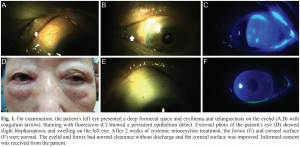
Giant Fornix Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Giant fornix syndrome (GFS) is an uncommon chronic inflammatory condition involving recurrent purulent conjunctivitis. First described in 2004 by Geoffrey Rose, GFS typically occurs in elderly patients with a deep superior forniceal anatomy, which predisposes patients to recurrent conjunctival infections. GFS manifests clinically with chronic conjunctival discharge, usually accompanied by secondary corneal and eyelid involvement. [1] The most common clinical findings of GFS are mucopurulent conjunctival discharge, conjunctival inflammation and injection, pseudomembrane formation in the conjunctival fornices, and ptosis. [2] Corneal features may include ocular surface disease, keratitis, and corneal scarring, with the potential for vision loss if left untreated. [1] GFS usually presents in the seventh to ninth decade of life, with a median age of 75. [2] Diagnosis, however, is frequently overlooked or delayed for years and may require multiple referrals due to the novel awareness of this condition and the limited data available.
Epidemiology
GFS classically affects patients in their seventh to ninth decade of life, with a median age of 75. According to several case series, GFS presented more commonly in females. [1] Data on the prevalence of GFS in the general population is limited, given under-diagnosis of this condition. [3]
Risk Factors
- An enlarged superior sulcus is typically found in patients with GFS, with the depth of the upper fornix often measuring 2-3 cm. [1]
- Age is a major risk factor, with GFS typically presenting in elderly patients.
- Female sex predilection is suggested by the greater incidence of GFS in females in published case series.
- Chronic colonization of the eyelashes and/or conjunctival fornices by commensal organisms predisposes a patient to develop GFS, with Staphylococcus aureus representing the most commonly isolated organism.
Pathophysiology
Although the mechanism of GFS is poorly understood, one theory describes a role for subclinical or clinically apparent inflammation caused by commensal organisms in the eyelid and/or conjunctiva. The inflamed tarsal conjunctiva may secrete proteinaceous exudates forming a coagulum in the patient's superior fornix. The coagulum is further colonized by bacteria, with Staphylococcus aureus being the most frequently implicated organism. Together, the coagulum and inflammatory cell exudate form a toxic environment to the ocular surface, exacerbating the tarsal inflammatory response. Inflammation increases the rugosity of the forniceal surface, resulting in more release of proteineous exudate by the tarsal inflammatory cells, perpetuating a vicious cycle of inflammation and subsequent toxic damage. Further, impaired lacrimal drainage may result in higher bacterial loads, exacerbating a patient's infectious risk and heightening the inflammatory response. [1]
The initial publication about GFS described "an usually deep upper fornix," frequently measuring 2-3 cm in patients with the diagnosis. It is thought that age-related disinsertion of the levator muscle aponeurosis from the tarsus plays a role, allowing the superior fornix to increase in depth. [1] While the initial description of GFS by Rose focused on the role of a deep superior sulcus, later publications also mention inferior forniceal involvement in the disease process. [2] Inflammation likely works in synchrony with abnormal forniceal anatomy to cause GFS, as inflammation induces an increased rugal surface area which promotes ptosis; additionally, prostaglandins released by the tarsal inflammatory cells may cause periorbitopathy, with resultant tissue atrophy and enophthalmos, further increasing the forniceal depth. [1] [3] Abnormal eyelid movements may also play a role. In summary, commensal-related inflammation may exacerbate a pre-existing abnormal forniceal anatomy, resulting in additional “dead space” that harbors bacterial colonies, continuing to propagate the disease.
