Trabecular Meshwork Bypass by Stent
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Prevalence and Impact
Glaucoma is the second leading cause of blindness in the world. Its current worldwide prevalence in the population aged 40-80 years old is estimated to be 3.54% with the highest incidence in the African and Asian racial groups.[1] Glaucoma is projected to affect 76 million people by 2020 and 111.8 Million in 2040. Although glaucoma is a progressive and irreversible disease, treatment can slow down its progression. Cataract and glaucoma frequently co-exist, especially in the elderly population. About 2.7 million cataract surgeries are performed each year, after which patients usually experience intraocular pressure (IOP) reduction.[2]
Background Information
IOP is determined by the inflow and outflow of aqueous fluid into and out of the anterior chamber (AC). A lower IOP deters glaucomatous progression.[3] The function of the aqueous humor is to provide nutrients and hydration to the AC while removing waste products. The aqueous humor is produced by the ciliary body epithelium and flows anteriorly to be drained through the trabecular meshwork (TM). It enters the Schlemm’s canal, passes through the scleral plexuses, and then flows into the episcleral veins.[4] Obstruction of flow leads to accumulation of aqueous humor in the anterior segment, with mechanical pressure transmission onto the vitreous humor and optic nerve.[5]
The most common culprit in glaucoma is increased resistance at the level of the TM. The TM is a filter-like structure that drains the aqueous humor from the AC. Its 360˚ circumference is divided into segments of high and low flow regions and it is a critical structure in the regulation of aqueous outflow.[6]
Patients who have been medicated for mild-moderate glaucoma and who needed to undergo cataract surgeries were shown to experience reduction in IOP after cataract removal. There has been a number of theories attempting to explain this phenomenon. Phacoemulsification involves the use of ultrasound energy to break down the lens. During the process, phacoemulsification energy and the ensued inflammation likely induces TM ultrastructural changes (stimulating cell division, phagocytosis, glycosaminoglycan deposit and other alterations) that reduce resistance to outflow.[7] Other potential mechanisms might include anterior segment volumetric changes and widening of the angle structures.
Trabecular micro-bypass stents have emerged as a relatively novel technology (first device approved in the US for trabecular bypass) that can yield favorable IOP reduction in eyes with mild-moderate open angle glaucoma undergoing cataract surgery. Currently both procedures are performed simultaneously.
Design
iStent
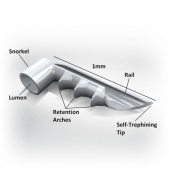
iStent (Glaukos Corp., Laguna Hills, CA) first generation trabecular micro-bypass implant is a snorkel-shaped device that is surgically embedded through the TM into the Schlemm’s canal. It provides direct passage for aqueous flow from the pressurized AC in glaucomatous eyes through the dysfunctional TM. There are currently two generations of iStents in use.
The first generation iStent was FDA approved in 2012. Its dimensions are 1mm x 0.33mm x 120 microns . It is made of surgical grade nonferromagnetic heparin-coated titanium, featuring three retention arches, promoting stable placement within the TM. The inserter system functions as a preloaded single use inserter with a rotator grip . There are two versions for left and right-handed surgeons. The device features a self-trephining tip that facilitates access into the TM via a sideways sliding technique.
The second generation “iStent Inject” system was FDA approved in 2018. The model is smaller in dimension. It has three sections: the flange which sits in the AC, the thorax which traverses the TM, and the head which sits in the Schlemm’s canal. The head features four outlets that are 50 microns each.
Hydrus
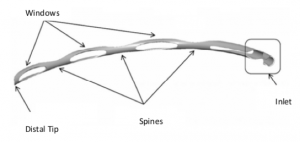
The Hydrus Microstent (Ivantis, Inc, Irvine, CA, USA) is an approximately 8 mm long, curved device that comprises alternating spines for structural support and windows for aqueous outflow. Because of its scaffold design, the microstent occupies the Schlemm’s canal, but does not block the collector channel ostia in the posterior portion of Schlemm’s canal. The microstent is made of nitinol, a nickel-titanium alloy that possesses super-elastic properties, allowing for return to its original shape after deformation. Once inserted, the microstent can dilate Schlemm’s canal by four to five times the natural width of the canal, countering the collapse of Schlemm’s canal induced by elevated IOP.[9][10]
Indications
The iStent and Hydrus are FDA approved in the U.S. and indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild to moderate open-angle glaucoma treated with topical hypotensive medication. Patients that could benefit from these procedures are those with primary open angle glaucoma (POAG), including pigmentary or pseudoexfoliative subtypes.[11] Although some evidence exists on use in patients with traumatic and steroid-induced glaucoma, POAG continues to be the most common approved indication.[12]
Contraindications
These devices are contraindicated in angle-closure with no access to the TM, neovascular glaucoma.[13] In addition it is not recommended or approved for patients with elevated episcleral venous pressure (such as thyroid eye disease and Sturge-Weber syndrome). It is also not studied for use in juvenile or childhood glaucoma, in eyes with chronic inflammatory disease, in patients with abnormal anterior segments, or in aphakic patients.
Clinical Evaluation
In order to determine candidacy for these procedures, a thorough glaucoma evaluation, including gonioscopy, must be performed. Gonioscopy will allow the clinician to assess angle structures and to examine the TM. It will also allow for the detection of peripheral anterior synechiae and rubeosis. The clinician needs to determine how wide the iridocorneal angle is, which has a bearing on whether to perform insertion before (when the cornea is clearest for most optimal intraoperative visualization) or after phacoemulsification (if the angle has a narrow approach, then it may be preferable to insert the device after phacoemulsification as lens removal tends to deepen the angle). Examining the TM for areas of increased pigmentation is important as some evidence suggests the proximity of a collector channel to those areas (anecdotal evidence.)
