Bardet-Biedl Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
This review describes the etiology, pathophysiology, and ocular and systemic findings in Bardet-Biedl syndrome (BBS).
Disease Entity
Disease
Bardet-Biedl syndrome (BBS) is a rare, autosomal recessive genetic disorder that can lead to dysfunction of multiple organ systems, including the kidneys, genitalia, brain, and eye.
Etiology
BBS is caused by mutations of proteins involved in function of the cilium, a specialized cellular organelle common to many cell types throughout the body. Patients with BBS are genetically heterogenous, the disease phenotype is widely variable among affected individuals[1] [2][3]. The overall most commonly exhibited feature among BBS patients is a retinal rod-cone dystrophy[4].
Pathophysiology
BBS is caused by pathogenic mutations in genes encoding proteins involved in the function of non-motile primary cilia. Rod and cone photoreceptors do not contain primary cilia, but rather have a primary cilia-like structure that spans the inner and outer segments. Photoreceptor outer segments have therefore been conceptualized as specialized sensory cilia, sometimes referred to as photoreceptor sensory cilia (PSC)[5]. Primary cilia and photoreceptor cilia are structurally similar, are composed of many of the same proteins, and are both dysfunctional in BBS[4].
Proteins affected by BBS are involved in intracellular protein trafficking along the photoreceptor connecting cilium, a process known as intraflagellar transport. In BBS mutant photoreceptors, proteins are mislocalized to incorrect cellular substructures[6]. For example, rhodopsin accumulates in rod inner segments and cell bodies at the expense of its localization to outer segments in some BBS mouse models[7][8][9][10]. Other work shows abnormal accumulation of 138 proteins in photoreceptor outer segments of BBS mutant mice compared to wild types[11]. The mislocalization and ectopic accumulation of proteins is thought to lead to inadequate cellular homeostasis and ultimately photoreceptor cell death[6][11][12].
Molecular genetics
At least twenty-six genes have been implicated in BBS (BBS1-BBS21)[1][3][4]. The most commonly mutated gene is BBS1 (23% of cases) followed by BBS10 (15%) and BBS2 (10%)[1]. The inheritance pattern of BBS follows a classic Mendelian autosomal recessive pattern in the majority of cases; however, more complex inheritance patterns have also been reported[6]. BBS phenotypes have been shown to be more severe in the presence of additional mutations in other BBS genes (i.e., modifier genes)[4].
Risk Factors and epidemiology
As BBS is an autosomal recessive disorder, patients may or may not have a family history of BBS. Both parents of an affected child must be carriers of a recessive allele for their child to inherit two pathogenic alleles. Consanguinity increases the risk of inheriting two copies of a recessive gene. Worldwide, the incidence ranges from 1:3700 in the Faroe Islands population to 1:160,000 in Northern Europeans[1][4]. Approximately 44 new cases are estimated to occur in the United States yearly[13].
Diagnosis
History and symptoms
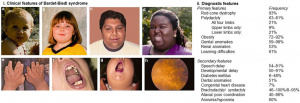
Patients may present with a wide variety of symptoms, consistent with the multi-system pathology involved in BBS (Table 1, Figure 1). BBS is the second-most common cause of syndromic retinitis pigmentosa after Usher syndrome[13]. Visual dysfunction can occur within the first decade of life, and nyctalopia is often the symptom that brings undiagnosed patients to medical attention[1][14]. Rod-cone degeneration typically results in night blindness and visual field constriction followed by loss of visual acuity and color vision, but this course varies among individuals with BBS and both rod and cone systems are typically affected early in the disease[15][16]. Legal blindness typically develops in the second or third decade of life.
| Primary features | Secondary features |
|---|---|
|
|
Physical examination
Ocular Findings
Retinal degeneration is the most highly-penetrant (i.e., most commonly exhibited) feature of BBS[4][17]. Retinal abnormalities in BBS are most often consistent with the phenotype seen in retinitis pigmentosa (RP), characterized by rod degeneration that precedes cone degeneration.[3] Certain BBS mutations can also cause isolated, non-syndromic RP or cone-rod degeneration[3][18].
