Sickle Cell Retinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Sickle Cell Retinopathy is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
ICD-10-CM Diagnosis Code H36
Description Synonyms
- Proliferative retinopathy due to sickle cell disease
- Retinal dystrophy due to systemic disorder
- Sickle cell proliferative retinopathy
- Sickle cell retinopathy
Disease
Sickle cell retinopathy is an ocular manifestation of the spectrum of sickle cell disease, an inherited group of hemoglobinopathies with numerous systemic and ocular presentations. Hemoglobin is an iron containing protein in red blood cells that transports oxygen. It consists of two alpha polypeptide chains, each of which pairs with a beta, gamma or delta chain. The predominant hemoglobin molecule in mature circulation is Hemoglobin A (HbA), which consists of two alpha and two beta subunits. Hemoglobin F consists of two alpha and two gamma chains, and is the predominant hemoglobin until 6 weeks of age. Hemoglobin A2 consists of two alpha and two delta chains and is found at low levels in normal adult human blood. A single nucleotide mutation (GAG to GTG) at the 6th position of the beta chain causes the substitution of the amino acid valine for glutamic acid. Heterozygosity for this mutation results in sickle cell trait and homozygosity results in sickle cell disease (SCD). Other mutations in the beta subunit can result in hemoglobin SC disease (HbSC) and sickle thaslassemia (HbSThal).
History
Retinal hemorrhage associated with SCD was first published in a case report by Cook in 1930. In 1937, Harden demonstrated a consistent finding of dilated and tortuous retinal vessels in patients with SCD, and this finding was further substantiated by Klinefelter in 1942. However, it was not until 1942 that the underlying mechanism of sickle cell retinopathy was proposed. Ray and Cecil suggested that occlusion of small vessels by sickled erythrocytes caused the observed changes in retina. This work was further driven by Verhoeff, who, in 1947, provided pathophysiological basis of vitreous hemorrhage, neovascularization and infarction due to sickled cells[1].
Epidemiology
In African-Americans in North America, the incidence of sickle cell trait (AS) is about 8%, while that of SCD is 0.4%. The incidence of SC, AC and S-Thal genotypes among African-Americans in North America is 0.2%, 2% and 0.03%, respectively. Importantly, the incidence of proliferative retinopathy is highest in patients with SC or S-Thal (33% and 14% respectively), while patients with SS have a 3% incidence of proliferative retinopathy[2]. The Jamaican Cohort Study showed that the prevalence of sickle cell retinopathy was 43% in SC and 14% in SS by age 20.5 years with an annual incidence of 2.5% for SC and 0.5% for SS. [3] Progression was associated with age, extent of sickle cell retinopathy and presence of retinopathy in the contralateral eye. Fluorescein angiographic findings of a type IIa border has been associated with higher progression to proliferative sickle cell disease .[4]
Pathophysiology
Normal blood cells that are round and oval can easily pass through smaller blood vessels including capillaries. However, local hypoxic conditions alter the shape of the red cells in SCD patients, leading to rigid, sickle-shaped red blood cells (RBCs), due to the irreversible conversion of soluble hemoglobin into crystalline hemoglobin. Changes in the adhesive properties of capillary endothelial cells due to hypoxia lead to deceased vascular flow and occlusion of vessels.[5]This trapping of sickle-shaped red cells in the small blood vessels in various structures of the eye, both in the anterior and posterior segments, leads to characteristic damage. The clinical manifestations vary depending on the presence or absence of vaso-proliferative changes. Hence, this disease can be classified as non-proliferative sickle cell retinopathy (NPSR) and proliferative sickle cell retinopathy (PSR).[6]
Non-proliferative Sickle retinopathy (NPSR):
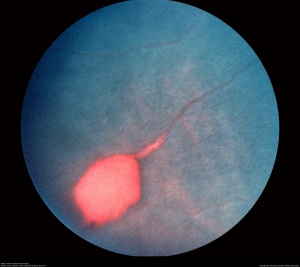
The retinal changes in NPSR occur secondary to vaso-occlusion and local ischemia. Occlusion of retinal vasculature first appears in the peripheral retina as “salmon patches”, which represent retinal hemorrhage from superficial blood vessels. These lesions are round or oval in shape and can be elevated or flattened (Figure 1).[7]Although they are initially red, they become salmon-colored due to the hemolysis of red blood cells. Over time, the hemorrhage is resorbed and the area appears normal with refractile deposits, which are iridescent spots of hemosiderin and macrophage deposition just beneath the internal limiting membrane. Migration and proliferation of retinal pigment epithelium (RPE) leads to the development of black sunburst spots which likely occur in response to hemorrhage.[8] Reduction in visual acuity is usually from the occlusion of perifoveal capillaries, causing a concave macular depression from RPE degeneration. Occlusions in the choroidal circulation with associated breaks in Bruch’s membrane are known to cause angioid streaks, a phenomenon seen in many retinal diseases including NPSR and PSR.
