Erdheim-Chester Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
2018 ICD-10 code: E88.89: Other specified metabolic disorders
Disease
Erdheim-Chester disease (ECD) is a rare systemic xanthogranulomatous, non-Langerhans histiocytic disease that most commonly affects the long bones and visceral organs but often has signs of ocular involvement. The disease was first described by Erdheim and Chester in 1930, and since this time only several hundred cases have been reported in the medical literature. Most patients with ECD will have osseous involvement (95%) at the time of diagnosis and the vast majority will also have at least one non-osseous site of involvement with 22% of cases demonstrating ocular involvement. The most common symptoms of the disease are bone pain (26%), neurological features including visual disturbances (23%), and diabetes insipidus from pituitary involvement (22%). The mean age of diagnosis of ECD is 53 years old with a male predominance.
Pathophysiology
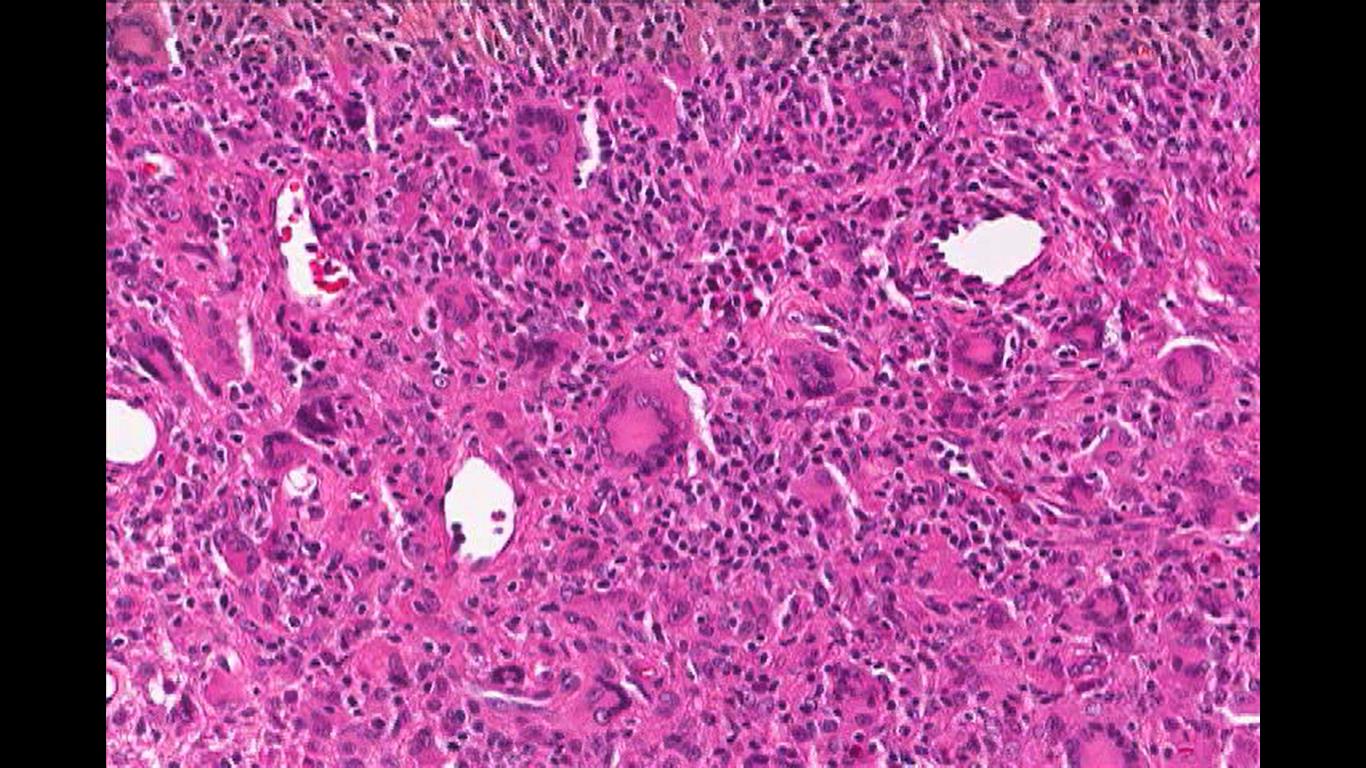
Erdheim-Chester disease is characterized by diffuse infiltration of the long bones, heart, lungs, kidneys, and retroperitoneum by histiocytes and lipid-laden macrophages. Histopathology classically demonstrates fibrosing xanthogranulomas with foamy histiocytes and Touton giant cells (image 1). Immunohistochemistry reveals that these cells express the histiocyte marker CD68, CD163 and Factor XIIIa, but unlike Langerhans cell histiocytosis (LCH), they do not express CD1a or S100, which is a key differentiating factor. Additionally, ECD cells lack of Birbeck granules.
