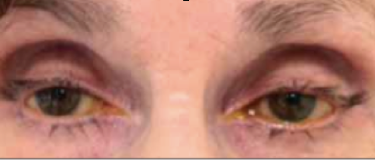
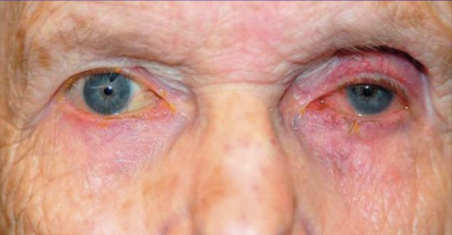
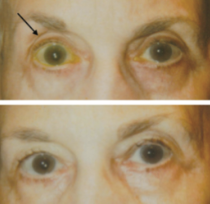
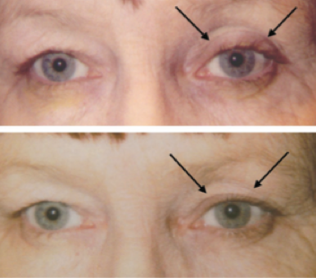
This entity was first described in an optometry journal case series by Peplinski and Smith in 2004[1], in which 3 separate patients treated with bimatoprost eye drops in one eye showed clear changes to their eyelid appearance and a deepening of the upper lid sulcus of that eye alone(See Fig 1). Of note, this case series also described the reversal of said signs and symptoms with discontinuation of the prostaglandin analogue. In 2008, Filippopoulos et al[2]described a case series of 5 patients on chronic daily unilateral bimatoprost therapy with similar changes (See Fig 2). After this, a plethora of case reports have been published describing this phenomenon not only with bimatoprost therapy but also as a result of treatment with all topical prostaglandin analogues[3][4][5][6]. This has spawned much research to determine the both the mechanism and the extent of such changes. PAP has now come to be regarded as a common side effect of prostaglandin analogue topical therapy, and has recently been added on the box as a side effect of the medication (See Fig 3).
Fig 1: Original case series of 3 patients showing PAP, courtesy of Peplinski et al.
Fig 2: Second case report of 5 patients showing PAP, courtesy of Filippopoulos et al.
Fig 3: Official Lumigan Product information, now updated to mention PAP related changes, courtesy of https://www.lumigan.com
Definition of Term
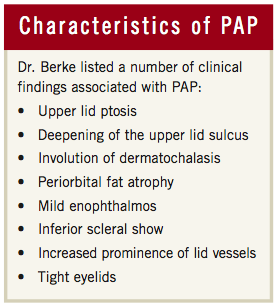
The specific term Prostaglandin Analogue Periorbitopathy was coined by glaucoma specialists Dr. Louis Pasquale and Dr. Stanley Berke. Other terms previously used in the literature included Deep Superior Sulcus Syndrome and DUES (Deepening of Upper Eyelid Sulcus), however it seems that the term PAP seems to encompass all of the eyelid and orbital changes that result from administration of topical prostaglandin analogue medication. The exact clinical findings associated with PAP are upper lid ptosis, deepening of the upper lid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos, inferior scleral show, increased prominence of lid vessels, and tight eyelids (See Fig 4). Other known side effects of prostaglandin analogues include lengthening of lashes and increased pigmentation of the iris and periorbital skin, which could possibly fit under the term PAP as well.
Fig 4: Characteristics of PAP as defined by Dr. Stanley Berke in the October 2012 issue of Review of Ophthalmology
Incidence
It is still unknown what percentage of patients experience Prostaglandin Associated Periorbitopathy, but it is safe to say that it is not a rare phenomenon. It has been claimed that once the clinician is looking for it, it can be noticed nearly 100 percent of the time[7], and is especially noticeable in those patients using a prostaglandin unilaterally. This is an important condition to be on the lookout for considering that topical prostaglandin therapy is the current first-line treatment for open-angle glaucoma. A new study by Shah et al[8] designed as a prospective cross-sectional survey using both external photography and external adnexal exam of 157 current, 15 past, and 171 never users of prostaglandin analaogues showed that current PG users had a 230-fold increased fisk of involution of dermatocholasis and a 249-fold increased risk of incremental loss of lower lid steatoblepharon. Additionally, upper lid ptosis, levator dysfunction, and lower lid retraction were also highly associated with current prostaglandin use.