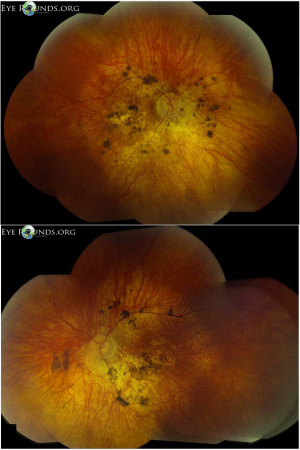
Fundus examination typically shows pigmentary degeneration with early macular atrophy and vascular attenuation. Pigmentary changes are most often described as pigment mottling without bone spicule morphology (Figure 2)[19]. Approximately 10% of patients have nystagmus[20]. Other ocular findings may include strabismus, cataracts, and high corneal astigmatism[21]. Chorioretinal coloboma have been reported as well [22].
Systemic Findings
A majority of patients also exhibit early obesity, polydactyly, and intellectual impairment, and many other features have been described (Table 1, Figure 1)[1][3].
Patients with mutations in BBS1 tend to have milder phenotype, including better retinal function and less obesity, than patients with mutations in other BBS genes[3][23][24]. Interestingly, children with BBS usually have a normal birth weight, and have rapid weight gain in early childhood. The early obesity at less than 6 years of age is atypical for childhood obesity, and should raise considerable suspicion for syndromic obesity.[24]
Clinical diagnosis
Diagnosis is confirmed clinically in patients that display at least four primary features or three primary and two secondary features of BBS (Table 1)[2]. A multidisciplinary diagnostic approach may be necessary in unclear cases.
Diagnostic procedures
Electroretinography (ERG) testing can support signs of retinal dysfunction particularly in early cases that have not yet manifested signs on retinal exam. Typically, ERG in someone with BBS shows a mixed rod-cone dystrophy, manifesting as diminished a-waves and b-waves at both scotopic and photopic light levels[15]. Interestingly, asymptomatic carriers of BBS can also exhibit abnormalities on flash and multifocal ERG testing[25][26].
Laboratory testing
Patients and their families should be referred to genetic counseling for evaluation upon clinical diagnosis[3]. The diagnosis may be supported by genetic testing demonstrating pathogenic mutations of known BBS genes. The diagnostic yield of finding a known causative mutation in a patient diagnosed with BBS is approximately 80%[2]. Given the genetic heterogeneity of BBS, testing should be done using a multigene panel or exome sequencing approach[1].
Differential diagnosis
Considering the wide variation in phenotypic presentation, the sensitivity of clinical diagnostic criteria may be low, especially in young children[1]. The differential diagnosis should include the numerous causes of syndromic and non-syndromic RP, including:
- Alström syndrome
- Joubert syndrome
- Leber congenital amaurosis
- McKusick-Kaufman syndrome
- Retinitis pigmentosa
- Senior-Løken syndrome
- Usher syndrome
Management
General treatment
There is no therapy to treat the cause of BBS, but multidisciplinary care is required to treat disease manifestations[2]. If present, diabetes, hypertension, and metabolic syndrome are managed intensively to minimize damage to other organ systems also involved in BBS, such as the kidney and retina[3]. Disease specific manifestations are managed by respective specialists. Kidney dysfunction is an especially common source of morbidity and mortality in BBS patients[4].
Obesity and hyperphagia in BBS are hypothesized to be a result of hypothalamic dysfunction. In 2020, treatment with Setmelanotide, an agonist to the melanocortin-4-receptor (MC4R), which regulates appetite and body weight, was shown to result in significant weight loss in patients with BSS over a 1-year period.[27] This drug continues to demonstrate positive outcomes in patients with BBS.[28]
Visual dysfunction is often significant in BBS. Initial ophthalmological evaluation in children should include assessment for strabismus, nystagmus, and decreased visual acuity, and if the patient is mature enough, electroretinography and visual field testing. Patients should be referred for low-vision services as is indicated. Annual or more frequent follow-up, as needed, with an ophthalmologist is recommended[1].