Standard of care evaluations for glaucoma include IOP trends, past/present topical treatments, corneal thickness, and structure-function testing. Optical coherence tomography (OCT) of the optic nerve head and retinal nerve fiber layer provides noninvasive, cross sectional imaging of tissues. These measures, in addition to visual field testing, assist in determining the severity of disease (mild, moderate or severe.) These factors have to be considered as the surgeon assesses the necessity and potential benefit of these procedures.
Surgical Technique and Peri-operative Management
iStent
Micro incisional glaucoma surgery (MIGS) relies on performing surgery through a small incision by utilizing the phacoemulsification incision. The goal is to lower IOP while keeping tissue manipulation to a minimum and thus enhancing the safety of the procedure, leading to a faster visual recovery compared to traditional incisional glaucoma surgeries. The iStent was the first MIGS procedure available to ophthalmologists.
Patients undergoing simultaneous cataract surgery and iStent implantation may be instructed to discontinue any ocular hypotensive medication in order to obtain a baseline washout IOP measurement if the severity of glaucoma allows (wash out period for different medication classifications as follows: one week for carbonic anhydrase inhibitors, two weeks for alpha-2 adrenergic agonists, and four weeks for either beta-blockers or prostaglandins).[14] Standard phacoemulsification is typically performed through a clear corneal (which is preferred over a limbal incision that is more likely to ooze blood making intraoperative gonioscopy more difficult due to blood in the interface between the cornea and the gonioscopy lens).
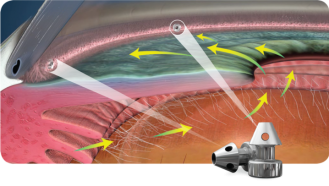
Key to the successful insertion of the iStent relies on the ability to visualize the AC angle during surgery. It is thus critical that surgeons familiarize themselves with the anatomy of the angle as well as with techniques for successful intraoperative gonioscopy. In the initial phases it is advantageous for surgeons to practice intraoperative gonioscopy skills during routine cataract surgery. As mentioned earlier, preoperative gonioscopy is essential before surgery.
iStent insertion can be performed prior to cataract extraction or after cataract removal. There are advantages and disadvantages to each approach. Performing MIGS prior to phacoemulsification allows for the best intraoperative view secondary to minimal corneal edema. On the other hand, the angle structures maybe more crowded particularly if the cataractous lens is intumescent with a larger volume and is pushing the iris forward. Performing MIGS after cataract extraction has the advantage of enhanced access to the TM due to angle deepening. However, the view maybe compromised by corneal edema.
For optimal intraoperative gonioscopy, the head of the patient has to be tilted away from the surgeon by approximately 30 to 45°. The operating microscope needs to be tilted in the opposite direction towards the surgeon by an equivalent angle. This would allow for the use of a direct gonioscopy lens to visualize the nasal angle.
The angle view has to be maintained throughout the procedure. Should the view be compromised due to patient movements, lens movements, or blood then the procedure should be halted. The surgeon should be mindful to avoid indenting the cornea with the gonioscopy lens as that would compromise visualization. The gonioscopy lens is typically removed from the cornea and the iStent inserter is introduced through the main corneal incision over the pupil. The gonioscopy lens is then reintroduced to the cornea. In the case of the first-generation implant, the TM is approached and engaged with the tip of the implant (at about 10-15° inclination) which is then advanced into the Schlemm’s canal and released . With the second-generation implant, the approach is direct . The protective sleeve is retracted once in the AC and the needle is used to engage the TM perpendicularly and then lightly dimple it before the implant is released. The process is repeated for the second implant.
Hydrus
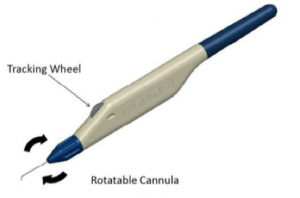
The delivery system is designed for use with either the right or left hand and possesses a rotatable sleeve for alignment of the cannula according to surgeon preference. Once this preference has been set, a 1.5 mm long clear corneal incision is made. The preloaded injector is placed through the wound, and the cannula tip is advanced through the TM until it enters Schlemm’s canal, and the bevel is flush against the entry point in the TM. The target tissue is then visualized using gonioscopy. Once the distal cannula tip is confirmed to be properly positioned, the tracking wheel is used to advance and release the microstent. Following injection, the device occupies about three clock hours, or 90 degrees, of Schlemm’s canal, and has a 1 mm inlet portion that resides within the anterior chamber.[8]
2-3 minute 3D animation
Postoperative Management
Cataract removal and device implantation require topical anesthesia in the majority of patients and is performed as an outpatient surgery. The eye is protected with a shield at night after surgery. Postoperatively restrictions are minimal and include strenuous work or physical labor that may increase intraocular pressure or venous pressure. Additionally, the patient should avoid any activities that may involve foreign contact to the eye such as contact sports and swimming.
After surgery, topical antibiotic and anti-inflammatory (steroid, NSAID) medications are used (some surgeons inject intracameral medications). Depending on the stage of the glaucoma and the individual target IOP for a patient, topical glaucoma therapy maybe reduced or withheld after surgery. In order to determine the efficacy of the surgical intervention, a washed out IOP may be necessary. As such, withholding glaucoma medication(s) may be advantageous if it is safe to do so for an individual patient (for example early glaucoma and/or IOP on target postoperatively). During postoperative follow up the need for topical glaucoma medications is determined based on IOP control and medications maybe resumed or discontinued as needed.
Complications
iStent
iStent implantation is considered generally safe. A randomized controlled trial of 240 eyes showed that iStent implantation combined with cataract surgery did not compromise vision for the 2-year study’s duration.[15][16] The most common complications reported post-operatively were malpositioning and obstruction by blood or iris. Management for such complications ranged from observation to stent repositioning/replacement. Of note, the incidence of such events was 3% to 4%. This may vary by surgeon experience, technique and type of implant.