Histopathology
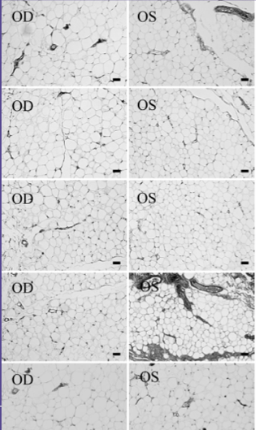
Histological analysis of orbital fat from eyes treated with prostaglandin analogues is thought to show a similar number of adipocytes as non-treated eyes however a significantly reduced size of individual adipocytes, suggesting overall fat atrophy rather than adipocyte death. One study by Park et al[9] looked at the histologic changes of orbital fat in patients unilaterally treated with a topical prostaglandin analogue. When looking at eye lid specimens in 11 patients who showed signs of a deep upper lid sulcus, he found that eyes treated with topical prostaglandin analogue therapy in general showed a statistically significant increase in mean adipocyte density of treated eyes, suggesting that in a given area there was a higher total number of adipocytes and thus a smaller size of each individual adipocyte (See Fig 5). Another finding on histopathologic analaysis of these specimens was the presence of clumped nuclei suggesting adipocyte atrophy (See Fig 6). Additionally, he showed that those treated with bimatoprost were most affected, followed by those treated with travoprost. Those treated with latanoprost showed an increased mean adipocyte density as well however this change was not found to be statistically significant. It is important to note that no significant markers of inflammation were observed on histologic analysis of the tissue specimens.
Fig 5: Histologic appearance of preaponeurotic fat of five different biopsy sites of a patient treated with bimatoprost in the left eye only. Right eye samples used as control. The cell density of the fat tissue is higher in the treated eye. Courtesy of Park et al.
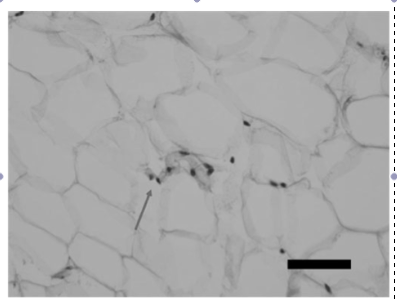
Fig 6: Microscopic findings of atrophied fat tissue in a bimatoprost treated eye as evidenced by clumped adipocyte nuclei, courtesy of Park et al.
Pathophysiology
There have been many proposed mechanisms for Prostaglandin Associated Periorbitopathy including mitochondrial apoptosis pathway of adipocytes, inflammatory fibrotic changes to the eyelid or to Mueller's muscle, atrophy of existing adipocytes, and inhibition of adipogenesis. Currently, it is most widely thought that preaponeurotic and deep orbital fat atrophy are likely the main contributors responsible for the majority of PAP changes. Fat atrophy would explain the relative enophthalmos, upper eyelid sulcus deepening, dermatochalsis incolution, and loss of lower eyelid fullness/convexity. Fat atrophy is also a likely mechanism considering that the phenomenon is reversible after discontinuation of the medication, and because no significant signs of inflammation have been identified on examination of tissue.
To understand the orbital changes associated with topical prostaglandin therapy, it is important to understand the penetration of prostaglandins when applied to the ocular surface. Pharmacokinetic studies of a single topical administration of 0.1% bimatoprost in male cynomolgus monkeys indicate that eyelid specimens contain more than 2,000 times higher concentrations of bimatoprost compared with aqueous and more than 16 times higher concentrations compared with iris and ciliary body[10]. Thus, there is significant periorbital absorption of prostaglandin analogue medication.