Experimental medical therapies
There are currently no clinical trials evaluating therapies for visual dysfunction in BBS. However, gene therapy for retinal dystrophy in BBS is currently being developed in animal models[29][30]. These experiments have utilized sub-retinal injections to deliver adeno-associated virus (AAV) vectors containing wild-type BBS genes to the retina, with some recovery of photoreceptor function[31]. There have been no clinical trials testing this approach in humans. Several other genetic, stem cell, and pharmacological interventions are also currently being explored[3][4]. In addition, there are several population based registry studies to better understand BBS and explore potential for future treatments.[32]
Surgery
Surgical intervention may be required for anatomic abnormalities, such as orodental, cardiovascular, and genitourinary malformations, but there is currently no role for surgery for retinal degeneration in BBS[1].
Prognosis
Visual prognosis is often poor, with most patients experiencing significant visual field loss and legal blindness by the second or third decade of life[17]. Systemic prognosis is variable and depends on the severity of disease manifestation in the various organ systems impacted by BBS.
Additional Resources
- Bardet Biedl Syndrome Foundation (bardetbiedl.org)
- The Clinical Registry Investigating Bardet-Biedl Syndrome (www.bbs-registry.org)
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Forsyth R, Gunay-Aygun M. Bardet-Biedl Syndrome Overview. 2003 Jul 14 [updated 2020 Jul 23]. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Mirzaa GM, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2021. PMID: 20301537.http://www.ncbi.nlm.nih.gov/books/NBK1363/
- ↑ 2.0 2.1 2.2 2.3 2.4 Forsythe, E., & Beales, P. L. (2013). Bardet-Biedl syndrome. European Journal of Human Genetics, 21(1), 8–13. https://doi.org/10.1038/ejhg.2012.115
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Forsythe, E., Kenny, J., Bacchelli, C., & Beales, P. L. (2018). Managing Bardet-Biedl Syndrome-Now and in the Future. Frontiers in Pediatrics, 6, 23. https://doi.org/10.3389/fped.2018.00023
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 Weihbrecht, K. (2020). Bardet-Biedl syndrome. In Genetics and Genomics of Eye Disease (pp. 117–136). Elsevier. https://doi.org/10.1016/B978-0-12-816222-4.00008-3
- ↑ Bujakowska, Kinga M., Qin Liu, and Eric A. Pierce. “Photoreceptor Cilia and Retinal Ciliopathies.” Cold Spring Harbor Perspectives in Biology 9, no. 10 (October 3, 2017). https://doi.org/10.1101/cshperspect.a028274.
- ↑ 6.0 6.1 6.2 Weihbrecht, K., Goar, W. A., Pak, T., Garrison, J. E., DeLuca, A. P., Stone, E. M., Scheetz, T. E., & Sheffield, V. C. (2017). Keeping an Eye on Bardet-Biedl Syndrome: A Comprehensive Review of the Role of Bardet-Biedl Syndrome Genes in the Eye. Medical Research Archives, 5(9). https://doi.org/10.18103/mra.v5i9.1526
- ↑ Abd-El-Barr, M. M., Sykoudis, K., Andrabi, S., Eichers, E. R., Pennesi, M. E., Tan, P. L., Wilson, J. H., Katsanis, N., Lupski, J. R., & Wu, S. M. (2007). Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet–Biedl syndrome. Vision Research, 47(27), 3394–3407. https://doi.org/10.1016/j.visres.2007.09.016
- ↑ Cognard, N., Scerbo, M. J., Obringer, C., Yu, X., Costa, F., Haser, E., Le, D., Stoetzel, C., Roux, M. J., Moulin, B., Dollfus, H., & Marion, V. (2015). Comparing the Bbs10 complete knockout phenotype with a specific renal epithelial knockout one highlights the link between renal defects and systemic inactivation in mice. Cilia, 4(1), 10. https://doi.org/10.1186/s13630-015-0019-8