Hyphema can be observed intraoperatively and early postoperatively.[17][18] It is often secondary to blood refluxing through the iStent. It is not regarded as a serious complication (but can hinder the view in surgery and should be cleared with viscoelastic injections before proceeding with the procedure) and can rather be an indication that the distal pathway is patent. The hyphema itself tends to resolve within days-week after the procedure and may affect visual acuity until its resolution.
Recurrent or persistent hyphema might compromise vision. One report has described recurrent hyphema 5 months post-operatively.[19] Gonioscopic examination revealed that the implant was malpositioned, being posteriorly angled toward the vasculature-rich ciliary body. The iStent had to be removed to resolve the hyphema. The stent’s malpositioning has not been a rarely reported complication; Fernandez-Barrientos et al. noted 17.6% cases of malpositioned iStent. Fea et al. similarly reported that 16.7% of their subjects experienced a malpositioned stent.[20][21] None of these patients, however, required another revision procedure.
There currently are no standard guidelines that define malpositioned stents. Confirmatory observations of the stent’s positioning can vary widely with different tools utilized. Gillmann et al. have recently shown that a significantly larger proportion of iStents (45.7%) tend to be completely burrowed within the trabeculum than observed gonioscopically, highlighting the value of anterior segment OCT in exploring the iStent’s location.[22] Adhering to proper technique during insertion will minimize those adverse events.
In rare circumstances, implanted iStents might not be locatable. In these cases, studies have shown that ultrasound biomicroscopy tend to locate the hidden stent more reliably than B-scan ultrasonography or OCT.[23][24]
Hydrus
Reported intraoperative complications in clinical studies of the Hydrus have been uncommon and typically involve transient hyphema or malpositioning of the device. Hyphema has been noted at a rate of about 1.1-2%, and usually resolves within one week post-operatively.[25][26][27] Malpositioning of the stent within the iris root or outside of Schlemm’s canal, which may require intraoperative scaffold repositioning, has also been reported.[26] Other noted complications include cyclodialysis cleft, iridodialysis, corneal abrasions, and Descemet membrane detachment.
IOP spikes are one of the most frequently reported complications of MIGS, but this has not been consistently demonstrated following Hydrus placement.[28] In the HYDRUS II study, there was a significantly higher incidence of elevated IOP (≥35 mmHg) the first day after surgery in 26% (13/50) of CS eyes, as opposed to 10% (5/50) in the Hydrus plus CS group. This was treated with systemic acetazolamide and resolved within the first few days of surgery.8 Similarly, in the HORIZON study, more IOP-related events, including spikes, hypotony, and secondary glaucoma filtration surgeries were reported in the cataract surgery alone group than in the Hydrus group.[25]
Focal peripheral anterior synechiae have been observed to develop in and around the microstent in about 10-20% of cases, but are generally not associated with device efficacy, IOP changes, or glaucoma medication use.[25] [27] [29] One case of iris tissue adhesion with microstent obstruction requiring argon laser trabeculoplasty has been noted.[26]
Long-term effects on endothelial cell loss (ECL) remain unclear at this time. In a study by Fea et al., at six-month follow-up, comparable ECL was found between phacoemulsification alone and phacoemulsification combined with Hydrus.[30] In contrast, the HMS eyes in the HORIZON study demonstrated more endothelial cell loss than the NMS group (mean change -14% vs. -10% from baseline, respectively), and at 24 months, 13.6% (47/346) of HMS eyes were noted to have ≥30% central ECL from baseline as opposed to 7.2% (12/167) of NMS eyes. This loss was noted to take place primarily within the first three months of surgery, and these patients are currently being monitored for further progression following study completion.
Outcomes
iStent
iStent Implantation with Cataract Surgery
The effect of cataract surgery on IOP reduction is well-studied. Peräsalo et al. reported 3.1 mmHg reduction in IOP 12 months post-phacoemulsification in 182 patients.[31] Mathalone et al. found a mean IOP reduction of 1.5 mmHg and 1.9 mmHg at 12 and 24 months, respectively.[32] They also observed a statistically significant reduction in ocular hypotensive medications at both follow-up time periods. The Ocular Hypertension Treatment Study Group conducted a larger (n=806 eyes), controlled, case series study to further evaluate the impact of cataract surgery on IOP.[33] They reported a statistically significant difference between pre- and post-operative IOP measurements (P < 0.001); the mean decrease in IOP was 4.0 mmHg (95% CI, 3.4-4.7). 39.7% of eyes that underwent surgery experienced ≥ 20% IOP reduction. They observed that the difference in IOP from baseline was still significant at 36 months post-operatively.
As the first MIGS device approved in the US, the IOP lowering effect of iStent implantation with cataract surgery has been extensively studied. Spiegel et al. provided one of the earliest reports.[34] Their study comprised 47 subjects with POAG and IOP above 18 mmHg, medicated with at least one ocular hypotensive medication. At 6 months follow-up, average IOP was reduced by 5.7 mmHg (± 3.8), a 25.4% decrease from baseline (P < 0.001). Such a reduction is greater than values previously reported in the literature for solo phacoemulsification procedure.
In 2011, an investigational device exemption (IDE) trial was conducted to determine the efficacy of solo cataract surgery (control) compared to simultaneous placement of iStent with phacoemulsification in lowering IOP.[35] A total of 116 patients were randomized to the stent arm, while 123 patients participated in the control arm. Patients in both groups presented with mild to moderate glaucoma. The study showed that 61% of the stent group exhibited unmedicated IOP ≤ 21 mmHg at 24 months post-operatively compared to 50% in the control group (P = 0.036). Long-term follow-up displayed similar safety profiles in both groups.