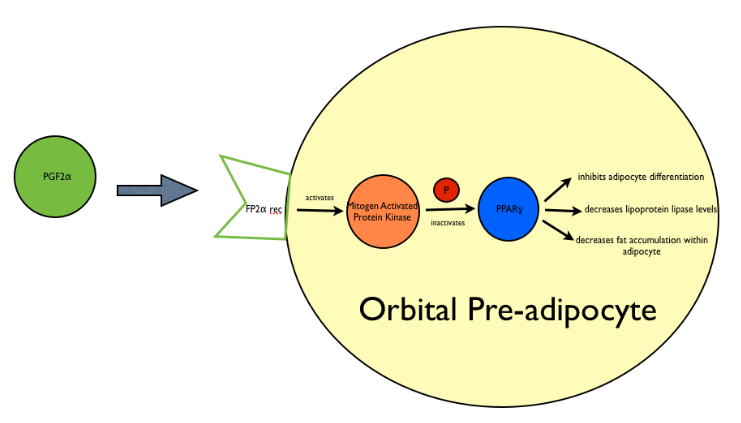
Prostaglandin analogues work via an FP-prostanoid receptor. The activation of this FP-receptor has been associated with inhibition of preadipocyte differentiation in several cell lines[11][12], which as a result prevents cells from expressing adipocyte-specific genes and accumulating fat droplets. Specifically, a study by Reginato et al[13] showed that PGF2alpha when combined with cell surface FP receptor activated mitogen-activated protein kinase, and phosphorylates and thus inhibits the PPAR-gamma receptor (a nuclear hormone receptor), resulting in the prevention of orbital fibroblasts to differentiate into adipocytes (See Fig 7). FP-receptor agonists have also been shown to down-regulate fatty acid binding protein expression, which is important for the uptake of free fatty acids and triglyceride synthesis in adipocytes.
Fig 7: Schematic representing mechanism of adipose changes in PAP. Prostaglandin molecules, specifically PGF2 and its analogues bind the Prostaglandin F receptor (FP) on orbital preadipocytes and activate Mitogen Activated Protein Kinase. This results in the phosphorylation and thus inactivation of Peroxisome Proliferator Activated Receptor Gamma (PPAR-gamma) and results in an overall inhibition of adipocyte differentiation, a decrease in levels of lipoprotein lipase, and a subsequent decrease of fat accumulation within the adipocyte.
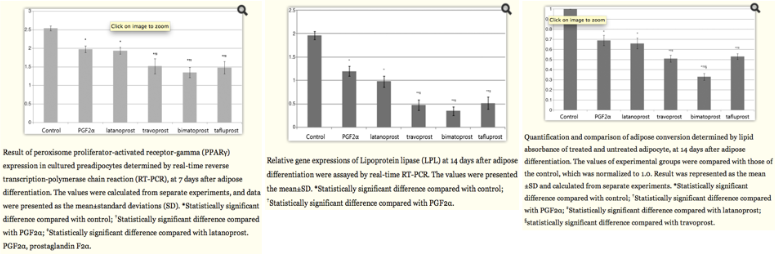
This mechanism was further supported in a study by an invitro study performed by Choi et al[14], in which human orbital adipose precursors were treated in vitro with PGF2alpha, latanoporst, travoprost, bimatoprost, and tafluporst, and the expresions of PPAR-gamma and lipoprotein lipase was determined by real time RT-PCR. This study showed that PGF2 alpha and the topical prostaglandin analogues downregulated the expression of PPAR-gamma and inhibited accumulation of intracytoplasmic lipid droplets and expression of LPL compared with untreated control. It also showed that bimatoprost downregulated these markers to the greatest extent, while latanoprost downregulated these markers the least (See Fig 8).
Fig 8: Results from an in vitro study of prostaglandin molecules and analogues on human orbital adipocytes. These graphs show an overall decrease in expression of PPAR-gamma by RT-PCR at day 7 (a genetic marker of adipogenesis) and an overall reduction in expression of lipoprotein lipase (LPL) by RT-PCR at day 14 (a terminal marker of adipocyte differentiation). Finally, an overall decrease of adipose conversion as determined by lipid absorbance was noted at day 14 as well. Courtesy of Choi et al.
Diagnosis
The diagnosis of Prostaglandin Associated Periorbitopathy is a clinical diagnosis made by a constellation of physical exam findings that occur after initiating topical prostaglandin analog therapy. The diagnosis may be very difficult to make and likely has been missed in several cases as most prostaglandin analogs are prescribed for bilateral use and as such would produce symmetrical changes which are often more difficult to recognize. Additionally, these changes occur relatively gradually and are usually found in elderly patients who may normally have some periorbital and orbital adipose volume depletion.
