Leber Congenital Amaurosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Leber congenital amaurosis (LCA) is a family of congenital retinal dystrophies that results in severe vision loss at an early age. Patients usually present at birth with nystagmus, sluggish or near-absent pupillary responses, severely decreased visual acuity, photophobia, and high hyperopia.[2] It is the most severe retinal dystrophy causing blindness by the age of 1 year in most cases and accounts for approximately 3% of children with severe visual impairment.[3]
History
The disease was first described by German ophthalmologist, Theodor Karl Gustav von Leber (February 29, 1840 - April 17, 1917), in 1869 in a blind child with wandering nystagmus, amaurotic pupils, and congenital retinitis pigmentosa.[4] It should not be confused with Leber Hereditary Optic Neuropathy, which tends to present at age 20 years, and was described by the same physician in 1871. Leber also described 'Leber miliary aneurysms' now thought to be a variant of Coats disease.[5]
In 1957, a non-recordable ERG (electroretinogram) was identified as a common feature essential to diagnosis of LCA. It was at this time that the disease was named. At the same time, a Swedish study identified the disease to be of autosomal recessive inheritance. This dystrophy has been described as a genetically heterogeneous recessive disease affecting 1 in 30000 to 1 in 81000 subjects.[6]
Primary Prevention
Genetic testing before pregnancy or prenatal testing can identify patients at risk of passing this condition on to offspring.
Etiology
Leber congenital amaurosis is a group of hereditary (usually autosomal recessive) retinal degenerative diseases. Various phenotypes (LCA1 to LCA19) with at least 29 genotypes have been identified that account for around 70-80% of cases, with thereby more genes yet to be identified. [7][8] These genes are known to be important in several retinal developmental and physiologic pathways including photoreceptor morphogenesis, phototransduction cilia, and the Visual Cycle.[3]
CEP290 (15%), GUCY2D (12%), and CRB1 (10%) and RPE65 (8%) are the most frequently mutated LCA genes.[7] Other genes with known mutations are SPATA7, AIPL1, LCA5, RPGRIP1, CRX, NMNAT1, IMPDH1, RD3, RDH12, LRAT, TULP1, KCNJ13, GDF6, CABP4, CNGA3, ALMS1, IQCB1, MYO7A. There are still unknown genes responsible for the other LCA subtypes.
Risk Factors
Risk factors are affected parent and/or parents who are carriers of a mutated gene responsible for one of the 17 LCA subtypes.
General Pathology
The histopathology showed the involvement of outer retina and photoreceptors and suggested that the LCA is a degenerative process rather than agenesis.[9]
Pathophysiology
The pathophysiology of LCA is related to the inability of the eye to undergo phototransduction due to a disruption of the Visual Cycle. The Visual Cycle is a series of enzymatic reactions between the retinal pigment epithelium (RPE) and the neurosensory retina to metabolize dietary vitamin A into 11-cis retinal to generate photopigment. Without 11-cis retinal, the phototransduction cascade cannot be initialized; thus, visual neuronal signals are not propagated to the visual cortex. A dysfunctional mutation of any of the genes encoding for proteins that catalyze any of the series of enzymatic reactions to generate 11-cis retinal can block the Visual Cycle and lead to symptoms of LCA.
The link between the hindrance of innate vitamin A metabolism within the eye and photoreceptor degeneration remains unclear and is currently an active area of biomedical research.
Epidemiology
The estimated birth prevalence of LCA is two to three per 100,000 births.[10][11] LCA represents almost 5% of all retinal dystrophies and 20% of children with visual impairment in special schools.[12]
Diagnosis
Clinical History
LCA is characterized by significant vision loss in infancy. It may be suspected in a young child with severe and early decreased visual response, sluggish or absent pupillary response, nystagmus, and an severely subnormal or extinguished ERG. Nystagmus is typically also present if not at birth, shortly after. Family history is typically consistent with autosomal recessive inheritance.
Diagnosis is clinical in this disorder, requiring thorough clinical evaluation and ophthalmic history. Diagnosis is supported by ERG and OCT (optical coherence tomography). Precise diagnosis requires molecular gene testing. Gene testing such as DNA microarray, next generation sequencing, linkage analysis and homozygosity/autozygosity mapping are used to identify specific gene mutations in LCA.