Somatic mutations in components of the MAPK signaling pathway are present in most patients with ECD. BRAF V600E is found in approximately half of ECD cases, and mutation of this serine-threonine kinase enhances cell proliferation and survival by activating the RAS/RAF/MEK/MAPK signaling pathway. Understanding the aberrant cell signaling pathway involved in this disease is key, as it allows for a more targeted therapeutic approach and the potential development of novel treatments in the future.
Epidemiology
ECD typically manifests in the 5th to 7th decades of life, with a mean age of diagnosis of 53 with a male predominance. Only a very small number of juvenile cases have been reported.
Diagnosis
Along with a thorough history of present illness and physical exam, orbital imaging is useful in the diagnosis of ECD. Both computed tomography (CT) and magnetic resonance imaging (MRI) of the orbits with contrast will reveal enhancing soft tissue-density masses in the intraconal retrobulbar space surrounding the optic nerve with obliteration of normal orbital fat. Less commonly there may be extraconal lesions present.
Systemic imaging can reveal bone findings on X-ray, such as symmetric diaphysial and metaphysial sclerosis. Pulmonary findings including, septal thickening of the lung, centrilobular nodular opacities, and interstitial opacities. Additionally, a variety of nonspecific retroperitoneal findings are almost always present, however the inferior vena cava and pelvic aspect of the ureters are typically spared. …. (summarize systemic imaging findings here)
Histopathological diagnosis is confirmed by biopsy of the firm, yellow intraconal masses that reveals foamy histiocytes with Touton giant cells, fibrotic and lymphocytic infiltrate, and positive immunostaining for CD68 and negative for CD1A.
History and Physical Examination
Systemically, ECD most commonly presents as mild juxta-articular pain in the lower extremities, however, the disease also affects the heart, lungs, and kidneys. Cardiac complications of the disease encompasses a wide range of pathologies, including valvular abnormalities, conduction defects, and fibrosis of the periaortic tissue. Pulmonary manifestations include dyspnea and/or cough from pleural and lung parenchymal tissue involvement. Kidney infiltration leads to renal insufficiency. Central nervous system involvement includes the pituitary and hypothalamus. The most common symptom of ECD is bone pain (26%). Also seen are neurological symptoms including visual disturbances (23%), and diabetes insipidus from pituitary involvement (22%).
The classic presentation of ocular involvement of ECD is of bilateral proptosis caused by infiltrating orbital masses, especially in the setting of associated periorbital xanthelasma. Proptosis can be severe and result in painless ophthalmoplegia and decreased visual acuity from diffuse intraconal orbital infiltration causing compressive optic neuropathy. In addition to proptosis, other ophthalmic manifestation of ECD include, xanthelasma of the eyelids (image 2), eyelid thinning, bullous chemosis, decreased visual acuity from exposure keratopathy and periorbital edema. Ophthalmic examination can reveal optic disc edema or atrophy and retinal striae from compression, bilateral lacrimal gland enlargement, and extension of mass lesions beyond the orbital apex and skull base into the intracranial space invading the cavernous sinus with multiple cranial nerve deficits and rarely extending further to the pituitary gland and optic chiasm. While vision loss in cases of orbital ECD occurs most commonly secondary to orbital or anterior visual pathway compression of the optic nerves, involvement of the central nervous system by masses affecting the posterior visual pathways have resulted in homonymous hemianopia.