History
Many patients may not actually complain of any symptoms despite clearly having the clinical signs of PAP as the changes occur gradually and are often symmetric. Some patients may complain of the onset of a droopy lid or their eyelid starting to get in the way of their vision when this was previously never an issue. It may be noted that performing Goldman applanation becomes increasingly difficult on patients with PAP as their orbits seem increasingly sunken in. Rarely, patients may complain of diplopia. Most commonly, patients refer to such changes as "tired-appearing eyes."
Physical examination
Several physical exam findings can be noted on patients with PAP. The MRD1 can be decreased as compared to measurements prior to the initiation of therapy. Hertel's exophthalmometry can reveal a mild degree of enophthalmos or at least a relative decrease in values as compared to baseline. MRD2 or a measurement of inferior scleral show may be increased as compared to baseline as well. Finally, prism alternate cover test or Maddox rod testing can reveal a relative muscle deficit, most commonly a slight limitation in abduction (See Fig 9, 10).
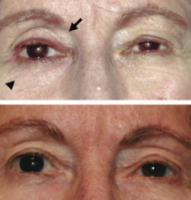
Fig 9: Patient treated for several years with bilateral bimatoprost therapy. Note the bilateral deepened upper lid sulcus and the bilateral mild ptosis.
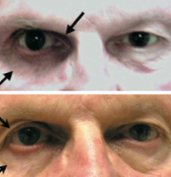
Fig 10: Patient treated with bimatoprost in the left eye only. Not the relative deepened sulcus, ptosis, and enophthalmos in addition to the injected ocular surface and prominence of lid vessels on the left side.
Imaging
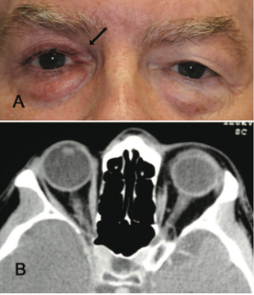
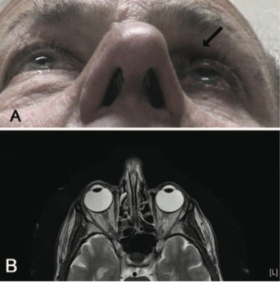
Imaging is not commonly done and is not usually indicated in patients suspected to have Prostaglandin Associated Periorbitopathy. However, often times imaging is performed in patients on unilateral therapy to rule out pathology of the contralateral eye. In this situation, CT or MRI of the orbits can reveal a difference in globe position of the two eyes with the treated eye often situated more posterior in the orbit, as well as a more apparent kinking of the optic nerve of the treated eye representing different axial positions of the two globes. Finally, it is possible to see relative lateral rectus laxity in the treated eye due to orbital fat volume depletion (See Fig 11, 12).
Fig 11: Patient presenting with "fullness of left upper lid" after using bimatoprost in the right eye for 4 years. CT orbits obtained to rule out pathology causing exophthalmos on the left shows a normal left orbit and a more posterior positioned globe on the right. Courtesy of Filippopoulos et al.
Fig 12: Patient initially presenting for 1 month of intermittent binocular diplopia with history of bimatoprost use in the left eye for 7 months. MRI brain, orbits and MRA head and heck were all performed, which were normal other than apparent enophthalmos and a kinking of the left optic nerve. Courtesy of Filippopoulos et al.
Biopsy
Biopsy is not routinely performed and is not indicated for PAP, however if specimens were obtained, it has been shown that orbital fat examination would show increased mean adipocyte density, decreased average adipocyte size, and clumped adipocyte nuclei, which implies fat atrophy[15]. Of note, it has not been shown that there are any significant signs of inflammation on such biopsies.