It should be noted that LCA exhibits wide variance in clinical phenotypes and can have genetic overlap with other inherited retinal diseases, complicating the diagnosis.[13]
No retinal lesion is diagnostic of LCA or specific for a certain subtype. Infants usually have normal fundus appearance and fundus abnormalities present later in life.
Signs[7]
- Abnormal or absent pupillary response
- Keratoconus
- Nystagmus noticed early in life, present from birth, can be pendular or roving and is present in all positions of gaze
- Photophobia
- Nyctalopia
- Vision loss (typically ranging from 20/200 to complete blindness)
- Hyperopia is commonly found but myopia has also been reported. High hyperopia (>5 diopters), which is thought to result from impaired emmetropization (the ability of the eye to accommodate to visual stimuli) is a consequence of early-onset visual impairment.
- Non-ophthalmologic features include mental retardation and olfactory dysfunction, in addition to stereotypical movements and behaviors
- Oculo-digital sign comprising eye poking, pressing, and rubbing likely producing mechanical retinal stimulation. The major sequel is enophthalmos, a physical defect in which the eye recedes into the orbit, presumably from atrophy of orbital fat. This behavior is performed to in an effort to stimulate vision by retinal stimulation.
- Intellectual Disability: Rarely, LCA is seen in association with neurodevelopmental delay, intellectual disability, and oculomotor apraxia-type behavior.
- Renal and olfactory dysfunction may be present [14]
Retinal exam
- The retina appears normal initially. Later, a variety of abnormalities may develop either in isolation or combination. They include:
- chorioretinal degeneration and atrophy centered around the fovea
- “bone-spicule" like pigmentation
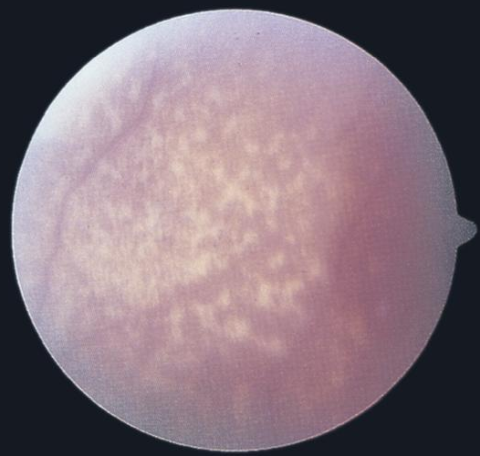
- subretinal flecks, "marbled" fundus
- pigmented nummular lesions at the level of the retinal pigment epithelium (RPE)
- optic disc abnormalities,
- “Coats like” reaction.
Symptoms
Visual impairment
Profound visual impairment is usually present from birth. One-third of individuals with LCA have no perception of light. Visual acuity is, with rare exceptions, 20/400 and below. The visual impairment is generally stable or very slowly progressive. Occasionally in the early stages, a mild degree of visual improvement is observed, followed by progressive degradation. Clinical diagnosis is based on clinical findings and ERG. LCA has retinal, ocular, and extraocular features and occasionally, systemic associations.[15] Recent evidence shows that not the genetics of LCA but the low vision can increase the risk for autism spectrum disorder. [16]
Diagnostic procedures
- ERG: Nonrecordable/Extinguished or severely reduced scotopic and photopic electroretinogram (ERG) is typical in LCA. Normal ERG responses rule out a diagnosis of LCA. Visual evoked responses are variable. ERG measures electrical response of retina which are decreased or absent in LCA.
- Fundus Autofluorescence (FAF): Autofluorescence measures lipofuscin accumulation in RPE which is related to shed photoreceptor disc elements. Amount of autofluorescence in LCA varies by subtype. For example, autofluorescence is normal in GUCY2D mutation but absent in RPE65 mutations. Abnormalities on FAF include a ring of macular hypo/hyper AF or peripheral hypo-AF. [17]
- OCT: In LCA, retinal atrophy is common with bowing of the macula and disruption of the retinal layer integrity.[18][19] In advanced LCA, retinal arterioles are attenuated reflecting overall disrupted metabolic status of the retina.[20]
Laboratory test
- Genetic testing is available for various genes.
