Joubert Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Joubert Syndrome is a congenital condition with a triad of major clinical findings: hypotonia in infancy, global developmental delay, and pathognomonic cerebellar and brainstem malformation (aplasia or hypoplasia of cerebellar vermis). Ocular phenotypes can present with oculomotor apraxia, strabismus, nystagmus, ptosis, retinal dystrophy, chorioretinal coloboma, optic nerve atrophy, and abnormal retinal pigmentation.
Disease Entity
Disease
Joubert syndrome (JS) is a rare, genetically heterogeneous disorder belonging to a group of inherited diseases caused by defect(s) in the primary cilia, which are also known as ciliopathies. The disease affects multiple organs, including the eye, kidney, and brain.
Etiology
First described by Marie Joubert in 1969, [1] JS now is known to have multiple genetic causes and subtypes, all of which affect the structure and/or function of primary cilia. Primary cilia are a specialized non-motile, microtubule-based cellular structure that has important roles in development, sensory signaling, and tissue homeostasis in mammalian cells.[2][3] In the vertebral eye, primary cilia are found in the cornea, lens, trabecular meshwork, photoreceptors, and retinal pigment epithelium.[4][5][6][7] As a result, syndromic ciliopathies often present with variable ocular manifestations.[4][8][9] Patients with JS classically present with the distinct cerebellar and brainstem malformation (“molar tooth sign” on MRI), hypotonia, and global developmental delay.
Risk Factors
Prevalence of JS is estimated to be 1 in 80,000 to 1 in 100,000, with notably higher prevalence in French Canadians.[10][8] Other ethnic foci include the Dutch ,[11] Ashkenazi Jews,[12] Canadian Hutterites,[13] and Japanese.[14]
Pathophysiology
Molecular studies show that dysfunctional cellular processes that compromise the structural and/or functional integrity of the primary cilia lead to retinal ciliopathies. JS is inherited primarily via an autosomal recessive pattern. Thirty-four genes have been reported to cause JS,[8] although 10-40% of all cases have an unknown genetic cause.[15] The major genes associated with JS include: CEP290, AHI1, TMEM67, CSPP1, and CPLANE1; all of which play a developmental and/or regulatory role in the primary cilium.[8][10] Mutations in CEP290 have been implicated in approximately 50% of JS.[16]
Retinal degeneration in JS is thought to be caused by abnormal photoreceptor development due to the defective primary cilia in the cells whose cellular processes are impaired, such as vesicular trafficking (AH1)[17] and ciliogenesis (CEP290).[18][4]
Diagnosis
History
Classic JS consists of a triad of major clinical findings: hypotonia in infancy, global developmental delay, and pathognomonic cerebellar and brainstem malformation (“molar tooth sign” on MRI) .[10] Atypical breathing patterns (alternating tachypnea and/or apnea) and abnormal eye movements, especially oculomotor apraxia, are also associated with JS.[19] [20] As the patient ages, hypotonia progresses to truncal ataxia, but breathing abnormalities usually improve.[10] Depending on the organs affected, JS can also present with renal disease (e.g., juvenile nephronophthisis), hepatic fibrosis, occipital encephalocele, polydactyly, oral hamartomas, endocrine disorders, and abnormal facies.[10][8]
Physical Examination
Patients with JS ocular phenotypes present with oculomotor apraxia (80% of patients), strabismus (74%), nystagmus (72%), ptosis (44%), retinal degeneration (38%), chorioretinal coloboma (30%), optic nerve atrophy (22%), and abnormal retinal pigmentation (4.5%).[8][21]
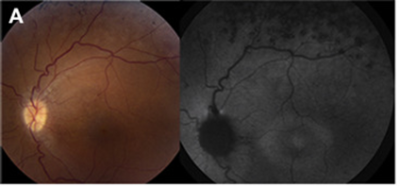
Retinal dystrophy can vary from early-onset severe rod–cone dystrophy to late-onset cone–rod dystrophy.[8][22] Retinal dystrophy can be appreciated on the fundus exam and fundus autofluorescence image (Figure 1). JS patients with retinal degeneration are less likely to have coloboma, and JS patients with colobomas are less likely to present with retinal degeneration, except in two reported cases of CEP290-related JS where the two co-exist.[22] Optic nerve atrophy can occur independently of retinal degeneration in JS patients.[22] Optic disc drusen have also been observed.[23][24] [25]