- ↑ Davis, R. E., Swiderski, R. E., Rahmouni, K., Nishimura, D. Y., Mullins, R. F., Agassandian, K., Philp, A. R., Searby, C. C., Andrews, M. P., Thompson, S., Berry, C. J., Thedens, D. R., Yang, B., Weiss, R. M., Cassell, M. D., Stone, E. M., & Sheffield, V. C. (2007). A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proceedings of the National Academy of Sciences of the United States of America, 104(49), 19422–19427. https://doi.org/10.1073/pnas.0708571104
- ↑ Nishimura, D. Y., Fath, M., Mullins, R. F., Searby, C., Andrews, M., Davis, R., Andorf, J. L., Mykytyn, K., Swiderski, R. E., Yang, B., Carmi, R., Stone, E. M., & Sheffield, V. C. (2004). Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proceedings of the National Academy of Sciences, 101(47), 16588–16593. https://doi.org/10.1073/pnas.0405496101
- ↑ 11.0 11.1 Datta, P., Allamargot, C., Hudson, J. S., Andersen, E. K., Bhattarai, S., Drack, A. V., Sheffield, V. C., & Seo, S. (2015). Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet-Biedl syndrome. Proceedings of the National Academy of Sciences of the United States of America, 112(32), E4400-4409. https://doi.org/10.1073/pnas.1510111112
- ↑ Swiderski, R. E., Nishimura, D. Y., Mullins, R. F., Olvera, M. A., Ross, J. L., Huang, J., Stone, E. M., & Sheffield, V. C. (2007). Gene Expression Analysis of Photoreceptor Cell Loss in Bbs4 -Knockout Mice Reveals an Early Stress Gene Response and Photoreceptor Cell Damage. Investigative Opthalmology & Visual Science, 48(7), 3329. https://doi.org/10.1167/iovs.06-1477
- ↑ 13.0 13.1 Stone, E. M., Andorf, J. L., Whitmore, S. S., DeLuca, A. P., Giacalone, J. C., Streb, L. M., Braun, T. A., Mullins, R. F., Scheetz, T. E., Sheffield, V. C., & Tucker, B. A. (2017). Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology, 124(9), 1314–1331. https://doi.org/10.1016/j.ophtha.2017.04.008
- ↑ Beales, P. L., Elcioglu, N., Woolf, A. S., Parker, D., & Flinter, F. A. (1999). New criteria for improved diagnosis of Bardet-Biedl syndrome: Results of a population survey. Journal of Medical Genetics, 36(6), 437–446.
- ↑ 15.0 15.1 Berezovsky, A., Rocha, D. M., Sacai, P. Y., Watanabe, S. S., Cavascan, N. N., & Salomão, S. R. (2012). Visual acuity and retinal function in patients with Bardet-Biedl syndrome. Clinics (Sao Paulo, Brazil), 67(2), 145–149. https://doi.org/10.6061/clinics/2012(02)09
- ↑ Fulton, A. B., Hansen, R. M., & Glynn, R. J. (1993). Natural course of visual functions in the Bardet-Biedl syndrome. Archives of Ophthalmology (Chicago, Ill.: 1960), 111(11), 1500–1506. https://doi.org/10.1001/archopht.1993.01090110066026
- ↑ 17.0 17.1 Adams, N. A., Awadein, A., & Toma, H. S. (2007). The retinal ciliopathies. Ophthalmic Genetics, 28(3), 113–125. https://doi.org/10.1080/13816810701537424
- ↑ Scheidecker, S., Hull, S., Perdomo, Y., Studer, F., Pelletier, V., Muller, J., Stoetzel, C., Schaefer, E., Defoort-Dhellemmes, S., Drumare, I., Holder, G. E., Hamel, C. P., Webster, A. R., Moore, A. T., Puech, B., & Dollfus, H. J. (2015). Predominantly Cone-System Dysfunction as Rare Form of Retinal Degeneration in Patients With Molecularly Confirmed Bardet-Biedl Syndrome. American Journal of Ophthalmology, 160(2), 364-372.e1. https://doi.org/10.1016/j.ajo.2015.05.007
- ↑ 19.0 19.1 Vislisel, J. M. (2013, October 17). Bardet-Biedl Syndrome. EyeRounds. https://webeye.ophth.uiowa.edu/eyeforum/atlas/pages/bardet-biedl.htm
- ↑ Khan, A. O., & Traboulsi, E. I. (2016, August 26). Bardet-Biedl Syndrome (BBS). American Academy of Ophthalmology. https://www.aao.org/disease-review/bardet-biedl-syndrome-bbs
- ↑ Yavuz Saricay L, Baldwin G, Moulton EA, Gonzalez E, Rajabi F, Hunter DG, Fulton AB. Refractive errors in patients with Bardet Biedl syndrome. Ophthalmic Genet. 2024 Oct;45(5):435-440.