Differential diagnosis
- Senior-Loken syndrome (Juvenile nephronophthisis with LCA):
- Juvenile nephronophthisis (medullary cystic renal disease)
- Early-onset retinal dystrophy
- Conorenal syndrome (Renal dysplasia, retinal pigmentary dystrophy, cerebellar ataxia, and skeletal dysplasia or Short-rib thoracic dysplasia 9 with or without polydactyly; SRTD9): Cone-shaped digital epiphyses
- Joubert syndrome:
- Nephronophthisis (a juvenile-onset cystic kidney disease)
- Hypoplasia of the cerebellar vermis
- Early-onset retinal dystrophy, and
- Either or both of the following: Episodic hyperpnea and/or apnea
- Peroxisome biogenesis disorders: A spectrum three phenotypes described before the biochemical and molecular basis of the disorders were known:
- Zellweger syndrome (ZS) - Features include retinal dystrophy, sensorineural hearing loss, developmental delay with hypotonia, and liver dysfunction. It is usually lethal during the first year of life.
- Neonatal adrenoleukodystrophy (NALD) - Retinal degeneration is associated with congenital liver and renal abnormalities.
- Infantile Refsum disease (IRD)
- Infantile neuronal ceroid-lipofuscinosis (CLN1, Santavuori-Haltia disease)
- Normal at birth
- Develop retinal vision impairment, loss of milestones, and progressive microcephaly by age six to 12 months
- Blindness by age two years, seizures and progressive mental deterioration
- Death generally occurs between ages three and 11 years
- Disorders of mitochondrial dysfunction
- Ptosis, external ophthalmoplegia, proximal myopathy and exercise intolerance, cardiomyopathy, sensorineural deafness, optic atrophy, pigmentary retinopathy
- Diabetes mellitus
- Early-onset retinitis pigmentosa (RP)
- Later age of onset than LCA
- Better preservation of central visual acuity than LCA
- No nystagmus.
- ERG: in the early stages of RP, the photopic component of the ERG typically shows some degree of sparing, while in LCA both the photopic and scotopic ERG are profoundly abnormal
- SECORD (severe early-childhood onset retinal dystrophy)[17]
- Achromatopsia
- Photophobia
- Specific ERG changes
- Congenital stationary night-blindness
- Myopia
- Specific ERG pattern
- Better visual acuity than LCA
- Abetalipoproteinemia
- Hyperthreoninemia
- Congenital Rubella Syndrome
- ERG is typically normal
Complications
Keratoconus is often associated with LCA and it has been postulated that the mechanism is possibly secondary to the oculodigital phenomenon. However, it is likely that development of keratoconus is due to a combination of genetic environmental and toxic (retinal death) factors.
Cataracts are also a known association of LCA. Etiology is similarly unclear but also likely due to a combination of genetic, environmental and toxic factors.
Management
To date no substantial treatment or cure for LCA exists for most forms (see medical therapy section below). Affected individuals benefit from correction of refractive error, use of low-vision aids when possible, and optimal access to educational and work-related opportunities.
There are several clinical trials, in different phases, involving specific mutation treatment [12] by gene replacement therapy or photo pigment supplementation.
Medical therapy
In 2017, the US Food and Drug Administration (FDA) approved voretigene neparvovec-rzyl (Luxturna Spark Therapeutics, Inc., Philadelphia, PA), for the treatment of biallelic RPE65 mutation-associated- LCA2. This was the first United States Food and Drug Administration (FDA)–approved gene therapy product for the eye. RPE65 mutation accounts for only a minority of patients with LCA. For the other mutations there is no effective therapy proven so far. However, reduction in light exposure is recommended to avoid photophobia.
Gene Therapy
The general theory behind gene therapy is that in a person with a known mutations of a coding region of a single gene, introduction of a normal allele can return cells to normal functioning. Gene therapy was first attempted in the Briard dog who was discovered to have similar clinical characteristics to humans of disease resulting from RPE65 gene mutation. In fact, there are a number of gene mutations in the RPE65 gene that are associated with inherited retinal dystrophies in both humans and dogs. The Briard dog was famously the first successful retinal gene therapy performed in animal models. Multiple studies have reported the restoration of vision in Briard dogs with RPE65 mutation using recombinant adeno-associated virus vectors as effective gene delivery vehicles for treatment of retinal diseases.[21] These breakthroughs served as the basis for clinical trials of gene therapy in humans.