Primary Prevention
Late diagnosis directly correlates with more severe disease and poorer quality of life. Therefore, it is essential that healthcare providers have a high index of suspicion for the diagnosis of GFS, when elderly patients present with recurrent purulent conjunctivitis. [4]
Diagnosis
The diagnosis of GFS is primarily clinical, but can be supported by the presence of an abnormally deep superior fornix. Due to under-recognition, missed and delayed diagnosis of GFS is common; patients have a mean duration of symptoms of 2 years before referral and diagnosis of GFS.[3][5] Given the recent recognition of GFS as a disease entity, there are no specific diagnostic criteria or universal grading systems for the severity of clinical signs observed in GFS. Although GFS has been claimed to occur in the setting of an upper forniceal depth greater than 25 mm, the presence of clinical signs is diagnostic regardless of forniceal depth. [3]
Symptoms
Clinical presentation mainly consists of chronically recurrent, purulent conjunctivitis with copious amounts of discharge. [1] Associated symptoms include pain, redness, irritation, dryness, tearing, burning, foreign body sensation, and decreased vision. [2]
Signs
The following signs may be observed in GFS:[1] [6] [7] [8] [9] [10]
- Unilateral or, less commonly, bilateral ocular involvement.
- Classically superior forniceal involvement, with or without inferior forniceal involvement. A single case involving solely the inferior fornix has been reported in a patient with a history of multiple lower eyelid procedures. [7]
- Tarsal papillary conjunctivitis with gross purulent ocular discharge and possible pseudomembrane formation.
- Ptosis secondary to severe superior tarsal conjunctivitis. [8]
- Punctate epithelial keratopathy, which may or may not be reversible and may lead to persistent dystrophic epithelium.
- Corneal changes in longstanding GFS, including vascularization, stromal scarring, and visual impairment. Severe corneal ulceration and corneal perforation may occur in severe cases.
- Yellow coagulum of debris within inflamed upper fornix. The most commonly cultured organism is Staphylococcus aureus.
- Signs of blepharitis, including erythema and telangiectasis.
- CT finding of free air in the superior conjunctival fornix. [9] [10]
History and Physical
When taking a history in a patient with suspected GFS, one should elicit a detailed history of presenting illness and medical history. History of chronic recurrent keratoconjunctivitis that may be refractory to treatment is commonly reported by patients. Duration of symptoms is an important piece of history taking, as GFS is a chronic disease. [6] Thorough determination of severity of ocular symptoms (e.g., redness, irritation, pain), exacerbating and alleviating factors (e.g., antibiotic treatment, eye washing), allergy history, and previous and current topical and systemic medications (e.g., antibiotics) should be performed.
Physical exam findings depend on disease severity. Slit lamp examination should be performed looking for thick mucopurulent discharge covering the conjunctiva and cornea. Eyelid eversion is helpful in revealing a thick pseudomembrane and a deep conjunctival fornix. Possible eyelid findings on anterior segment examination include blepharoptosis, crusting, scalloping of the eyelid margins, and blepharitis. Application of pressure on the lacrimal sac can be helpful in eliciting mucus reflux through the lacrimal puncta. A follicular reaction on the upper and lower palpebral conjunctiva and diffuse punctate epithelial erosions may be seen, with potential development of corneal stromal scarring. Fundus examination is unremarkable. [1] [6]
Tests
The purulent conjunctival discharge can be cultured and frequently shows a high bacterial load in GFS patients. Commensal organisms are frequently detected, with Staphylococcus aureus and Staphylococcus epidermidis representing the most commonly isolated organisms. Immunophenotypic analysis of the purulent conjunctival discharge can be performed and has been consistent with an inflammatory process in previous studies. A conjunctival biopsy would reveal intense chronic inflammation with abundant plasma cells and small, well-differentiated lymphocytes with germinal centers. [2]
Differential Diagnosis
The differential diagnosis for chronic conjunctivitis is broad. One of the most common causes of chronic conjunctivitis is ocular allergy, which may present similarly to GFS resulting in misdiagnosis. Ocular allergy, however, is usually associated with other atopic diseases such as asthma and dermatitis. Other common causes of recurrent conjunctivitis include blepharitis, dry eye, chronic use of ophthalmic medications, episcleritis, scleritis, contact lenses and ophthalmic solutions.[11] Another cause of recurrent conjunctivitis is chronic dacryocystitis. Organisms implicated in this condition are Staphylococcus epidermidis and Staphylococcus aureus, similar to GFS. Patients with unrelenting conjunctivitis despite treatment of dacryocystitis should be assessed for GFS, as should those with recurrent conjunctivitis despite patent nasolacrimal ducts. [2]
Management
Treatment
While GFS can be managed medically and/or surgically, GFS has proven to be difficult to cure. To accomplish effective medical treatment that adequately controls inflammation and prevents recurrences, a combination of topical and systemic medications has to be used lifelong at frequent intervals. Risks of such treatment must be carefully assessed and are a downside of this approach compared to surgical management. In patients with uncontrolled symptoms despite medical treatment, surgical treatment should be considered. [1] [2] [3] [4]
Medical treatment includes: [1] [2] [4] [8] [12] [13]
- Removal of coagulum and irrigation and/or physical sweeping of conjunctival fornices
- This acts to decrease bacterial load, which gives the topical antibiotic better access to the bacteria.