- ↑ Chattannavar G, Ger M, Balasubramanian J, Mandal S, Jalali S, Takkar B, Pisuchpen P, de Guimaraes TAC, Capasso JE, Kumar Padhy S, Levin AV. Bardet-biedl syndrome with chorioretinal coloboma: a case series and review of literature. Ophthalmic Genet. 2024 Oct 15:1-7.
- ↑ Daniels, A. B., Sandberg, M. A., Chen, J., Weigel-DiFranco, C., Fielding Hejtmancic, J., & Berson, E. L. (2012). Genotype-phenotype correlations in Bardet-Biedl syndrome. Archives of Ophthalmology (Chicago, Ill.: 1960), 130(7), 901–907. https://doi.org/10.1001/archophthalmol.2012.89
- ↑ 24.0 24.1 Pomeroy J, Krentz AD, Richardson JG, Berg RL, VanWormer JJ, Haws RM. Bardet-Biedl syndrome: Weight patterns and genetics in a rare obesity syndrome. Pediatr Obes. 2021 Feb;16(2):e12703
- ↑ Cox, G. F., Hansen, R. M., Quinn, N., & Fulton, A. B. (2003). Retinal function in carriers of Bardet-Biedl syndrome. Archives of Ophthalmology (Chicago, Ill.: 1960), 121(6), 804–810. https://doi.org/10.1001/archopht.121.6.804
- ↑ Kim, L. S., Fishman, G. A., Seiple, W. H., Szlyk, J. P., & Stone, E. M. (2007). Retinal dysfunction in carriers of bardet-biedl syndrome. Ophthalmic Genetics, 28(3), 163–168. https://doi.org/10.1080/13816810701537440
- ↑ Haws R, Brady S, Davis E, Fletty K, Yuan G, Gordon G, Stewart M, Yanovski J. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020 Nov;22(11):2133-2140
- ↑ Forsythe E, Haws RM, Argente J, Beales P, Martos-Moreno GÁ, Dollfus H, Chirila C, Gnanasakthy A, Buckley BC, Mallya UG, Clément K, Haqq AM. Quality of life improvements following one year of setmelanotide in children and adult patients with Bardet-Biedl syndrome: phase 3 trial results. Orphanet J Rare Dis. 2023 Jan 16;18(1):12.
- ↑ Seo, S., Mullins, R. F., Dumitrescu, A. V., Bhattarai, S., Gratie, D., Wang, K., Stone, E. M., Sheffield, V., & Drack, A. V. (2013). Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Investigative Ophthalmology & Visual Science, 54(9), 6118–6132. https://doi.org/10.1167/iovs.13-11673
- ↑ Simons, D. L., Boye, S. L., Hauswirth, W. W., & Wu, S. M. (2011). Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proceedings of the National Academy of Sciences of the United States of America, 108(15), 6276–6281. https://doi.org/10.1073/pnas.1019222108
- ↑ Dhooge, P. P. A., Valkenburg, D., & Hoyng, C. B. (2020). Gene therapy for inherited retinal diseases. In Genetics and Genomics of Eye Disease (pp. 279–295). Elsevier. https://doi.org/10.1016/B978-0-12-816222-4.00017-4
- ↑ https://clinicaltrials.gov/ct2/results?cond=Bardet-Biedl+Syndrome&term=&cntry=&state=&city=&dist=