Around the same time, Samuelson et al. conducted a similarly structured (but larger; n=240) randomized controlled trial comprising 29 US investigational sites with a follow-up period of 12 months.[36] Inclusion criteria were primarily a need for cataract surgery with a best corrected visual acuity (BCVA) of 20/40 or worse, mild-moderate open angle glaucoma, and a medicated IOP of ≤ 24 mmHg. Main exclusion criteria were the presence of severe glaucomatous field defects, prior glaucoma surgeries other than iridectomy, monocular subjects, and glaucoma of other etiologies than primary open-angle. With both groups unmedicated at 12 months, 72% of patients who underwent iStent displayed IOP ≤ 21 mmHg compared to 50% in the cataract-only group (P < 0.001). A secondary efficacy outcome of the study was the proportion of patients with ≥ 20% IOP reduction at one year without ocular hypotensive medications. The difference between both groups’ outcomes was still statistically significant (P= 0.003) with 66% of the iStent group achieving the outcome, compared to 48% in the cataract-only group.
The iStent phacoemulsification approach has not only shown more favorable IOP outcomes (than cataract surgery alone) but also lead to a significant reduction in the number of hypotensive medications used in the long run. An Italian study of 36 patients with follow-up at 1 day,[37] 1 week, and 1, 2, 3, 6, 9, 12, and 15 months post-operatively. At 15 months, the mean IOP for the iStent phacoemulsification group was 14.8 ± 1.2 mmHg, while that for the phacoemulsification group was 15.7 ± 1.1 mmHg (P= 0.031). By 15 months, the average numbers of ocular medications for the combined surgery and control groups were 0.4±0.7 and 1.9±1.0, respectively (P= 0.007). The reduction in IOP and number of glaucoma medications was reproduced in another smaller long term study (53 months) after combined phacoemulsification/iStent implant procedure.[38]
A meta-analysis comprised of 17 studies (n=2495) showed that both solo phacoemulsification and combined iStent/cataract surgery displayed significant decrease in IOP and long-term number of medications used.[39] The combined surgery approach led to a statistically greater reduction in IOP than the cataract-only surgery. Solo cataract surgery caused an average decrease of 4% in IOP, the addition of the iStent produced a 9% reduction. The addition of a second iStent (still with phacoemulsification) reduced IOP by a mean of 27%.
iStent Implantation as a Solo Surgery
Standalone iStent implantation has been studied in case series of patients with POAG who underwent only first generation iStent implantation (without cataract extraction) with a 24% IOP reduction from a baseline average IOP of 20.2 mmHg (± 6.3).[40] Another case series confirmed the results and reported 27.3% reduction 12 months post-operatively.[41]
Ahmed et al. included 39 phakic eyes with POAG in a non-randomized study to explore the efficacy of simultaneous implanting of 2 iStents.[42] All subjects were on two IOP medications before surgery. With an average baseline IOP of 22.2 mmHg, one month and 12 months assessments showed IOP reduction to a mean of 14.0 mmHg (± 2.2) mmHg and 13.0 mmHg (± 2.4), respectively; all patients showed IOP <18 mmHg by one year.
Fea et al. investigated whether Solo iStent placement yielded more favorable glaucoma management on the long run than chronic hypotensive medications.[43] They conducted a multicenter, randomized study at eight European investigational sites (Italy, Spain, Poland, Germany, United Kingdom, and Armenia; n=192). The trial compared the stent group to a matched group of patients treated with a prostaglandin/beta-blocker combination. The study’s primary efficacy measure was the percentage of subjects achieving IOP reduction of ≥ 20% at one-year. 94.7% of the unmedicated eyes in the stent group achieved the primary efficacy measure, compared to 91.8% in the medicated group, which was not statistically significant. An analysis of a subset of patients who achieved ≥ 50% decrease in IOP, however, revealed a statistically significant difference of 17.5% in favor of the stent group (P=0.02).
Another similarly structured long term clinical trial investigated the 5-year efficacy and safety of 2 stents implantation in comparison to a matched group of patients medicated with once daily topical prostaglandin.[44] The study incorporated 101 phakic eyes diagnosed with POAG who were randomly assigned to each treatment arm. At the conclusion of the study the mean diurnal IOP was similarly reduced in both groups (35.3% [iStent] and 35.1% [prostaglandin]). 17% of the stent implanted eyes had required additional medication to control IOP by 5 years, compared to 44% in the prostaglandin-treated eyes (P=0.017). Whereas 77% of the stent group maintained an IOP of 6-18 mmHg at the study’s conclusion without additional medications, only 54% from the prostaglandin-treated group did (P= 0.04). Both groups displayed comparably favorable safety profiles.
Another study examined the efficacy of adding a third iStent as standalone surgery.[45] Subjects received one, two, or three iStents, with 30 patients in each group. The single iStent group experienced 31% IOP reduction at 6 months post-operatively. On the other hand, both groups that received two or three iStents exhibited an identical 41% IOP reduction.
A meta-analysis comprised of 248 subjects examined five studies, one of which explored the efficacy of three iStents implantation.[46] The study concluded that there were significant reductions in IOP with 1, 2, or 3 iStents. Analysis revealed 22% weighted mean IOP reduction after the implantation of a single iStent at 18 months. Evaluation at the end of the same time period showed 30% and 41% decrease in IOP with 2 implants and 3 implants, respectively.
Hydrus
Hydrus vs. Selective Laser Trabeculoplasty (SLT)
In the sole study comparing Hydrus versus SLT at the time of this writing, Fea et al. studied the efficacy of standalone Hydrus device implantation compared to SLT in a 12-month, prospective, non-randomized, interventional case series of 56 patients with uncontrolled mild to moderate POAG[26]. In total, 25 eyes underwent SLT, while 31 received Hydrus implantation. There were no statistically significant differences in baseline demographic characteristics, intraocular pressure, or mean number of medications between groups. Washout of all glaucoma medications took place after the procedures. Both groups saw significant decreases in IOP compared to baseline, though the between-group differences in both mean and percentage IOP reduction were statistically insignificant at all follow-up visits. At 12 months, 90% of Hydrus patients and 88% of SLT patients achieved IOP reduction greater than 20%. Despite essentially equivalent outcomes in IOP among the treatment groups, there was a statistically significant difference in drug reduction at 12 months between groups (-1.4 ± 0.97 in Hydrus patients vs.-0.5 ± 1.05 in SLT patients), and only Hydrus patients achieved a significant within-group drug reduction from baseline at 12 months. Furthermore, 47% of Hydrus patients were medication-free at 12 months, versus only 4% of SLT patients.