Electroretinogram (ERG) studies may also be abnormal in JS patients. In one NIH study, the majority of JS patients exhibited abnormal ERG readings. JS patients who have severely reduced or non-recordable ERG readings are found to have mutations in CEP290, CEP164, AHI1, MKS1, and INPP5E.[22]
Children with JS have delayed development of best visual acuity compared to normal. Specifically, the transition from non-quantifiable acuity (e.g. fix and follow; central steady and maintained; blink to light) to quantifiable acuity is delayed. Compared to a normal child who develops complete visual ability between age 2-3, children with JS visually mature around age 4-6.[22] While visual acuity generally does not worsen with age, those with retinal degeneration experience declining visual acuity over time.[22]
In JS patients, oculomotor abnormalities are common, including oculomotor apraxia, decreased vestibulo-ocular reflex cancellation, decreased smooth pursuit, compensatory head thrusts or catch-up saccades, and nystagmus.[22][23][26][9] Head titubation, characterized by high frequency (~3Hz), small amplitude (5-10°), horizontal head tremor, may be observed in the first months of life.[27]
Diagnostic procedures
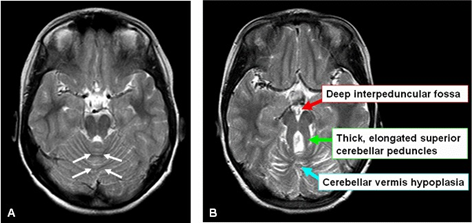
The pathognomonic neuro-radiological sign on the brain MRI, the “molar tooth sign,” indicates the cerebellar vermis hypoplasia and brainstem abnormalities (deep interpeduncular fossa, and thick and elongated superior cerebellar peduncles) (Figure 2). The fourth ventricle can have an appearance of 'bat wing'.
Laboratory test
Targeted genetic testing of the common gene mutations can aid in the diagnosis of JS. If the gene panel returns negative, exon-sequencing or even whole-genome sequencing can be used to establish a genetic cause.[28]
Differential diagnosis
- Cerebellar vermis malformations without the Molar Tooth Sign (e.g. Dandy-Walker)
- X-linked cerebellar hypoplasia
- Meckel-Gruber syndrome
- Bardet–Biedl syndrome
- Leber congenital amaurosis
Management
Medical therapy
Ophthalmologic management includes corrective lenses for refractive errors and neuro-ophthalmological rehabilitation for oculomotor disorders. Regular ophthalmic monitoring throughout the amblyogenic period is recommended, which may last longer in JS patients.[22]
Genotyping can aid in disease diagnosis and monitoring disease trajectory. For example, because JS caused by mutations in CEP290, AHI1, and INPP5E commonly manifest with retinal degeneration, affected patients should have regular monitoring for retinal health.[22]
Surgery
Surgery is indicated for symptomatic strabismus and ptosis, depending on the severity and amblyogenic potential.[10][22]
Prognosis
Because JS has different phenotypic subtypes that affect different organs, prognosis and mortality in JS is largely dependent on the severity of the organs affected. It has been reported that JS patients with retinal dystrophy are more likely to have multicystic renal disease and thus higher mortality rate than JS patients without retinal dystrophy.[19] Children with JS are more likely to have delayed visual development and a longer amblyopic period.[22]
Further reading
Joubert syndrome: https://radiopaedia.org/articles/joubert-syndrome-1
References
- ↑ Joubert M, Eisenring JJ, Robb JP, Andermann F. Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnea, abnormal eye movements, ataxia, and retardation. Neurology. 1969 Sep;19(9):813-25. doi: 10.1212/wnl.19.9.813. PMID: 10488899. https://pubmed.ncbi.nlm.nih.gov/10488899/
- ↑ Gerdes, J. M., Davis, E. E., & Katsanis, N. (2009). The vertebrate primary cilium in development, homeostasis, and disease. Cell, 137(1), 32–45. https://doi.org/10.1016/j.cell.2009.03.023.