Differential Diagnosis
While the diagnosis of PAP is usually straight forward as changes begin to occur about 1-2 months after starting treatment, it is important to consider any causes of enophthalmos in the differential diagnosis as well as a relative enophthalmos caused by a contralateral exophthalmos. Common causes of ipsilateral enophthalmos and contralateral exophthalmos that should be considered in patients presenting with possible PAP. [16]
Causes of Ipsilateral Enophthalmos[16]
- Conditions that increase orbital volume
- Orbital fracture
- Bony remodeling as seen in silent sinus syndrome or silent brain syndrome
- Conditions that cause orbital tissue atrophy
- Age-related involutional changes
- Giant Fornix Syndrome as described by Rose et al (chronic unilateral relapsing coniunctivitis incited by age related disinsertion of the levator resulting in increased fornix depth--bacteria can become sequestered in the upper fornix which can lead to colonization)
- Scirrhous metastatic disease--fibrous and contraction of orbital tissue most commonly seen with breast cancer but also noted in lung and stomach cancers
- Post surgical changes as seen after fracture repair, blepharoplasty, or enucleation
- Linear scleroderma
- Hemifacial atrophy
- Age-related involutional changes
Causes of Contralateral Exophthalmos[16]
- Thyroid eye disease
- Orbital cellulitis
- Orbital pseudotumor/Non-specific orbital inflammation
- Retrobulbar hemorrhage
- Vascular Malformation/varix/cavernoma/CC-fistula
Diagnostic Exam
While a thorough exam is always required for patients with any new onset complaint, the following should be performed at each visit to document the extent of prostaglandin related change that each patient is experiencing. This can help determine whether or not discontinuation of the medication is warranted (See Table 1).
| Table 1: Thorough external exam to document changes resulting from prostaglandin administration [17] | |
|---|---|
| Visual Acuity | Vertical palpebral fissure |
| Pupils | MRD1 |
| Tonometry | Superior scleral show |
| Confrontational Visual Fields | Inferior scleral show |
| External adnexal exam | levator function |
| Firmness/Tenderness to Retropulsion | Lagophthalmos |
| Hertel's exophthalmometry | Distraction test for lower lid laxity |
| Lid Crease | External Photographs |
Laboratory test
Currently, no laboratory tests are indicated for diagnosis of prostaglandin associated periorbitopathy.
Management
Management of Prostaglandin Associated Periorbitopathy can usually be achieved by simple discontinuation of therapy.
Discontinuation of therapy
Typically, discontinuation of the medication results in partial to complete reveral of PAP characteristics. This change has been noted to occur as quickly as 4-6 weeks[18]. Much like observation of oset of PAP, observation of reversal of PAP is more noticeable in those treated unilaterally, so thorough documentation and measurements as listed above can be helpful is assessing the extent of atrophy reversal (See Fig 13, 14, 15, 16). If the patient cannot stop a prostaglandin analogue altogether, it has been shown that switching from bimatoprost to latanoprost has been somewhat effective in reversing signs of PAP[19]. In a prospective study of 13 patients who experienced PAP on bimatoprost, 11 of them had either a decrease or disappearance of their symptoms after switching to latanoprost in only 2 months. This change was reported to have maintained over a 6 month follow up.
Fig 13: Patient on bimatoprost OU initially (top) and 1 month after discontinuation of medication (bottom). Note normalization of sulcus. Courtesy of Peplinski et al.
Fig 14: Patient initially on bimatoprost OS (top) and six weeks after discontinuation of medication (bottom). Note the re-appearance of baseline pre-existing dermatochalsis. Courtesy of Peplinski et al.
Fig 15: Patient on bimatoprost OD (top) and 3 months after switching to brimonidine (bottom). Courtesy of Filippopoulos et al.
Fig 16: Patient on bimatoprost OD (top) and 6 months after discontinuation (bottom). Courtesy of Filippopoulos et al.
Medical therapy
Currently, no topical or systemic medication is recommended to counteract the effects of prostaglandins. This may be an area for future research.
Medical follow up
In the case that patients need to stay on their prostaglandin analogue drop, they should be cautioned that they may begin to experience ptosis or diplopia which can begin to interfere with daily functioning. While an exact interval of followup for patients with PAP has not been determiend, it is recommended to continue regular visits with their glaucoma specialist with additional evaluations at the onset of any visually disturbing symptoms.
Surgery/Injections
Currently, surgery, injections of fillers, or micro fat grafting is not recommended for PAP as signs seem to resolve with discontinuation of agent. This may also be an area for future research.
Additional Applications
Cosmesis
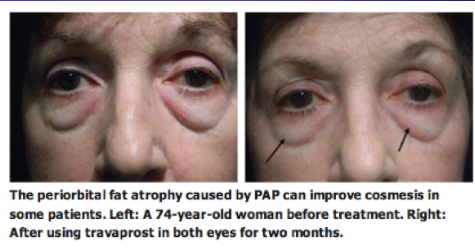
Currently, the use of prostaglandins for cosmetic management of facial fat is under investigation. One company, Topokine Thereapeutics, is currently in Phase 2 of investigation for a gel aimed to reduce submental fat. Additionally, it should be noted that some of the Prostaglandin Associated Periorbitopathy changes have been recieved favorably by patients (See Fig 17).