Differential Diagnosis
The differential diagnosis of ECD includes other causes of proptosis and infiltrative mass lesions of the orbit. Because many patients present with bilateral proptosis as well as systemic symptoms suggestive of endocrinopathies, thyroid eye disease is often initially suspected as a cause of these patients symptoms. Thyroid eye disease, however, presents with inflammation and enlargement of extraocular muscles and orbital fat, and does not present with xanthogranulomatous orbital infiltration. Thyroid function studies are usually normal in patients with ECD.
Sarcoidosis is another systemic disease with ocular manifestations including orbital infiltration as discrete mass lesions that can cause proptosis. Sarcoidosis is well known as one of the masquerade syndromes bc it has many presentations. This common autoimmune illness can present most commonly with lacrimal gland enlargement but can also present with eyelid granulomas, optic nerve infiltration, and has been documented to rarely involve all other orbital structures.
Nonspecific orbital inflammation (NSOI), also known as orbital inflammatory pseudotumor, is the most common cause of painful orbital mass in adults. It is a benign inflammatory process that is characterized by a polymorphous lymphoid infiltrate with unknown etiology, however, there is a strong association with rheumatologic diseases. This disease classically presents with the abrupt onset of pain, proptosis, erythema, and swelling. CT and MRI studies can show various patterns of involvement of the orbit including enhancement of the lacrimal glands, enlargement and enhancement of the extraocular muscles without sparing the tendon, diffuse orbital infiltration, discrete orbital masses or optic perineuritis. Due to the inflammatory nature of the disease, erythrocyte sedimentation rate and C reactive protein are commonly elevated.
IgG4 related disease (IgG4-RD) is a systemic disease characterized by tumor like swelling of involved organs due to a lymphocytic infiltration of IgG4 positive plasma cells resulting in a variable degree of fibrosis. Histopathologically, this pattern of fibrosis is described as “storiform” which helps to differentiate it from ECD. Orbital involvement of IgG4-RD most commonly involves the lacrimal gland, soft tissue surrounding the orbit, and extraocular muscles leading to a presentation of proptosis, but does not generally infiltrate the orbit with mass lesions like ECD. Similar to ECD, this disease may involve the retroperitoneal organs, but the most frequent non-ocular systemic manifestations are type I autoimmune pancreatitis, sclerosing cholangitis, and retroperitoneal fibrosis.
Metastatic tumors to the orbit may present similarly to ECD, however, these lesions are typically unilateral. The onset of symptoms is insidious, with proptosis, and sometimes the presence of subcutaneous nodules. The most common primary tumors that metastasize to the orbit are breast, lung, and prostate cancers. Characteristic imaging findings are of enhancing, infiltrative mass lesions with bony involvement and destruction of normal anatomical landmarks.
Other Orbital Tumors
Primary orbital tumors such as hemangiomas, venolymphatic lesions, and rhabdomyosarcoma, can usually be differentiated from ECD by their primary orbital involvement, near exclusive unilaterality, younger patient demographics, and imaging characteristics. Lacrimal gland neoplasms can also be differentiated from ECD by their unilaterality, location and imaging characteristics with bony destruction pain being common in malignant forms. Paranasal sinus tumors involving the orbit arise in the maxillary, ethmoid, sphenoid, or frontal sinuses with the vast majority of tumors arising from the maxillary sinus. They secondarily invade the orbit via direct extension through the bony walls of the orbit or hematogenously via emissary veins.
Langerhans Cell Histiocytosis
Langerhans Cell Histiocytosis (LCH) and ECD are both histiocytic diseases that can involve multiple sites, most commonly bones. Langerhans cell histiocytosis (LCH) is a neoplastic proliferation of the specialized dendritic cells that present antigen to naïve T cells. These cells are predominantly found in the skin and derived from bone marrow monocytes. LCH is characterized by the unique histopatholocial findings of Birbeck granules on electron microscopy and central nuclear grooves that are not seen in ECHD. LCH shows CD1a and S100 positive cells by immunohistochemistry. LCH is a family of diseases that includes eosinophilic granuloma, Hans-Schuller-Christian disease and Letterer-Siwe and are more common in children than ECD.
