Rosai Dorfman Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Rosai-Dorfman Disease (“Sinus histiocytosis with massive lymphadenopathy” or SHML) is a rare benign tumor characterized by an abundance of histiocytes (tissue resident macrophages, dendritic cells) in the involved tissue. Typically manifests as painless lymphadenopathy, but extranodal orbital disease also occurs, and may even occur without lymph node involvement.
Disease Entity
Rosai-Dorfman Disease (RDD) Other names include Rosai-Dorfman-Destombes Disease as well as Sinus Histiocytosis with Massive Lymphadenopathy. [1]
Disease
Rare non-malignant histiocyte proliferation, typically presenting with painless bilateral cervical lymphadenopathy. [2] Commonly seen with extranodal involvement. Presently designated an R-group histiocytosis [3], previously histiocytosis X.
Etiology
There are 4 classes, based on predisposing factors. [4]
Heredity: A number of associated kinase mutations have been identified, including ARAF, MAP2K1, NRAS, KRAS. Germline mutations in SLC29A3 may cause familial RDD.
Neoplasia: Hodgkin or non-Hodgkin lymphoma, myelodysplastic syndrome, cutaneous clear cell sarcoma, histiocytosis.
Immune-Related: Systemic lupus erythematosus, rheumatoid arthritis, juvenile idiopathic arthritis, autoimmune hemolytic anemia.
IgG4-Related: Polyclonal hypergammaglobulinemia has been noted in up to 90% of cases. [3]
Incidence
1 per 200,000 in US. [4]
43% of cases have extranodal involvement. [3] 11% of cases involve the eye, orbit, or ocular adnexa. [5]
Risk Factors
Most common in children, young adults. Mean age of onset is 20. [5] More common in males, particularly those of African descent. Cutaneous form more common in women of Asian descent. [6]
Patients tend to have co-existing, possibly underlying, autoimmunity. Most commonly autoimmune hemolytic anemia, systemic lupus erythematosus, juvenile idiopathic arthritis, and IgG4 related disease. [5]
General Pathology
Non-specific inflammatory infiltration of large histiocytic cells with hypochromatic nuclei, abundant emperipolesis. S100 +, CD 68+, CD14+, CD1a -, CD207- [3][4] No specific cytogenetic findings. [5] In lymphatic tissue, histiocytes found in sinuses, plasma cells and B-cells seen in cortex. [3][4]
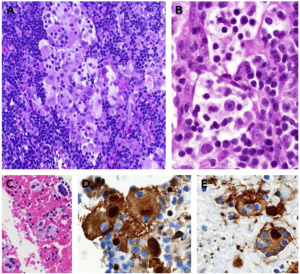
Pathophysiology
Unknown. Proposed mechanisms include chronic occult infection or exaggerated immune response to an undetermined trigger prompting histiocytosis. [4] [5]
Diagnosis
History
Painless or painful swelling of the eyelids, conjunctiva, lacrimal gland or orbital soft tissues. [6] Mass effect may cause proptosis or dystopia causing symptoms of exposure, diplopia, headaches, symptoms of optic nerve compression in severe cases. [8] May be bilateral or unilateral. Painless ipsilateral cervical lymphadenopathy likely present.
Occasionally there is a history of recent fever and upper respiratory infection. [5] Less commonly with constitutional symptoms including malaise, night sweats, weight loss. If multi-system, may have other organ systems involved.
Diagnosis is based on correlation of clinical, historical, radiologic, laboratory, and pathologic studies. Histologic examination is required.
Radiology
Imaging with CT, MRI, and ultrasonography may be helpful to characterize the lesion. [9] [10] Osteolytic bone lesions are present in less than 10% of cases. [5] Soft tissue lesions are enhancing on CT and MRI. PET imaging demonstrates a hypermetabolic state.
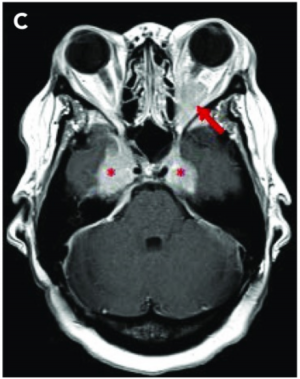
Laboratory
Laboratory findings are non-specific, include elevated ESR, leukocytosis, elevated ferritin. [3] Polyclonal hypergammaglobulinemia is present in 90% of cases.[5] Rheumatoid factor and ANA may be positive. Normocytic or microcytic anemia may be present if there is associated autoimmune hemolytic anemia.
Differential diagnosis
Given the vague presentation and lack of hard definite clinical characteristics, the differential is necessarily broad.
Consider neoplastic etiologies such as Langerhans cell sarcoma, Hodgkin lymphoma, metastatic carcinoma. [5]
Non-neoplastic considerations should include Langerhans cell histiocytosis, granulomatosis with polyangiitis, IgG4 related disease, juvenile xanthogranuloma, Erdheim-Chester disease, sarcoidosis, tuberculosis, histoplasmosis, HHV-6, EBV, HIV.