Fig 17: Patient who displayed a mild reduction in prominence of lower lid fat after bilateral bimatoprost therapy.
Thyroid Eye Disease
Because thyroid eye disease involves orbitopathy featuring fatty hypertrophy, proptosis, eyelid retraction and optic nerve compression, it seems as though the fat atrophy effects of prostaglandin analogues may counteract such pathogenesis. Currently many studies are being performed to demonstrate the effect of prostaglandin analogues on the course of thyroid eye disease, however at this time no results have been published.
Additional Resources
- Lumigan
- Xalatan
- EyeNet
- PAP: New Concerns for Prostaglandin Use
- Prostaglandin Associated Periorbitopathy: A Post-marketing surveillence observation
- Topokine Therapeutics
- International Glaucoma Review
- EyeTube Video by Dr. Stanley Berke
- MedScape: The coming age of enophthalmos
References
- ↑ Peplinksi LS, Albani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci 2004;81:574-7.
- ↑ Filippopoulos T, Paula JS, Torun N, Hatton MP, Pasquale LR, Grosskreutz CL. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg 2008;24:302-307
- ↑ Tappeiner C, Perren B, Iliev ME, Frueh BE, Goldblum D. Orbital fat atrophy in glaucoma patients treated with topical bimatoprost--can bimatoprost cause enophthalmos? Klin Monbl Augenheilkd 2008;225:443-445
- ↑ Lee JW, Kim DY, Lee YK. Two cases of deepening of the upper lid sulcus from topical bimatoprost therapy. J Korean Ophtalmol Soc 2007;48:332-336
- ↑ Yam JC, Yuen NS, Chan CW. Bilateral deepening of upper lid sulcus from topical bimatoprost therapy. J Ocul Pharmacol There 2009;25:471-472.
- ↑ Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol 2009;53:176-179.
- ↑ Berke SJ. PAP: New concerns for prostaglandin use. Review of Ophthalmology 2012; Vol 19 Issue 10:70.
- ↑ Shah M, Lee G, Lefabvre DR, Kronberg B, Loomis S, Brauner S, Turalba A, Rhee DJ, Freitag SK, Pasquale LR. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS ONE 8(5):e61638.
- ↑ Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol 55:22-27.
- ↑ Woodward DF, Krauss AH, Chen J, et al. Pharmacologiccal characterization of a novel antiglaucoma agent, Bimatoprost (ANG 192024). J Pharmacol Exp Ther 2003;305:772-85.
- ↑ Miller CW, Casimir DA, Ntambi JM. The mechanism of inhibition of 3T3-L1 preadipocyte differentiation by prostaglandin F2alpha. Endocrinology 1996;137:5641-50.
- ↑ Serrero G, Lepak NM. Prostaglandin F2alpha receptor (FP receptor) agonists are potent adipose differentiation inhibitors for primary culture of adipocyte precursors in defined medium. Biochem Biophys Res Commun 1997;233:200-2.
- ↑ Reginato MJ, Krakow SL, Bialey ST, Lazar MA. Prostaglandins promote and lbock adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem. 1998;273:1855-1858.
- ↑ Choi HY, Lee JE, Lee JW, Park HJ, Lee JE, Jung HE. In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadipocytes. J Ocul Pharmacol Ther. 2012;28(2):146-52.
- ↑ Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol 55:22-27.
- ↑ Jump up to: 16.0 16.1 16.2 Jayaram A. Fig 13 Prostaglandin Associated Periorbitopathy. EyeWiki. https://eyewiki.org/File:PAPFig13.png. Accessed April 18, 2023.
- ↑ Jayaram A. Fig 14 Prostaglandin Associated Periorbitopathy. EyeWiki. https://eyewiki.org/File:PAPFig14.png. Accessed April 18, 2023.
- ↑ Peplinksi LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci 2004;81:574-577.
- ↑ Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013;57(2):179-84.