The FDA approval of sequential and bilateral injection of voretigene neparvovec-rzyl to treat visually impaired patients who carry an RPE65 mutation was based on 1-year data from the only randomized controlled phase III clinical study to date, which demonstrated significant vision improvement as a result of the treatment. [22] Patients with LCA2, a mutation of RPE65 gene were treated with injection of adenovirus vector carrying a normal copy of the RPE65 gene. Multiyear follow-up evaluation of the patients from two other trials (ClinicalTrials.gov NCT00481546 and NCT00643747), however, revealed progressive decline of clinical benefits including retinal sensitivity, visual acuity, and functional gain following an initial peak seen at 6– 12 months after the treatment.[23]
Many additional gene therapy programs targeting both inherited retinal diseases and other ocular diseases are underway with animal models including treatment for GUCY2D, AIPL1 and CEP290 mutations found in other LCA subtypes. These studies are also using viral vectors to deliver normal genes and are showing promise in the rescue of rod-cone photoreceptors.
Medical follow up
Close follow-up of infants with LCA is recommended with diagnostic ERG. Fundus photos and detailed retinal examination may be helpful. Low vision referral may be warranted. Genetic counseling is recommended for families and patients. At conception, each sibling of an individual with recessively inherited LCA has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier. Carrier testing for at-risk family members and prenatal testing for pregnancies at increased risk may be possible if the disease-causing mutation in the family are known. Pre-implantation genetics may be considered in this patient population.
Surgery
Gene therapy is a technique where the genes are delivered through vector virus injected subretinally.
Prognosis
The disease has three subtypes: stable, progressive decline and appreciable improvement. Three separate studies with a total of 90 patients with LCA documented the prevalence of patients in each category. In summary, 15%, 75%, and 10% of cases have showed deterioration, stability and improvement, respectively. With the exception of AIPL1 (progressive decline) and RPGRIP1 (stable), the category to which patients belonged was independent on the subtype of LCA each patient had.[7][24][25][26]
References
- ↑ American Academy of Ophthalmology. Leber congenital amaurosis, marbleized fundus type. https://www.aao.org/image/leber-congenital-amaurosis-marbleized-fundus-type-2 Accessed July 31, 2019.
- ↑ Wagner, R. S., Caputo, A. R., Nelson, L. B., Zanoni, D. High hyperopia in Leber's congenital amaurosis. Arch. Ophthal. 103: 1507-1509, 1985.
- ↑ Jump up to: 3.0 3.1 Daich Varela M, Jeste M, de Guimaraes TAC, Mahroo OA, Arno G, Webster AR, Michaelides M. Clinical, Ophthalmic, and Genetic Characterization of RPGRIP1-Associated Leber Congenital Amaurosis/Early-Onset Severe Retinal Dystrophy. Am J Ophthalmol. 2024 Oct;266:255-263. doi: 10.1016/j.ajo.2024.05.007. Epub 2024 May 19. PMID: 38768745.
- ↑ Leber, T., Uber retinitis pigmentosa und angeborene amaurose. Graefes Arch Clin Exp Ophthalmol, 1869. 15: p. 1-25.
- ↑ Sen M, Honavar SG. Theodor Karl Gustav von Leber: The Sultan of Selten. Indian J Ophthalmol. 2022;70(7):2218-2220. doi:10.4103/ijo.IJO_1379_22
- ↑ Chung DC, Traboulsi EI. Leber congenital amaurosis: clinical correlations with genotypes, gene therapy trials update, and future directions. J AAPOS. 2009;13(6):587-592. doi:10.1016/j.jaapos.2009.10.004
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Kumaran, N., Moore, A. T., Weleber, R. G., & Michaelides, M. (2017). Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. British Journal of Ophthalmology, 101(9), 1147. doi:http://dx.doi.org.libproxy.uams.edu/10.1136/bjophthalmol-2016-309975
- ↑ Viswarubhiny S, Anjanamurthy R, Vanniarajan A, Bharanidharan D, Perumalsamy V, Sundaresan P. Clinical exome sequencing facilitates the understanding of genetic heterogeneity in Leber congenital amaurosis patients with variable phenotype in southern India. Eye Vis (Lond). 2021;8(1):20. Published 2021 May 6. doi:10.1186/s40662-021-00243-5
- ↑ Sullivan TJ, Heathcote JG, Brazel SM, Musarella MA. The ocular pathology in Leber's congenital amaurosis. Aust N Z J Ophthalmol. 1994;22(1):25-31. doi:10.1111/j.1442-9071.1994.tb01691.x
- ↑ Koenekoop RK. An overview of Leber congenital amaurosis: a model to understand human retinal development. Surv Ophthalmol. 2004;49(4):379-398. doi:10.1016/j.survophthal.2004.04.003
- ↑ Verma A, Perumalsamy V, Shetty S, Kulm M, Sundaresan P. Mutational screening of LCA genes emphasizing RPE65 in South Indian cohort of patients. PLoS One. 2013;8(9):e73172. Published 2013 Sep 16. doi:10.1371/journal.pone.0073172
- ↑ Jump up to: 12.0 12.1 Chacon-Camacho, O.F. and J.C. Zenteno, Review and update on the molecular basis of Leber congenital amaurosis. World J Clin Cases, 2015. 3(2): p. 112-24.