Proliferative Sickle Retinopathy (PSR):
As noted above, the ocular complications of PSR are more characteristically seen in HbSC and SThal disease, rather than in SS disease. As localized vascular occlusion leads to the changes described above in NPSR, chronic changes of local hypoxia and ischemia lead to the upregulation of vascular growth factors, which may result in retinal neovascularization, pre-retinal or vitreous hemorrhage and tractional retinal detachment.
Goldberg classified PSR into 5 different stages[9][10]
| Stage I | Peripheral arterial occlusion. |
|---|---|
| Stage II | Peripheral arteriovenous anastomoses, representing dilated pre-existing capillaries (hairpin loop) |
| Stage III | Neovascular and fibrous proliferation (sea fan) (Figure 2) occurring at the posterior border of nonperfusion. A subsequent white sea fan appearance is due to auto-infarction of the neovasculature (Figure 3). |
| Stage IV | Vitreous hemorrhage |
| Stage V | Tractional retinal detachment |
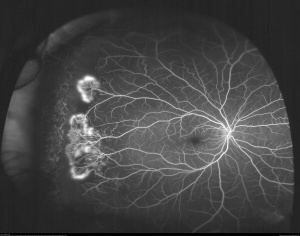
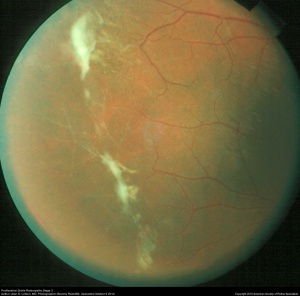
While extra-retinal fibrovascular proliferation is seen in other disease entities, most commonly diabetic retinopathy, neovascularization in PSR typically begins peripherally, rather than centrally as in diabetic retinopathy. Visual loss in PSR occurs most commonly due to vitreous hemorrhage and tractional retinal detachment.[11]
Clinical Signs/Symptoms
Vaso-occlusion of conjunctival vessels leads to the development of “comma” shaped vessels due to the accumulation of sickled RBCs at the distal end of the capillaries.[12]
Vascular changes in the optic disc are transient, but present as dark red lesions also visible as dilated dark capillary vessels on fluorescein angiography.[13]
Patients may see flashes, floaters or dark shadows, which may be indicative of vitreoretinal traction or detachment.
Presence of traumatic hyphema in African Americans warrants testing the patients for sickle cell hemoglobinopathies due to ocular complications that can result from sickle red blood cells. To control intraocular pressure, use of carbonic anhydrase inhibitors, especially acetazolamide, is contraindicated as it induces systemic acidosis and increases the local concentration of ascorbic acid in the aqueous humor; hence, the resulting low pH enhances sickling of RBCs. Instead of acetazolamide, the use of methazolamide is recommended.[14] [15]
Diagnosis
- History of sickle cell disease
- Presence of comma-shaped blood vessels in the bulbar conjunctiva (“comma sign”) is common. There are multiple anterior segment findings associated with sickle cell disease. [16] Posterior segment findings are divided into non-proliferative and proliferative. A small percentage of eyes may have angioid streaks on funduscopic examination.