Eosinophilic granuloma is a benign proliferation of Langerhans cells in the bone. It classically presents with a pathologic fractures in adolescents without skin involvement. Biopsy shows Langerhans cells with mixed inflammatory cells, specifically numerous eosinophils. Hand-Schuller-Christian disease is a malignant proliferation of Langerhans cells. This disease classically presents with exophthalmos, a scalp rash, lytic skull defects, and diabetes insipidous in children. Letterer-Siwe disease a malignant proliferation of Langerhans cells that classically presents with a skin rash and cystic skeletal defects in infants under the age of 2. While multiple organs are involved, ocular manifestation is not common.
Histopathology
Image 1: Touton Giant Cell, a foamy lipid laden macrophage. Image courtesy of Dr. William Morris, M.D.
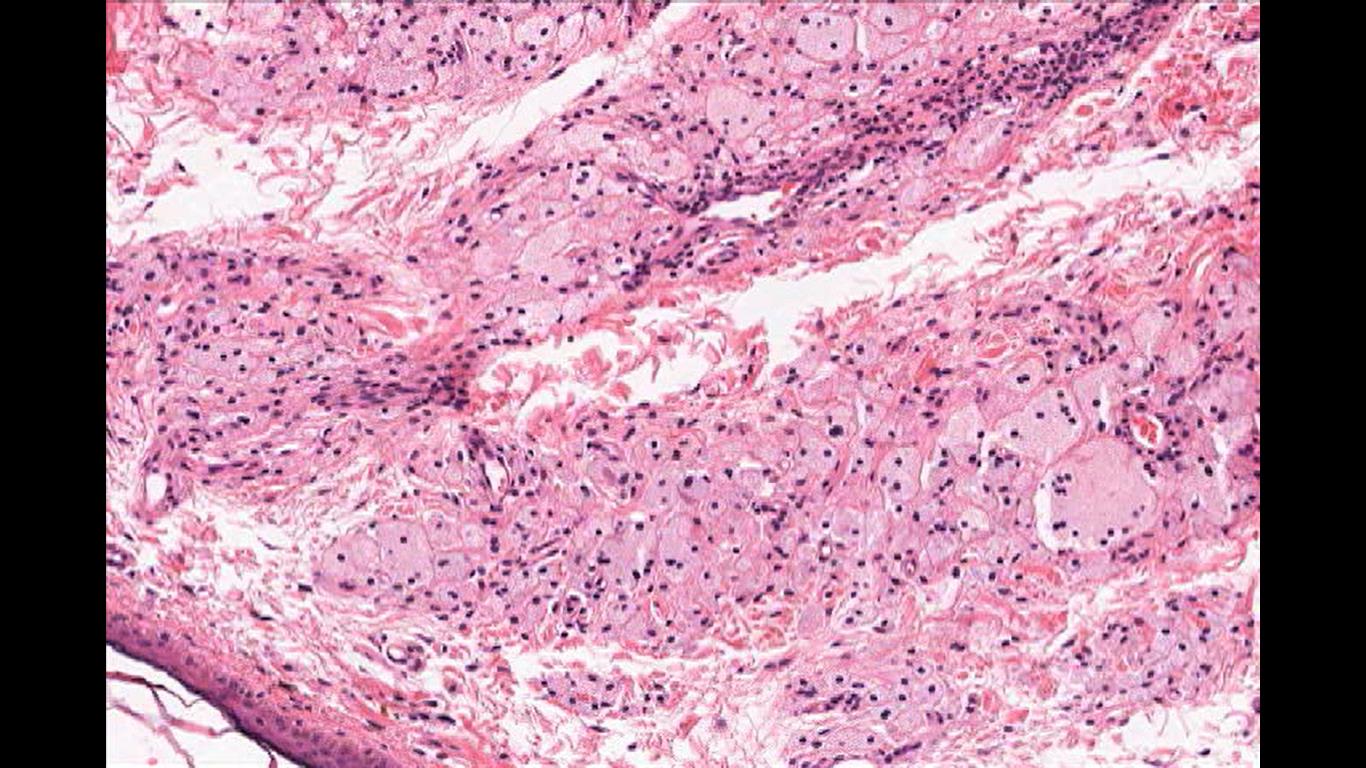
Image 2: Xanthelasma Cells, cutaneous manifestation of lipid laden foamy cells in the epidermis. They manifest as yellowish white plaque like lesions in the eyelid skin, commonly in the medial canthus. Image courtesy of Dr. William Morris, M.D.