- If followed by reconstruction of the upper eyelid, this step may prevent recurrent infections.
- Dilute antiseptic
- Topical antibiotics
- The most commonly implicated organism in GFS is Staphylococcus aureus, a Gram positive coccus which is sensitive to many topical antibiotics including penicillins, cephalosporins, and macrolides. Methacillin resistance should be considered, based on patient history, local resistance patterns, and initial therapeutic response. If MRSA is suspected, then antibiotics such as vancomycin, trimethoprim-sulfamethoxazole, and/or tetracyclines may be considered.
- Gram-negative organisms such as Pseudomonas aeruginosa and Serratia marcesans have also been detected in GFS. [1] [8]
- Given bacterial colonization of a privileged environment within the forniceal coagulum, high-dose antibiotics must be used. Low-dose antibiotics have been shown to be unsuccessful in eradicating bacteria.
- Subconjunctival antiobiotics and/or systemic antibiotics
- 10% povidone-iodine washings
- Povidone-iodine washings should be performed in conjunction with antibiotics and steroids in refractory GFS cases.
- Generally, 10% povidone-iodine washings have been known to reduce bacterial colonies by 91% on the conjunctival surface. [12]
- Topical steroids can be added to control inflammation.
- Artificial tear drops can be administered to lubricate the ocular surface and aid with corneal healing.
Surgical treatment: [1] [2] [3] [4] [14]
- Forniceal reduction
- Reducing the length of the superior conjunctival fornix corrects the abnormal anatomy seen in patients with GFS. This surgical intervention has shown promising results.
- Surgical steps:
- 1. Resection of upper and/or lower eyelid palpebral conjunctiva and pseudomembrane removal.
- 2. Excision of the underlying inflamed conjunctival bed using electrocautery.
- 3. Subconjunctival antibiotic injection (e.g., cefazolin, vancomycin).
- In a case series where 6 patients underwent forniceal reduction surgery, forniceal depth was reduced by an average of 4.75 mm. All 6 patients reported symptomatic improvement postoperatively. Five of the 6 patients had complete resolution and 1 in 6 patients reported initial complete improvement prior to a relapse of symptoms. No surgical complications occurred in any patient. [4]
- In an article which compared the results of 2 case series where multiple different surgical forniceal reduction approaches were employed, it was concluded that: [3]
- Addition of lower forniceal reduction did not alter surgical outcomes.
- Using a Putterman clamp instead of artery clips for conjunctival resection improved the surgical outcomes.
- Sometimes eyelid or lacrimal surgery is also performed to improve tear drainage.
- Preoperative culture of the coagulum or discharge can and should be used to guide intraoperative and postoperative antibiotic therapy.
It is important to emphasize that symptoms may persist despite the use of several antibiotics, addition of steroids, and even performance of dacryocystorhinostomy.