Hydrus vs. iStent
The COMPARE study was the first to compare two different MIGS devices in standalone surgery, rather than in combination with phacoemulsification. This was a prospective, multicenter, randomized controlled single-masked clinical trial in which 152 eyes with open-angle glaucoma (primary and select secondary diagnoses, including pseudoexfoliative and pigmentary glaucoma) were randomized 1:1 to either receive one Hydrus device or two iStents, with medication washout performed prior to surgery.[47] For all endpoints at 12 months, Hydrus outcomes were superior to those of the two-iStent group, even on subgroup analysis. Baseline diurnal IOP (DIOP) was similar between the two groups, both pre- and post-medication washout, as were number of glaucoma medications used (2.5, 2.7). Patients in the Hydrus group had a greater reduction in mean IOP than those in the iStent group (-1.7 vs. -1.0 mm Hg), though this was not statistically significant. However, the Hydrus group achieved a greater decrease in number of medications than the iStent group (-1.6, -1.0), and had a higher proportion of patients who were medication-free at 12 months compared to the iStent group (46.6% vs. 24.0%). Among all patients at 12 months (including those who were not medication-free), mean IOP was lower (17.3, 19.2), change in IOP was greater (-8.2, -5.1), and more patients in the Hydrus group achieved >20% IOP reduction from the washed out baseline DIOP (39.7%, 13.3%). Conversely, there were fewer Hydrus patients that needed 3 or more medications at 12 months than iStent patients (8.2%, 29.3%).
Of note, among the first 40 subjects to reach 12 months’ follow-up, approximately 20% of eyes in the 2 iStent group had IOP elevation refractory to maximum tolerated medical therapy. Investigators were reluctant to perform a medication washout in these eyes at this time, causing a deviation from the original study design in which the washout requirement was waived for the 64th patient to reach the 12-month follow-up and all patients after that. This protocol change eliminated the ability to directly compare device related IOP reductions and limits ability to reach definitive conclusions about the efficacy of the two devices.
Hydrus vs. Canaloplasty
Gandolfi et al. studied the efficacy of Hydrus implantation vs. canaloplasty (CP) in 45 eyes in a nonrandomized, single-center, retrospective case series.[29] Twenty-one eyes underwent Hydrus implantation, while 24 eyes received CP. Both groups had similar starting BCVA, IOP, and visual field mean deviations (MD). Pre-operative diagnoses included POAG, pseudoexfoliation syndrome, and pigmentary glaucoma, and outcomes were analyzed after a minimum follow-up of 24 months. Following their procedures, both groups experienced a statistically significant reduction in IOP, though there was no significant between-group difference in final IOP. Both groups also achieved a reduction in number of medications, with no significant difference in number of medications used by the end of the study (0.7 HM, 0.9 CP). Finally, the eyes were described as “complete” success, “qualified” success, or “failure” if, two years after surgery, they needed no hypotensive medications, some hypotensive medications, or further glaucoma surgery to attain the target IOP, respectively. Complete success was achieved in 33.3% of HM eyes and 50% of CP eyes. On the other hand, 57.1% of HM eyes were a qualified success, versus 41.7% of CP eyes. Rates of failure were similar between both groups (2 eyes in each group). A history of prior laser trabeculoplasty (ALT or SLT) was not associated with a higher rate of failure.
Hydrus with Cataract Surgery vs Cataract Surgery Alone
In several studies, concurrent Hydrus device implantation with cataract surgery (CS) appears to be superior in lowering IOP and reducing the number of glaucoma medications than CS alone, and equivalently safe. In the HYDRUS II study, a 2-year prospective, multicenter, randomized controlled single-masked clinical trial, Pfeiffer et al. analyzed 100 eyes with open-angle glaucoma, who all underwent washout of ocular hypotensives prior to surgery. [27]Patients were randomized 1:1 to undergo CS with the microstent or CS alone. Washout of hypotensive medications was repeated at 12 and 24 months. After accounting for study dropout and need for additional incisional glaucoma surgery, 90 patients remained in the study after 24 months. Washed out mean DIOP in the Hydrus plus CS group was significantly lower at 24 months compared with the CS group (16.9 +/- 3.3 mmHg vs. 19.2 +/- 4.7 mmHg; P = 0.0093). The proportion of patients with a decrease in washed out DIOP of 20% or more compared to baseline was significantly higher in the Hydrus plus CS group at 24 months compared with the CS group (80% vs. 46%; P = 0.0008). More patients in the Hydrus plus CS group were medication-free at 24 months than patients in the CS group (73% vs. 38%; P = 0.0008).