Management
As discussed earlier, there is no cure for ECD, nor is there a consensus on optimal management of ECD with orbital involvement. However, most studies regarding treatment involve a multidisciplinary approach including medical management, surgical therapy, and radiation therapy. Each is discussed below.
Medical management
There are three main classes of medication aimed at treating ECD: corticosteroids, chemotherapeutic agents, and immunomodulators.
The role of corticosteroids in ECD is for the acute reduction of orbital edema and exophthalmos causing compressive optic neuropathy. They are not considered to be adequate monotherapy for ECD , as they do not inhibit lesion progression and have not been shown to result in increased visual acuity.
Chemotherapeutic agents are used to treat ECD with the goal of reducing cell division in order to slow disease progression. This class includes used to treat ECD include cladribine and vemurafenib. Cladribine is a purine analogue that targets monocytes, and in one case study resulted in significant resolution of both proptosis and improvement in visual acuity. However, this result was not repeatable among other patients and was thought to be the result of a specific mutation with subsequent monocyte activation in this particular patient, highlighting the fact that immunohistochemistry and genetic testing may be important in tailoring treatment. Vemurafenib is a serine/threonine kinase inhibitor that has demonstrated clinical utility in V600E BRAF mutant cells. One study describes dramatic clinical and radiographic improvement in patients treated with vemurafenib and clinical trials are currently underway.
Immunomodulators, specifically interferon alpha (IFN-α) have been used to treat ECD. IFN-α is normally present during cell line differentiation and it functions to promote the terminal differentiation among dendritic cells and histiocytes. Early results with this particular therapy are promising, resulting in retro-orbital disease regression in one month. An additional immunomodulatory drug, canakinumab, is a recombinant form of the IL-1 receptor agonist (IL-1RA). In normal physiology, IL-1RA production is stimulated by IFN-α. Preliminary research shows that canakinumab may be most useful in patients with stable, non-life threatening forms of ECD that are not aggressive enough to warrant systemic chemotherapy such as cladribine.
While several options exist for medical management of the disease, the current drugs are limited and more often than not, surgical therapy is necessary.
Surgical management
Surgical treatment of orbital involvement of ECD generally includes mechanical debulking of intraorbital masses and orbital decompression to relieve pressure in the orbit and around the optic nerves. While this procedure may improve pain, and prevent progressive vision loss from compressive optic neuropathy, it is not curative as complete surgical excision is not possible due to the infiltrative nature of the disease.
Additional treatment
Use of external beam radiation therapy (EBRT) at a range of doses has been used as a symptomatic measure in an effort to treat pain associated with orbital involvement. Unfortunately, it appears that the pain relief is short lived, and there is no indication that radiation improves ocular symptoms nor prevents progression of the disease. Additionally, a small number of patients have developed bilateral radiation retinopathy resulting in permanent secondary loss of vision.
Prognosis
There is no known cure for this disease, nor its ocular manifestations with a 5-year survival rate of 70% . Overall survival appears to be correlated with extent of organ involvement and mortality is more common with significant systemic manifestations of disease. As opposed to other xanthogranulomatous diseases that affect the anterior orbit, ECD lesions are generally more diffuse and more likely to result in loss of vision.