Follow-up
Close follow-up is recommended to monitor for scleritis and orbital cellulitis given the inflammatory nature of the disease and the effect of longstanding conjunctival infection on the ocular surface. [4] In a retrospective case series for GFS patients treated with different combinations of medical and surgical treatments, the median duration of follow up was 4 months. [2]
Prognosis
In the initial study by Rose, 12 patients with GFS were described. Of these, 7 were managed only with medical treatment, while 5 patients additionally required surgical treatment. Of these 12 patients, 8 received chronic medical treatment, with none of the patients experiencing a flare-up in a follow up period of 4-48 months. [1] In a retrospective case series where 6 patients were treated with surgical forniceal reduction, 100% of patients achieved symptom resolution by one month postoperatively and none had a recurrence of GFS signs or symptoms. [4]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Rose GE. The giant fornix syndrome: An unrecognized cause of chronic, relapsing, grossly purulent conjunctivitis. Ophthalmology. 2004 Aug; 111(8):1539-1545. (Rose)
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Turaka K, Penne RB, Rapuano CJ, Ayres BD, Abazari A, Eagle RC Jr, Hammersmith KM. Giant fornix syndrome: A case series. Ophthal Plast Reconstr Surg. 2012; 28(1):4-6. (Turaka)
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Farmer LD, Rajak SN, McNab AA, Hardy TG, Selva D. Surgical correction of giant fornix syndrome. Ophthal Plast Reconstr Surg. 2016; 32(2):142-144. (Farmer)
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Nabavi CB, Long JA, Compton CJ, Vicinanzo MG. A novel surgical technique for the treatment of giant fornix syndrome. Ophthal Plast Reconstr Surg. 2013; 29(1):63-66. (Nabavi)
- ↑ Gupta S, Prasad TSB. Giant fornix syndrome. BMJ. 2015; 351:h6274.
- ↑ Jump up to: 6.0 6.1 6.2 Lee KW, Jung JW. Systematic minocycline treatment of methicillin-resistant staphylococcus aureus in giant fornix syndrome. Koren J Ophthalmol. 2016; 30(5):394-395. (case report sent as letter to the editor)
- ↑ Jump up to: 7.0 7.1 Jones LD, Ghosh Y, Ahluwalia H, et al. Iatrogenic giant fornix syndrome of the lower eyelid. Ophthal Plast Reconstr Surg. 2007;23:256-7.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 To J, Macsai M, Phelps PO. Chronic Conjunctivitis in an Older Patient With Ptosis. JAMA Ophthalmol. 2020 Jan 1;138(1):97-98. doi: 10.1001/jamaophthalmol.2019.4439. PMID: 31725837.
- ↑ Jump up to: 9.0 9.1 Turner SJ, Sharma V, Hunter PA. Giant fornix syndrome: a recently described cause of chronic purulent conjunctivitis and severe ocular surface inflammation, with a new diagnostic sign on CT. Eye (Lond). 2006 Dec;20(12):1481-3.
- ↑ Jump up to: 10.0 10.1 Czyz CN, Kondapalli SSA, Mazzuca DE J, Allen SH, Cahill KV. Variant of Giant Fornix Syndrome Masquerading as Intraocular Free Air on Computed Tomography. J Emerg Med. 2013;44(3):E311-E313.
- ↑ Morrow GL, Abbott RL. Conjunctivitis. Am Fam Physician. 1998 Feb 15;57(4):735-46. PMID: 9490996.
- ↑ Jump up to: 12.0 12.1 Karim R, Mandal N, Tuft S. Management of giant fornix syndrome with irrigation with povidone-iodine. BMJ Case Rep. 2018:1-3.
- ↑ Taylor JB, Fintelmann RE, Jeng BH. Subconjunctival injections and povidone-iodine washings for the treatment of giant fornix syndrome. Cornea. 2011 Apr;30(4):479-80.
- ↑ Vahdani K, Lim LA, Thaller VT. Fornix Reduction for Treatment of Giant Fornix Syndrome. Ophthalmic Plast Reconstr Surg. 2016 Jul-Aug;32(4):313-4.