Management
Evidence to guide treatment is lacking and largely anecdotal, therefore we currently rely on expert recommendations.
Observation without treatment is advised when possible, as spontaneous resolution is common [11].
Medical Therapy
Patients are likely to be corticosteroid responsive. [7] Steroid sparing therapies should be pursued if long-term suppression is required, including certain chemotherapeutics and immunomodulatory drugs. Rituximab, imatinib, cyclophosphamide, methotrexate, cladribine, sirolimus, IFN-alpha, have reportedly been used with varying degrees of success. [12] [13] [14] [15] [16][17]
Surgical Treatment
Surgical excision is curative if disease is unifocal.[6] Debulking is effective [7] [18] [19], particularly if the tumor is disrupting vision or there is risk or evidence of optic nerve compression. Radiotherapy alone has not shown consistent results and is likely most useful for palliative treatment. [20]
Prognosis
Generally self-limiting. Most common outcome is spontaneous remission without recurrence. [3]
A subset experience recurrence or persistence.[21] Cosmetic appearance may be an issue. In orbital disease, the biggest concerns are mass effect causing diplopia, exposure, and/or optic nerve compression in rapidly progressive cases.
Rarely, the course may be aggressive and fatal. [5] Poor prognostic factors include autoimmune cytopenias as well as disseminated involvement including kidneys, lower respiratory tract, liver.
References
- ↑ Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87(1):63-70.
- ↑ Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Cai Y, Shi Z, Bai Y. Review of Rosai-Dorfman Disease: New Insights into the Pathogenesis of This Rare Disorder. Acta Haematol. 2017;138:14-23.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 5.9 Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1117-1121.
- ↑ Jump up to: 6.0 6.1 6.2 Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Forest F, N’guyen AT, Fesselet J, et al. Meningeal Rosai-Dorfman disease mimicking meningioma. Ann Hematol. 2014;93(6):937-940.
- ↑ Tran HM, Chinichian S, Storkersen K, Tokuhara K. An unusual case of extranodal Rosai-Dorfman disease manifesting as an epibulbar mass. Case Rep Ophthalmol. 2015;6(3):351-355.
- ↑ McAlister WH, Herman T, Dehner LP. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Pediatr Radiol. 1990;20(6):425-432.
- ↑ Bösmüller H, Nann D, Horger M, Fend F. Erdheim-Chester disease and Rosai-Dorfman disease: Pathological, radiological and clinical features of adult non-Langerhans cell histiocytosis. Pathologe. 2015;36(5):458-466.
- ↑ Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (rosai‐dorfman disease): Report of a case and literature review. Am J Hematol 2001;69(1):67-71.
- ↑ Galicier L, Boutboul D, Oksenhendler É, Fieschi C, Meignin V. Rosai-Dorfman disease: Sinusal histiocytosis with massive lymphadenopathy. Presse Med. 2017;46(1):107-116.
- ↑ Garces S, Medeiros LJ, Patel KP, et al. Mutually exclusive recurrent KRAS and MAP2K1 mutations in Rosai-Dorfman disease. Mod Pathol. 2017;30(10):1367-1377.
- ↑ Petschner F, Walker UA, Schmitt-Gräff A, Uhl M, Peter HH. "Catastrophic systemic lupus erythematosus" with Rosai-Dorfman sinus histiocytosis. Successful treatment with anti-CD20/rutuximab [in German]. Dtsch Med Wochenschr. 2001;126(37):998-1001.
- ↑ Utikal J, Ugurel S, Kurzen H, et al. Imatinib as a treatment option for systemic non-Langerhans cell histiocytoses. Arch Dermatol. 2007;143(6):736-740.
- ↑ Cooper SL, Arceci RJ, Gamper CJ, Teachey DT, Schafer ES. Successful treatment of recurrent autoimmune cytopenias in the context of sinus histiocytosis with massive lymphadenopathy using sirolimus. Pediatr Blood Cancer. 2016;63(2):358-360.
- ↑ Newman SB, Sweet DL, Vardiman JW. Sinus histiocytosis with massive lymphadenopathy: response to cyclophosphamide therapy. Cancer Treat Rep. 1984;68(6):901-902.
- ↑ Chen HH, Zhou SH, Wang SQ, Teng XD, Fan J. Factors associated with recurrence and therapeutic strategies for sinonasal Rosai-Dorfman disease. Head Neck. 2012;34(10):1504-1513
- ↑ Masoomian B, Lally SE, Shields JA, Shields CL. Ophthalmic Manifestations of Rosai-Dorfman Disease in Five Patients. J Curr Ophthalmol. 2020;32(3):238-243.
- ↑ Maklad AM, Bayoumi Y, Tunio M, Alshakweer W, Dahar MA, Akbar SA. Steroid-resistant extranodal rosai-dorfman disease of cheek mass and ptosis treated with radiation therapy. Case Rep Hematol. 2013;2013:428297.
- ↑ Agarwal A, Pathak S, Gujral S. Sinus histiocytosis with massive lymphadenopathy--a review of seven cases. Indian J Pathol Microbiol. 2006;49(4):509-515.