- ↑ Wheway, G., Parry, D. A., & Johnson, C. A. (2014). The role of primary cilia in the development and disease of the retina. Organogenesis, 10(1), 69–85. https://doi.org/10.4161/org.26710 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4049897/
- ↑ Jump up to: 4.0 4.1 4.2 May-Simera, H. L., Wan, Q., Jha, B. S., Hartford, J., Khristov, V., Dejene, R., Chang, J., Patnaik, S., Lu, Q., Banerjee, P., Silver, J., Insinna-Kettenhofen, C., Patel, D., Lotfi, M., Malicdan, M., Hotaling, N., Maminishkis, A., Sridharan, R., Brooks, B., Miyagishima, K., … Bharti, K. (2018). Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells. Cell reports, 22(1), 189–205. https://doi.org/10.1016/j.celrep.2017.12.038 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6166245/
- ↑ Sugiyama Y, Stump RJ, Nguyen A, Wen L, Chen Y, Wang Y, Murdoch JN, Lovicu FJ, McAvoy JW. Secreted frizzled-related protein disrupts PCP in eye lens fiber cells that have polarised primary cilia. Dev Biol. 2010 Feb 15;338(2):193-201. doi: 10.1016/j.ydbio.2009.11.033. Epub 2009 Dec 5. PMID: 19968984; PMCID: PMC2815166. https://pubmed.ncbi.nlm.nih.gov/19968984/
- ↑ Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y. OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet. 2012 Aug 1;21(15):3333-44. doi: 10.1093/hmg/dds163. Epub 2012 Apr 27. PMID: 22543976; PMCID: PMC3392109. https://pubmed.ncbi.nlm.nih.gov/22543976/
- ↑ Grisanti L, Revenkova E, Gordon RE, Iomini C. Primary cilia maintain corneal epithelial homeostasis by regulation of the Notch signaling pathway. Development. 2016 Jun 15;143(12):2160-71. doi: 10.1242/dev.132704. Epub 2016 Apr 27. PMID: 27122169; PMCID: PMC4920172. https://pubmed.ncbi.nlm.nih.gov/27122169/
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Wang SF, Kowal TJ, Ning K, Koo EB, Wu AY, Mahajan VB, Sun Y. Review of Ocular Manifestations of Joubert Syndrome. Genes (Basel). 2018 Dec 4;9(12):605. doi: 10.3390/genes9120605. PMID: 30518138; PMCID: PMC6315342. https://pubmed.ncbi.nlm.nih.gov/30518138/
- ↑ Jump up to: 9.0 9.1 Khan AO, Oystreck DT, Seidahmed MZ, AlDrees A, Elmalik SA, Alorainy IA, Salih MA. Ophthalmic features of Joubert syndrome. Ophthalmology. 2008 Dec;115(12):2286-9. doi: 10.1016/j.ophtha.2008.08.005. PMID: 19041481. https://pubmed.ncbi.nlm.nih.gov/19041481/
- ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 10.5 10.6 Parisi M, Glass I. Joubert Syndrome. 2003 Jul 9 [Updated 2017 Jun 29]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1325/ https://www.ncbi.nlm.nih.gov/books/NBK1325/
- ↑ Kroes HY, Monroe GR, van der Zwaag B, Duran KJ, de Kovel CG, van Roosmalen MJ, Harakalova M, Nijman IJ, Kloosterman WP, Giles RH, Knoers NV, van Haaften G. Joubert syndrome: genotyping a Northern European patient cohort. Eur J Hum Genet. 2016 Feb;24(2):214-20. doi: 10.1038/ejhg.2015.84. Epub 2015 Apr 29. PMID: 25920555; PMCID: PMC4717206. https://pubmed.ncbi.nlm.nih.gov/25920555/