In a 2-year retrospective, multicenter case-series, Fea et al. analyzed the effectiveness of the Hydrus microstent combined with cataract surgery in 92 eyes, including 6 that had undergone prior glaucoma surgery (4 trabeculectomies, 1 tube shunt, 1 cyclodiode laser).[48] On subgroup analysis, 42 eyes fell in group 1 (IOP ≤ 18 mmHg), while the other 50 fell in group 2 (IOP ≥ 19 mmHg); baseline characteristics aside from IOP and glaucoma medication usage (1.86 +/- 0.9, 2.40 +/- 1.1) were similar between the groups. Mean follow-up among all patients was 20.0 +/- 7.3 months, with 67 patients ultimately completing the 2-year visit. The mean baseline IOP was 19.4 +/- 4.4 mm Hg, which decreased to 15.6 +/- 2.9 mm Hg at 6 months and was sustained at 2 years (P < .0001). The amount of IOP reduction was positively correlated with baseline IOP (R2 = 0.721). Mean number of medications decreased significantly from 2.1 +/- 1.0 to 0.7 +/- 1.0 at 2 years (P < .0001). At the final follow-up visit, 64% of patients were medication free. On subgroup analysis, in group 1, the mean IOP of 15.7 mm Hg did not change significantly throughout the follow-up period (P > .05). However, the number of medications was statistically significantly reduced to a mean of 0.2 +/- 0.5 at 1 year and 0.5 +/- 0.7 at 2 years (P < .0001). Moreover, 69% of patients were off medication at the final study visit. In group 2, mean IOP decreased significantly from the baseline of 22.6 to 16.0 +/- 3.2 mm Hg at 1 year and 15.7 +/- 2.3 mm Hg at 2 years (P < .0001). The number of medications used also significantly decreased to a mean of 1.0 +/- 1.2 at 2 years. Moreover, 60% of patients were off medications at the final follow-up. Based on success criterion 1 (IOP </=18), the Kaplan-Meier survival rate was 70% of patients at 1 year and 52% at 2 years. Based on success criterion 2 (IOP </=15), the survival was reduced to 36% at 1 year and 25% at 2 years. One patient required a trabeculectomy (at 18 months) during the follow-up.
In the HORIZON study, a 2-year prospective, multicenter, randomized controlled single-masked clinical trial, Samuelson et al. analyzed 556 eyes.[25] Based on randomization that was stratified by site and by history of SLT, 369 of the eyes were assigned to the Hydrus microstent (HMS) group, and the other 187 were to receive cataract surgery alone with no microstent (NMS). Medication washout was performed at 12 and 24 months. For all endpoints at 24 months, HMS outcomes were superior to NMS, even on subgroup analysis. 77.3% of HMS patients achieved a reduction of unmedicated mean DIOP of >20%, versus only 57.8% of NMS patients. HMS eyes also saw a greater mean reduction of unmedicated mean DIOP than NMS eyes (-7.6 vs. -5.3 mm Hg). While patients in both groups were using an average of 1.7 glaucoma medications prior to the study, HMS patients were only using 0.3 on average, while NMS patients were taking 0.7. Furthermore, the proportion of patients who were medication-free at 24 months was higher in the HMS group (78 percent) than in the NMS group (48 percent). Five year outcomes of the HORIZON study found that patients receiving the Hydrus implant were less likely to suffer visual field progression compared to eyes receiving cataract surgery alone.[49]
Cost-Efficacy of MIGS vs. Topical Treatment
The iStent has been studied from a cost-efficacy stand point as a long-term solution for IOP control compared to topical treatment. Surgical treatments like iStent implantation circumvent some of the non-adherence challenges in patients on chronic medical treatment. In addition, the iStent effects on cost of therapy and quality of life measures by reducing chronic dependency on topical drops has been studied.
One study looked at a comparative cost analysis between iStent implantation and topical medical treatment in Canada.[50] The study was performed over a 15-year horizon on patients with mild to moderate POAG (83% and 17% respectively). Direct medical costs such as ophthalmology visits and diagnostic exams as well as indirect costs such as transportation and nursing care were considered. The cost of drops was calculated using the Ontario Drug Benefit Formulary for all existing glaucoma medications (taking into consideration waste, dispensing fees, and predicted markup value). It was found that over the study’s time frame, the iStent led to healthcare costs totaling C$9,394.1, compared to C$12,302.4 in matched patients treated with medications alone.
Data were also measured in incremental cost-utility ratio (difference in costs of treatment divided by difference in quality-adjusted life-years [QALY]) for iStent and Cataract extraction vs. Cataract surgery alone (control). Results showed that over a 15-year period, iStent showed a higher QALY score compared to the control group. Using a probabilistic analysis, it was concluded that iStent with cataract surgery would promote more savings and increase the QALY score and was an economically favorable treatment.
A similar study was performed to compare three types of treatments: iStent, laser trabeculoplasty, and topical combination medications (timolol/dorzolamide/brimonidine, timolol/dorzolamide/latanoprost, and timolol/dorzolamide/bimatoprost) in a Colombian healthcare payer system.[51] The analysis was done over a lifetime horizon with a patient baseline age of 40 years. Patients in this study had glaucoma stages ranging from mild to severe. The analysis of QALYs related to economic cost and loss of visual acuity was measured. It was shown that over a lifetime horizon, the iStent was expected to have a the most discounted QALY score followed by topical treatment then laser trabeculoplasty. Over a 40-year period across the study population, the iStent was $13,252,318 lower than the cost of laser trabeculoplasty and $6,403,534 lower than that of timolol/dorzolamide/brimonidine, $22,311,064 lower than that of timolol/dorzolamide/latanoprost, and $29,156,113 lower than that of timolol/dorzolamide/bimatoprost. The study concluded iStent was cost effective for preventing disease progression and increasing savings.
In a cost-utility analysis using data from randomized controlled trials, the Hydrus microstent was found to be cost-effective when compared to cataract surgery alone within 5 years of follow-up.[52]
Future Direction
Pipeline trabecular micro-bypass products are expected in the coming years. Further follow-up is needed to elucidate adverse effects in the long term, but its overall favorable safety profile and ability to spare conjunctiva should there be need for future incisional glaucoma surgery bode well for the addition of these devices to the surgical arsenal of glaucoma management.
References
- ↑ Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081‐2090. doi:10.1016/j.ophtha.2014.05.013.