- ↑ Zobor D, Brühwiler B, Zrenner E, Weisschuh N, Kohl S. Genetic and Clinical Profile of Retinopathies Due to Disease-Causing Variants in Leber Congenital Amaurosis (LCA)-Associated Genes in a Large German Cohort. Int J Mol Sci. 2023 May 17;24(10):8915. doi: 10.3390/ijms24108915. PMID: 37240262; PMCID: PMC10219005.
- ↑ Huang CH, Yang CM, Yang CH, Hou YC, Chen TC. Leber's Congenital Amaurosis: Current Concepts of Genotype-Phenotype Correlations. Genes (Basel). 2021 Aug 19;12(8):1261. doi: 10.3390/genes12081261. PMID: 34440435; PMCID: PMC8392113.
- ↑ Daiger SP, S.L., Bowne SJ. Available from: http: // www.retnet.org.
- ↑ Sallum JMF, Pellissari MC, Carreiro LR, de Vasconcellos CFC. Screening for Autism Spectrum Disorder in Children and Adolescents With Leber's Congenital Amaurosis. Am J Ophthalmol. 2024 May 20;265:257-274. doi: 10.1016/j.ajo.2024.05.020. Epub ahead of print. PMID: 38777102.
- ↑ Jump up to: 17.0 17.1 Sather R 3rd, Ihinger J, Simmons M, Lobo GP, Montezuma SR. The Clinical Findings, Pathogenic Variants, and Gene Therapy Qualifications Found in a Leber Congenital Amaurosis Phenotypic Spectrum Patient Cohort. Int J Mol Sci. 2024 Jan 19;25(2):1253. doi: 10.3390/ijms25021253. PMID: 38279252; PMCID: PMC10816538.
- ↑ Lee YJ, Jeong HC, Kim JH, Jo DH. Clinical Characterization, Natural History, and Detailed Phenotyping of NMNAT1-Associated Leber Congenital Amaurosis. Am J Ophthalmol. 2024 Dec 20:S0002-9394(24)00582-8. doi: 10.1016/j.ajo.2024.12.016. Epub ahead of print. PMID: 39710161.
- ↑ Sahli E, Kiziltunc PB, Idil A. A Report on Children with CEP290 Mutation, Vision Loss, and Developmental Delay. Beyoglu Eye J. 2023 Sep 13;8(3):226-232. doi: 10.14744/bej.2023.37233. PMID: 37766766; PMCID: PMC10521126.
- ↑ Zhou Y, Huang L, Xie Y, Liu W, Zhang S, Liu L, Lin P, Li N. Clinical and genetic studies for a cohort of patients with Leber congenital amaurosis. Graefes Arch Clin Exp Ophthalmol. 2024 Sep;262(9):3029-3038. doi: 10.1007/s00417-024-06450-9. Epub 2024 Apr 25. PMID: 38662103; PMCID: PMC11377616.
- ↑ Le Meur G, Stieger K, Smith AJ, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14(4):292-303. doi:10.1038/sj.gt.3302861
- ↑ Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2- hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849–60
- ↑ Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015;372(20):1887–97. 13.
- ↑ Brecelj J, S.-K.B., ERG and VEP follow-up study in children with Leber’s congenital amaurosis. Eye (Lond), 1999. 13: p. 47-54.
- ↑ Heher KL, T.E., Maumenee IH, The natural history of Leber’s congenital amaurosis. Age-related findings in 35 patients. Ophthalmology, 1992. 99: p. 241-245.
- ↑ Fulton AB, H.R., Mayer DL, Vision in Leber congenital amaurosis. Arch Ophthalmol, 114(681-703).