- ↑ Edvardson S, Shaag A, Zenvirt S, Erlich Y, Hannon GJ, Shanske AL, Gomori JM, Ekstein J, Elpeleg O. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am J Hum Genet. 2010 Jan;86(1):93-7. doi: 10.1016/j.ajhg.2009.12.007. Epub 2009 Dec 31. Erratum in: Am J Hum Genet. 2010 Feb;86(2):294. Shanske, Alan L [added]. PMID: 20036350; PMCID: PMC2801745. https://pubmed.ncbi.nlm.nih.gov/20036350/
- ↑ Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, Greenberg CR, McLeod DR, Bernier FP, Chudley AE, Müller T, Shboul M, Logan CV, Loucks CM, Beaulieu CL, Bowie RV, Bell SM, Adkins J, Zuniga FI, Ross KD, Wang J, Ban MR, Becker C, Nürnberg P, Douglas S, Craft CM, Akimenko MA, Hegele RA, Ober C, Utermann G, Bolz HJ, Bulman DE, Katsanis N, Blacque OE, Doherty D, Parboosingh JS, Leroux MR, Johnson CA, Boycott KM. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet. 2011 Dec 9;89(6):713-30. doi: 10.1016/j.ajhg.2011.11.005. PMID: 22152675; PMCID: PMC3234373. https://pubmed.ncbi.nlm.nih.gov/22152675/
- ↑ Suzuki T, Miyake N, Tsurusaki Y, Okamoto N, Alkindy A, Inaba A, Sato M, Ito S, Muramatsu K, Kimura S, Ieda D, Saitoh S, Hiyane M, Suzumura H, Yagyu K, Shiraishi H, Nakajima M, Fueki N, Habata Y, Ueda Y, Komatsu Y, Yan K, Shimoda K, Shitara Y, Mizuno S, Ichinomiya K, Sameshima K, Tsuyusaki Y, Kurosawa K, Sakai Y, Haginoya K, Kobayashi Y, Yoshizawa C, Hisano M, Nakashima M, Saitsu H, Takeda S, Matsumoto N. Molecular genetic analysis of 30 families with Joubert syndrome. Clin Genet. 2016;90:526–35. https://pubmed.ncbi.nlm.nih.gov/27434533/
- ↑ MedlinePlus. Joubert syndrome. Retrieved 2021 Oct 17. https://medlineplus.gov/genetics/condition/joubert-syndrome/#causes
- ↑ Waters AM, Beales PL. "Ciliopathies: an expanding disease spectrum". Pediatric Nephrology.2011 Jul;26(7):1039-56 doi:10.1007/s00467-010-1731-7. PMC 3098370. PMID 21210154 https://pubmed.ncbi.nlm.nih.gov/21210154/
- ↑ Westfall JE, Hoyt C, Liu Q, Hsiao YC, Pierce EA, Page-McCaw PS, Ferland RJ. Retinal degeneration and failure of photoreceptor outer segment formation in mice with targeted deletion of the Joubert syndrome gene, Ahi1. J Neurosci. 2010 Jun 30;30(26):8759-68. doi: 10.1523/JNEUROSCI.5229-09.2010. PMID: 20592197; PMCID: PMC2923804. https://pubmed.ncbi.nlm.nih.gov/20592197/
- ↑ Rachel, R. A., Yamamoto, E. A., Dewanjee, M., Munasinghe, J., May-Simera, H. L., Dong, L., & Swaroop, A. (2012). CEP290 is required for photoreceptor ciliogenesis and other cilia related functions. Cilia, 1(Suppl 1), P98. https://doi.org/10.1186/2046-2530-1-S1-P98 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3555976/
- ↑ Jump up to: 19.0 19.1 Saraiva JM, Baraitser M. Joubert syndrome: a review. Am J Med Genet. 1992 Jul 1;43(4):726-31. doi: 10.1002/ajmg.1320430415. PMID: 1341417. https://pubmed.ncbi.nlm.nih.gov/1341417/