- ↑ Jiménez-Román, J., Prado-Larrea, C., Laneri-Pusineri, L., & Gonzalez-Salinas, R. (2018). Combined Glaucoma and Cataract: An Overview. Difficulties in Cataract Surgery. doi:10.5772/intechopen.73584.
- ↑ Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268‐1279. doi:10.1001/archopht.120.10.1268.
- ↑ Fautsch, Michael P, and Douglas H Johnson. “Aqueous humor outflow: what do we know? Where will it lead us?.” Investigative ophthalmology & visual science vol. 47,10 (2006): 4181-7. doi:10.1167/iovs.06-0830.
- ↑ Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK. Aqueous outflow - A continuum from trabecular meshwork to episcleral veins. Prog Retin Eye Res. 2017;57:108‐133. doi:10.1016/j.preteyeres.2016.12.004.
- ↑ Vranka, Janice A et al. “Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma.” Experimental eye research vol. 133 (2015): 112-25. doi:10.1016/j.exer.2014.07.014.
- ↑ Geyer, O., Mathalone, N., & Buckman, G. (2006). Reply: Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. Journal of Cataract & RefractiveSurgery,32(2),183. doi:10.1016/j.jcrs.2005.12.040.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 FDA Summary of Safety and Effectiveness Data (SSED) – Hydrus microstent.
- ↑ Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol 1973; 75: 365-383.
- ↑ Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clinical Ophthalmology 2016; 10: 189-206.
- ↑ Voskanyan, L., García-Feijoó, J., Belda, J. I., Fea, A., Jünemann, A., & Baudouin, C. (2014). Prospective, Unmasked Evaluation of the iStent® Inject System for Open-Angle Glaucoma: Synergy Trial. Advances in Therapy, 31(2), 189–201. doi:10.1007/s12325-014-0095-y.
- ↑ Saheb, H., & Le, K. (2014). iStent trabecular micro-bypass stent for open-angle glaucoma. Clinical Ophthalmology, 1937. doi:10.2147/opth.s45920.
- ↑ Bovee, C. E., & Pasquale, L. R. (2016). Evolving Surgical Interventions in the Treatment of Glaucoma. Seminars in Ophthalmology, 32(1), 91-95. doi:10.1080/08820538.2016.1228393.
- ↑ Arriola-Villalobos, P., Martínez-de-la-Casa, J. M., Díaz-Valle, D., García-Vidal, S. E., Fernández-Pérez, C., García-Sánchez, J., & García-Feijoó, J. (2013). Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. British Journal of Ophthalmology, 97(10), 1250–1255. doi:10.1136/bjophthalmol-2012-302394.
- ↑ Samuelson, T. W., Katz, L. J., Wells, J. M., Duh, Y.-J., & Giamporcaro, J. E. (2011). Randomized Evaluation of the Trabecular Micro-Bypass Stent with Phacoemulsification in Patients with Glaucoma and Cataract. Ophthalmology, 118(3), 459–467. doi:10.1016/j.ophtha.2010.07.007.
- ↑ Craven, E. R., Katz, L. J., Wells, J. M., & Giamporcaro, J. E. (2012). Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. Journal of Cataract & Refractive Surgery, 38(8), 1339–1345. doi:10.1016/j.jcrs.2012.03.025.
- ↑ Duch, S., Buchacra, O., Milla, E., & Oana Stirbu. (2011). One-year analysis of the iStent trabecular microbypass in secondary glaucoma. Clinical Ophthalmology, 321. doi:10.2147/opth.s15025.
- ↑ Patel I, de Klerk TA, Au L. Manchester iStent study: early results from a prospective UK case series. Clin Exp Ophthalmol. 2013;41(7):648‐652. doi:10.1111/ceo.12098.
- ↑ Khouri, A. S., & Megalla, M. M. (2016). Recurrent hyphema following iStent surgery managed by surgical removal. Canadian Journal of Ophthalmology / Journal Canadien d’Ophtalmologie, 51(6), e163–e165. doi:10.1016/j.jcjo.20.
- ↑ Fernández-Barrientos, Y., García-Feijoó, J., Martínez-de-la-Casa, J. M., Pablo, L. E., Fernández-Pérez, C., & García Sánchez, J. (2010). Fluorophotometric Study of the Effect of the Glaukos Trabecular Microbypass Stent on Aqueous Humor Dynamics. Investigative Opthalmology & Visual Science, 51(7), 3327. doi:10.1167/iovs.09-3972.
- ↑ Fea, A. M. (2010). Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. Journal of Cataract & Refractive Surgery, 36(3), 407–412. doi:10.1016/j.jcrs.2009.10.031.
- ↑ Gillmann K, Bravetti GE, Mermoud A, Mansouri K. A Prospective Analysis of iStent Inject Microstent Positioning: Schlemm Canal Dilatation and Intraocular Pressure Correlations. J Glaucoma. 2019;28(7):613‐621. doi:10.1097/IJG.0000000000001273.
- ↑ Giamporcaro, Belda Sanchis, J. I., Chang, L., Pablo, L., Voskanyan, L., Katz, L. J., … Fea, A. (2014). Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clinical Ophthalmology, 875. doi:10.2147/opth.s59932.
- ↑ Ichhpujani P, Katz LJ, Gille R, Affel E. Imaging modalities for localization of an iStent(®). Ophthalmic Surg Lasers Imaging. 2010;41(6):660‐663. doi:10.3928/15428877-20100929-02.
- ↑ Jump up to: 25.0 25.1 25.2 25.3 Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology 2018; https://doi.org/10.1016/j.ophtha.2018.05.012
- ↑ Jump up to: 26.0 26.1 26.2 26.3 Fea AM, Ahmed IIK, Lavia C, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: one year results. Clin Exp Ophthalmol 2017; 45(2): 120-127.
- ↑ Jump up to: 27.0 27.1 27.2 Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 2015; 122(7): 1283-1293
- ↑ Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS One 2017; https://doi.org/10.1371/journal.pone.0183142.