- Fluorescein angiography to examine blood flow in retina and choroid
- Detailed structural examination with spectral-domain optical coherence tomography (SD-OCT)[17]
Differential Diagnosis
Sickle cell retinopathy should be distinguished from the conditions listed below. A prior history of sickle cell disease helps rule out many of these conditions, although it is possible for these conditions to occur simultaneously with NPSR/PSR.
- Diabetic retinopathy
- Retinal vascular occlusion
- Talc emboli
- Hypertensive retinopathy
- Sarcoidosis
- Eales disease
- Infectious diseases (e.g. dengue)
- Ocular ischemic syndrome
- Retinopathy of prematurity
- Familial exudative vitreoretinopathy
- Chronic myelogenous leukemia
- Hyperviscosity syndrome
Management
Screening
The goal of screening is to identify patients with retinopathy and to schedule follow-up or treatment as warranted by their level of disease. Recently, imaging techniques have led to identification of retinal thinning and ischemia in patients who do not have significant or even any apparent retinopathy.
Ocular Imaging with SDOCT has been shown to be helpful in identification of patients with retinal thinning related to sickle cell retinopathy. Retinal thinning has been associated with decreased retinal sensitivity as shown by microperimetry.[17] [18] SDOCT imaging has also identified differences in degrees of retinal thinning amongst hemoglobin subtypes. Age, stage of retinopathy, hemoglobin SS subtype and some systemic conditions are associated with more severe retinal thinning. Despite these retinal changes, good visual acuity may be maintained. Of course, when retinal arterial occlusions involving major retinal vessels occur, visual acuity is affected. OCT angiography (OCTA) has also shown significant changes even in children without structural damage. [19]
There is no therapy at present for non-proliferative sickle cell retinopathy.
Therapy for PSR stage 3 is to prevent bleeding and development of retinal detachment. Complete ophthalmic examination at least yearly is recommended for sickle cell patients (SC, SS, S-thal, etc). SDOCT and OCTA imaging add to the clinical staging of the disease and may be performed. Baseline fluorescein angiography may be performed to examine blood flow in the retina and choroid as needed.
Systemic therapy
Since humans have an inherent ability to produce fetal hemoglobin, emerging therapies that stimulate fetal hemoglobin have been shown to be beneficial. For example, hydroxycarbamide treatment has been demonstrated to have a beneficial effect in children in preventing sickle cell retinopathy.[20] Similarly, reducing HbSS red blood cells by exchange transfusion has also shown to be beneficial.[21][22]Hyperbaric oxygen therapy may reverse the pathology and improve visual acuity.[23] [24] Hydroxyurea is often used and is believed to increase the levels of HgbF. Gene editing with CRISPR-Cas9 is also being studied to increase fetal hemoglobin levels.[25]
Anti-VEGF
As complications of PSR arise, intravitreal administration of anti-vascular endothelial growth factor (anti-VEGF) therapy[26] and/or scatter laser photocoagulation[27] may lead to regression of neovascularization. The high incidence of neovascularization and spontaneous regression complicates treatment modalities.
Laser
Feeder vessel photocoagulation to close sea fan structures with argon laser is largely of historic significance.[28] Scatter laser for treatment of seafans has been shown to be effective and safer than feeder vessel techniques. [29] In the focal scatter laser study, eyes treated with focal scatter developed new sea fans in 34.4% versus 41.3% in control eyes. In addition, prolonged visual loss from vitreous hemorrhage occurred less often in treated than control eyes (1 % vs. 6.7 %) and incidence of vitreous hemorrhage was also lower (Chi squared 4.5; p ≤ 0.05).
Scatter laser to surround seafans is the current treatment for seafans. [30] [31] [32] In the event of vitreous hemorrhage, patients need to be examined at regular intervals to document clearance and improvement in visual acuity.
Surgical
Surgical indications include non-clearing vitreous hemorrhage, tractional retinal detachment of surgical severity or combined tractional and rhegmatogenous retinal detachment. Pre-operative systemic management should be optimized to reduce the risk of ocular and systemic comorbidity, and consultation with a hematologist or primary care provider should be considered. During surgery, oxygenation and hydration should be optimized to prevent this risk of a sickle crisis. To reduce the risk of anterior segment ischemia, a scleral buckle should be used with caution. Intraocular pressure should be monitored to reduce the risk of ischemia intraoperatively.