- ↑ Tusa RJ, Hove MT. Ocular and oculomotor signs in Joubert syndrome. J Child Neurol. 1999 Oct;14(10):621-7. doi: 10.1177/088307389901401001. PMID: 10511333. https://pubmed.ncbi.nlm.nih.gov/10511333/
- ↑ Chawla, R., Bypareddy, R., Vekaria, L., Venkatesh, P., & Ananthashayana, V. H. (2017). Fundus findings in a case of Joubert syndrome. Indian journal of ophthalmology, 65(4), 329–330. https://doi.org/10.4103/ijo.IJO_441_16
- ↑ Jump up to: 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 Brooks BP, Zein WM, Thompson AH, Mokhtarzadeh M, Doherty DA, Parisi M, Glass IA, Malicdan MC, Vilboux T, Vemulapalli M, Mullikin JC, Gahl WA, Gunay-Aygun M. Joubert Syndrome: Ophthalmological Findings in Correlation with Genotype and Hepatorenal Disease in 99 Patients Prospectively Evaluated at a Single Center. Ophthalmology. 2018 Dec;125(12):1937-1952. doi: 10.1016/j.ophtha.2018.05.026. Epub 2018 Jul 25. PMID: 30055837. https://www.aaojournal.org/article/S0161-6420(18)30686-9/fulltext
- ↑ Jump up to: 23.0 23.1 Sturm, V., Leiba, H., Menke, M. et al. Ophthalmological findings in Joubert syndrome. Eye 24, 222–225 (2010). https://doi.org/10.1038/eye.2009.116 https://www.nature.com/articles/eye2009116#citeas
- ↑ Yilmaz S, Biler ED, Solmaz AE, Serdaroglu G, Tekin HG, Gokben S. Optic disc drusen mimicking papilledema in an infant with Joubert syndrome. Genet Couns. 2015;26(1):35-9. PMID: 26043505. https://pubmed.ncbi.nlm.nih.gov/26043505/
- ↑ Apostolou T, Nikolopoulou N, Theodoridis M, Koumoustiotis V, Pavlopoulou E, Chondros D, Billis A. Late onset of renal disease in nephronophthisis with features of Joubert syndrome type B. Nephrol Dial Transplant. 2001 Dec;16(12):2412-5. doi: 10.1093/ndt/16.12.2412. PMID: 11733635. https://pubmed.ncbi.nlm.nih.gov/11733635/
- ↑ Gill H, Muthusamy B, Atan D, Williams C, Ellis M. Joubert syndrome presenting with motor delay and oculomotor apraxia. Case Rep Pediatr. 2011;2011:262641. doi:10.1155/2011/262641. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3350021/
- ↑ Poretti A, Christen HJ, Elton LE, Baumgartner M, Korenke GC, Sukhudyan B, Hethey S, Cross E, Steinlin M, Boltshauser E. Horizontal head titubation in infants with Joubert syndrome: a new finding. Dev Med Child Neurol. 2014 Oct;56(10):1016-20. doi: 10.1111/dmcn.12489. Epub 2014 May 10. PMID: 24814865. https://pubmed.ncbi.nlm.nih.gov/24814865/
- ↑ Vilboux T, Doherty DA, Glass IA, Parisi MA, Phelps IG, Cullinane AR, Zein W, Brooks BP, Heller T, Soldatos A, Oden NL, Yildirimli D, Vemulapalli M, Mullikin JC, Nisc Comparative Sequencing Program, Malicdan MCV, Gahl WA, Gunay-Aygun M. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med. 2017 Aug;19(8):875-882. doi: 10.1038/gim.2016.204. Epub 2017 Jan 26. PMID: 28125082. https://pubmed.ncbi.nlm.nih.gov/28125082/