- ↑ Jump up to: 29.0 29.1 Gandolfi SA, Ungaro N, Ghirardini S, Tardini MG, Mora P. Comparison of surgical outcomes between canaloplasty and Schlemm’s canal scaffold at 24 months’ follow-up. J Ophthalmol 2016: 3410469. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4771907/pdf/JOPH2016-3410469.pdf
- ↑ Fea AM, Consolandi G, Pignata G, et al. A comparison of endothelial cell loss in combined cataract and MIGS (Hydrus) procedure to phacoemulsification alone: 6-month results. J Ophthalmol 2015; http://dx.doi.org/10.1155/2015/769289
- ↑ Peräsalo, R. (2009). Phaco-emulsification of cataract in eyes with glaucoma. Acta Ophthalmologica Scandinavica, 75(3), 299–300. doi:10.1111/j.1600-0420.1997.tb00778.x.
- ↑ Mathalone, N., Hyams, M., Neiman, S., Buckman, G., Hod, Y., & Geyer, O. (2005). Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients. Journal of Cataract & Refractive Surgery, 31(3), 479–483. doi:10.1016/j.jcrs.2004.06.046.
- ↑ Arriola-Villalobos, P., Martínez-de-la-Casa, J. M., Díaz-Valle, D., García-Vidal, S. E., Fernández-Pérez, C., García-Sánchez, J., & García-Feijoó, J. (2013). Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. British Journal of Ophthalmology, 97(10), 1250–1255. doi:10.1136/bjophthalmol-2012-302394.
- ↑ Spiegel D, García-Feijoó J, García-Sánchez J, Lamielle H. Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther. 2008;25(5):453‐464. doi:10.1007/s12325-008-0062-6.
- ↑ Craven ER, Katz LJ, Wells JM, Giamporcaro JE; iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339‐1345. doi:10.1016/j.jcrs.2012.03.025.
- ↑ Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE; US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459‐467. doi:10.1016/j.ophtha.2010.07.007.
- ↑ Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407‐412. doi:10.1016/j.jcrs.2009.10.031.
- ↑ Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96(5):645‐649. doi:10.1136/bjophthalmol-2011-300218.
- ↑ Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with Phacoemulsification versus Phacoemulsification Alone for Patients with Glaucoma and Cataract: A Meta-Analysis. PLoS One. 2015;10(7):e0131770. Published 2015 Jul 6. doi:10.1371/journal.pone.0131770.
- ↑ Spiegel D, Wetzel W, Haffner DS, Hill RA. Initial clinical experience with the trabecular micro-bypass stent in patients with glaucoma. Adv Ther. 2007;24(1):161‐170. doi:10.1007/BF02850004.
- ↑ Buchacra O, Duch S, Milla E, Stirbu O. One-year analysis of the iStent trabecular microbypass in secondary glaucoma. Clin Ophthalmol. 2011;5:321‐326. doi:10.2147/OPTH.S15025.
- ↑ Ahmed II, Katz LJ, Chang DF, et al. Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma. J Cataract Refract Surg. 2014;40(8):1295‐1300. doi:10.1016/j.jcrs.2014.07.004.
- ↑ Fea AM, Belda JI, Rękas M, et al. Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875‐882. Published 2014 May 7. doi:10.2147/OPTH.S59932.
- ↑ Fechtner, Robert D., et al. “Five-Year, Prospective, Randomized, Multi-Surgeon Trial of Two Trabecular Bypass Stents versus Prostaglandin for Newly Diagnosed Open-Angle Glaucoma.” Ophthalmology Glaucoma, vol. 2, no. 3, 2019, pp. 156–166. doi:10.1016/j.ogla.2019.03.004.
- ↑ Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911‐1917. doi:10.1016/j.jcrs.2012.07.017.
- ↑ Malvankar-Mehta MS, Chen YN, Iordanous Y, Wang WW, Costella J, Hutnik CM. iStent as a Solo Procedure for Glaucoma Patients: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(5):e0128146. Published 2015 May 27. doi:10.1371/journal.pone.0128146.
- ↑ Ahmed IIK, Fea AM, Au L, et al. A prospective randomized trial comparing Hydrus and iStent micro-invasive glaucoma surgery implants for standalone treatment of open-angle glaucoma: The COMPARE study. Ophthalmology 2019; https://doi.org/10.1016/j.ophtha.2019.04.034
- ↑ Fea AM, Rekas M, Au L. Evaluation of a Schlemm canal scaffold microstent combined with phacoemulsification in routine clinical practice: Two-year multicenter study. J Cataract Refract Surg 2017; 43(7): 886-891.
- ↑ Montesano G, Ometto G, Ahmed IIK, Ramulu PY, Chang DF, Crabb DP, Gazzard G. Five-Year Visual Field Outcomes of the HORIZON Trial. Am J Ophthalmol. 2023 Jul;251:143-155. doi: 10.1016/j.ajo.2023.02.008. Epub 2023 Feb 21. PMID: 36813144.
- ↑ Patel V, Ahmed I, Podbielski D, Falvey H, Murray J, Goeree R. Cost-effectiveness analysis of standalone trabecular micro-bypass stents in patients with mild-to-moderate open-angle glaucoma in Canada. J Med Econ. 2019;22(4):390‐401. doi:10.1080/13696998.2019.1572013.
- ↑ Ordóñez JE, Ordóñez A, Osorio UM. Cost-effectiveness analysis of iStent trabecular micro-bypass stent for patients with open-angle glaucoma in Colombia. Curr Med Res Opin. 2019;35(2):329‐340. doi:10.1080/03007995.2018.1506022.
- ↑ Sood S, Heilenbach N, Sanchez V, et al. Cost-effectiveness Analysis of Minimally Invasive Trabecular Meshwork Stents with Phacoemulsification. Ophthalmol Glaucoma. 2021;S2589-4196.