Complications and contraindications
Due to the risk of retinal detachment post-treatment, scatter photocoagulation is the preferred method of intervention to cause regression of sea fan structures if the sea fans do not auto-infarct. However, retinal tear and rhegmatogenous retinal detachments may occur after scatter photocoagulation. The rate of surgical complications in patients with PSR has been reported to be as high as 50% for traction/rhegmatogenous retinal detachment in a recent study.[33] Scleral buckling surgery also carries higher intra- and post-operative risk due to the induction of ocular ischemia and is generally not recommended. Pars plana vitrectomy has been reported with good outcomes. During vitrectomy, it is important to keep the intraocular pressure in the low normal range to avoid contributing to retinal infarction.
References
- ↑ Part II: Ocular lesions associated with sickle-cell disease. Acta Ophthalmologica. 27 MAY 2009 ed1959:18.
- ↑ Fekrat SG, Morton F . Sickle Retinopathy New York: Thieme Medical Publishers.; 1998.
- ↑ Downes SM, Hambleton IR, Chuang EL et al: Incidence and natural history of proliferative sickle cell retinopathy: Observations from a cohort study. Ophthalmology 112(11):1869-1875, 2005.
- ↑ Downes SM, Hambleton IR, Chuang EL et al: Incidence and natural history of proliferative sickle cell retinopathy: Observations from a cohort study. Ophthalmology 112(11):1869-1875, 2005.
- ↑ Michiels C, Arnould T, Remacle J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochimica et biophysica acta 2000;1497:1-10.
- ↑ Emerson GG, Lutty GA. Effects of sickle cell disease on the eye: clinical features and treatment. Hematology/oncology clinics of North America 2005;19:957-73, ix.
- ↑ Jampol LM, Condon P, Dizon-Moore R, Serjeant G, Schulman JA. Salmon-patch hemorrhages after central retinal artery occlusion in sickle cell disease. Archives of ophthalmology 1981;99:237-40.
- ↑ Friberg TR, Young CM, Milner PF. Incidence of ocular abnormalities in patients with sickle hemoglobinopathies. Annals of ophthalmology 1986;18:150-3.
- ↑ Goldberg MF. Natural history of untreated proliferative sickle retinopathy. Archives of ophthalmology 1971;85:428-37.
- ↑ Goldberg MF. Classification and pathogenesis of proliferative sickle retinopathy. Am J Ophthalmol (1971) vol. 71 (3) pp. 649-65.
- ↑ Penman AD, Serjeant GR. Recent advances in the treatment of proliferative sickle cell retinopathy. Current opinion in ophthalmology 1992;3:379-88.
- ↑ Roy MS, Rodgers GP, Podgor MJ, Noguchi CT, Nienhuis AW, Schechter AN. Conjunctival sign in sickle cell anaemia: an in-vivo correlate of the extent of red cell heterogeneity. The British journal of ophthalmology 1985;69:629-32.
- ↑ Nia J, Lam WC, Kleinman DM, Kirby M, Liu ES, Eng KT. Retinopathy in sickle cell trait: does it exist? Canadian journal of ophthalmology Journal canadien d'ophtalmologie 2003;38:46-51.
- ↑ Fritch CD. Traumatic hyphema. Annals of ophthalmology 1976;8:1223-5.
- ↑ Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Survey of ophthalmology 2002;47:297-334.
- ↑ Lim JI. Ophthalmic manifestations of sickle cell disease: update of the latest findings. Curr Opin Ophthalmol. 2012 Nov;23(6):533-6.
- ↑ Jump up to: 17.0 17.1 Chow CC, Genead MA, Anastasakis A, Chau FY, Fishman GA, Lim JI. Structural and functional correlation in sickle cell retinopathy using spectral-domain optical coherence tomography and scanning laser ophthalmoscope microperimetry. American journal of ophthalmology 2011;152:704-11.