Conclusion
Erdheim-Chester Disease is a rare, systemic xanthogranulomatous disease that is thought to arise due to somatic mutations in signaling pathways. This is a systemic disease that commonly presents with bone pain, symptoms of visceral organ dysfunction, and ocular involvement. Ocular involvement can manifest as orbital lesions, eyelid lesions, proptosis, pain, and decreased visual acuity. Consequently, ophthalmologists can play a critical role in the diagnosis and treatment of this disease. There is no known cure for ECD, nor a consensus on symptom management, however there is ongoing research aimed at new drug development and gaining a further understanding of the disease.
References
1. Achar A, Naskar B, Mondal PC, Pal M. MULTIPLE GENERALIZED XANTHOGRANULOMA IN ADULT: CASE REPORT AND TREATMENT. Indian Journal of Dermatology. 2011;56(2):197-199. doi:10.4103/0019-5154.80416.
2. Arnaud L, Gorochov G, Charlotte F, et al. Systemic perturbation of cytokine and chemokine networks in Erdheim-Chester disease: a single-center series of 37 patients. Blood 2011; 117:2783.
3. Braiteh, F., Boxrud, C., Esmaeli, B., & Kurzrock, R. (2005). Successful treatment of Erdheim-Chester disease, a non–Langerhans-cell histiocytosis, with interferon-α. Blood, 106(9), 2992-2994. Accessed March 15, 2018.https://doi.org/10.1182/blood-2005-06-2238.
4. Cavalli G, Guglielmi B, Berti A, et al. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis 2013; 72:1691.
5. Chester W. Uber lipoidgranulomatose. Virchows Arch A Pathol Anat Histol 1930; 279:561.
6. Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44.
7. Diamond EL, Dagna L, Hyman DM, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124(4):483-492. doi:10.1182/blood-2014-03-561381.
8. Dutton JJ. Orbit and lacrimal gland. In: Yanoff M, Duker JS, editors. Ophthalmology. Mosby, Inc., St. Louis, 2004, p.729-743
9. Ebbo M, Patient M, Grados A, et al. Ophthalmic manifestations in IgG4-related disease: Clinical presentation and response to treatment in a French case-series. Kers. J, ed. Medicine. 2017;96(10):e6205. doi:10.1097/MD.0000000000006205.
10. Font RL, Smith SL, Bryan RG. Malignant epithelial tumors of the lacrimal gland: a clinic-pathologic study of 21 cases. Arch Ophthalmol. 1998 May; 116(5): 613-16.
11. Jaffe R. The diagnostic histopathology of Langerhans cell histiocytosis. In: Histiocytic Disorders of Children and Adults. Basic Science, Clinical Features, and Therapy, Weitzman S, Egeler RM (Eds), Cambridge University Press, Cambridge 2005. p.14.
12. Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 2014; 11:460.
13. Merritt, H., Pfeiffer, M. L., Richani, K., & Phillips, M. E. (2016). Erdheim-Chester disease with orbital involvement: Case report and ophthalmic literature review. Orbit, 35(4), 221-226. doi:10.1080/01676830.2016.1176211
14. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R: Inflammatory Pseudotumor. Radiographics, online publication 2003;23:719-720
15. Pasadhika S, Rosenbaum JT. Ocular Sarcoidosis. Clinics in chest medicine. 2015;36(4):669-683. doi:10.1016/j.ccm.2015.08.009.
16. Perry CB, Lenci L, Shriver EM. Orbital Lymphatic Malformation (Lymphangioma). Jan 21, 2015; Available from: http://EyeRounds.org/cases/201-Lymphatic-Malformations.htm
17. Rootman, Jack. Diseases of the orbit: a multidisciplinary approach. Lippincott Williams & Wilkins, Philadelphia, 2nd ed, 2003.
18. Shields JA, Shields CL, Epstein J, et al. Primary epithelial malignancies of the lacrimal gland. The 2003 Ramon L. Font Lecture. Ophthalmic Plast Reconstr Surg 2004;20:10–21
19. Weber AL, Romo LV, Sabates NR. Pseudotumor of the orbit: clinical, pathologic, and radiologic evaluation. Radiol Clin North Am 1999;37:151-168