- ↑ Lim JI, Cao D. Analysis of Retinal Thinning Using Spectral-domain Optical Coherence Tomography Imaging of Sickle Cell Retinopathy Eyes Compared to Age- and Race-Matched Control Eyes. Am J Ophthalmol. 2018;192:229-238.
- ↑ Ong SS, Linz MO, Li X et al. Retinal thickness and microvascular changes in children with sickle cell disease evaluated by OCT and OCTA. Am J Ophthalmol 2020; ;209:88-98.
- ↑ Estepp JH, Smeltzer MP, Wang WC, Hoehn ME, Hankins JS, Aygun B. Protection from sickle cell retinopathy is associated with elevated HbF levels and hydroxycarbamide use in children. British journal of haematology 2013;161:402-5.
- ↑ Gustave BW, Oliver SC, Mathias M, et al. Reversal of paracentral occlusive retinopathy in a case of sickle cell disease using exchange transfusion. Ophthalmic surgery, lasers & imaging retina 2013;44:505-7.
- ↑ McKinney CM, Siringo F, Olson JL, Capocelli KE, Ambruso DR, Nuss R. Red cell exchange transfusion halts progressive proliferative sickle cell retinopathy in a teenaged patient with hemoglobin SC disease. Pediatric blood & cancer 2015;62:721-3.
- ↑ Canan H, Ulas B, Altan-Yaycioglu R. Hyperbaric oxygen therapy in combination with systemic treatment of sickle cell disease presenting as central retinal artery occlusion: a case report. Journal of medical case reports 2014;8:370.
- ↑ Freilich DB, Seelenfreund MH. The use of hyperbaric oxygen in the treatment of retinal detachment in patients with sickle cell disease. Israel journal of medical sciences 1972;8:1458-61.
- ↑ Sharma A, Boelens JJ, Cancio M, Hankins JS, Bhad P, Azizy M, Lewandowski A, Zhao X, Chitnis S, Peddinti R, Zheng Y, Kapoor N, Ciceri F, Maclachlan T, Yang Y, Liu Y, Yuan J, Naumann U, Yu VWC, Stevenson SC, De Vita S, LaBelle JL. CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease. N Engl J Med. 2023 Aug 31;389(9):820-832. doi: 10.1056/NEJMoa2215643. PMID: 37646679.
- ↑ Mitropoulos PG, Chatziralli IP, Parikakis EA, Peponis VG, Amariotakis GA, Moschos MM. Intravitreal Ranibizumab for Stage IV Proliferative Sickle Cell Retinopathy: A First Case Report. Case reports in ophthalmological medicine 2014;2014:682583.
- ↑ Moshiri A, Ha NK, Ko FS, Scott AW. Bevacizumab presurgical treatment for proliferative sickle-cell retinopathy-related retinal detachment. Retinal cases & brief reports 2013;7:204-5.
- ↑ Jampol LM, Condon P, Farber M, Rabb M, Ford S, Serjeant G. A randomized clinical trial of feeder vessel photocoagulation of proliferative sickle cell retinopathy. I. Preliminary results. Ophthalmology 1983;90:540-5.
- ↑ Farber MD, Jampol LM, Fox P, et al. A randomized clinical trial of scatter photocoagulation of proliferative sickle cell retinopathy. Arch Ophthalmol 1991; 109: 363–367.
- ↑ Rednam KR, Jampol LM, Goldberg MF. Scatter retinal photocoagulation for proliferative sickle cell retinopathy. American journal of ophthalmology 1982;93:594-9.
- ↑ Jampol LM, Farber M, Rabb MF, Serjeant G. An update on techniques of photocoagulation treatment of proliferative sickle cell retinopathy. Eye 1991;5 ( Pt 2):260-3.
- ↑ Myint KT, Sahoo S, Thein AW, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. The Cochrane database of systematic reviews 2015;10:CD010790.
- ↑ Chen RW, Flynn HW, Jr., Lee WH, et al. Vitreoretinal management and surgical outcomes in proliferative sickle retinopathy: a case series. American journal of ophthalmology 2014;157:870-5 e1.