Pituitary Adenoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Pituitary adenomas are a collection of tumors that arise from the pituitary gland. These tumors are the most common cause of optic chiasm compression in adults.[1][2] Ophthalmologic findings typically involve visual field defects (e.g., optic neuropathy, junctional visual loss, bitemporal hemianopsia), although less commonly patients may also have efferent complaints (e.g., ocular motility deficits from cavernous sinus involvement, Nystagmus). Rarely compression of the third ventricle can produce Increased intracranial pressure. Pituitary adenoma typically presents with gradual and progressive visual or endocrinological symptoms/signs but acute presentations may occur from hemorrhage or necrosis within a pre-existing tumor (i.e., pituitary apoplexy).[3][4][5]
Disease Entity
General
Epidemiology
Pituitary adenomas are reported to account for 12-15% of symptomatic intracranial neoplasms and are the most common tumor of the sella turcica region.[2][6] Overall, it is estimated that in upwards of 25% of the population have pituitary adenomas although most of these are incidentally found.[3] One review found an average incidence of 16.7% (14.4% in autopsy studies and 22.5% in radiologic studies).[7]
Pituitary adenomas usually occur in adults; they rarely occur in childhood.[1][3] Various articles have reported both male and female predilections.[7][8] While most pituitary adenomas occur in isolation, 3% of cases are associated with multiple endocrine neoplasia (MEN) type 1.[3][9]
Classification
In 2017, the World Health Organization (WHO) updated their classification of pituitary tumors. Previously, pituitary tumors were classified by their hormone content and structural features. Currently, rather than classifying a tumor as “hormone-producing” or “functional” adenoma, pituitary tumors are defined by their cell lineage and are further divided based on immunohistological stains and tumor markers.
The WHO currently recommends against using the term “atypical adenoma” as there is no accurate prognosis with this distinction but evaluating the potential for aggressiveness of the tumor (e.g., tumor invasion, mitotic count and Ki-67 labeling index) may still be useful.[6] The term, “pituitary carcinoma” is reserved for cases with metastatic spread, either local or systemic invasion, and comprise of <1% of tumors. Distinction of densely vs sparsely granulated adenomas is mainly through low molecular weight cytokeratin (LMWK) immunohistochemistry.[6]
Size
Historically, pituitary adenomas have been described by their size. This is performed radiographically with CT or MRI.[3][7]
- Macroadenomas: ≥10 mm
- Microadenomas: <10 mm
Hormonal Activity
- Functional (secreting) tumors are associated with hormonal secretion. Because of the clinical effects of hormonal disturbances, these tumors are more likely to be detected while they are still small. They are divided according to the hormone(s) produced. Prolactinomas are the most common type of functional pituitary adenoma.
- Nonfunctional (nonsecreting) tumors do not produce excess hormones. They may cause hypopituitarism due to mass effect. Gonadotrophic adenomas are the most common type of nonfunctional pituitary adenoma.[10]
Staining Pattern
Previously, pituitary adenomas were also divided by their staining pattern on histology[3] but this classification is no longer clinically significant due to the more specific immunohistochemistry stains that can differentiate the hormones being secreted.[5] These stains do not correlate well with other features of pituitary tumors, such as the type of secreted hormone and cell type.[2]
- Acidophils contain eosinophilic granules in their cytoplasm that stain brightly with acid fuschin.
- Basophils contain basophilic granules in their cytoplasm that stain brightly with aniline blue.
- Chromophobes contain agranular cytoplasm. This term is often associated with nonfunctioning adenomas, although some hormone-secreting adenomas may also have chromophobic features.
Pathology
Pathogenesis
Many animal studies have suggested one or more genetic factors that may contribute to the formation of pituitary adenomas. For example, the pituitary tumor-transforming gene (PTTG) has been shown to be overexpressed in hormone-secreting adenomas.[11] Mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene are associated with an increased risk of aggressively invasive growth hormone-secreting pituitary adenomas.[12] There is evidence as well that adult pituitary stem cells may contribute to the development of pituitary adenomas.[13] Various cell markers have also been implicated in the behaviors of various pituitary tumors, including type of hormone produced, recurrence, regression, and response to treatment.[10][14][15]
Thyroid transcription factor (TTF-1) is identified by the WHO 2017 classification that some posterior lobe adenomas have been shown to express.[6]
Morphology
Most pituitary adenomas are soft, well-circumscribed lesions that are confined to the sella turcica. As these adenomas grow, they tend to extend superiorly and may eventually compress the optic chiasm and various cranial nerves. Expansion may lead to bony erosion of the anterior clinoid processes and sella turcica, as well as extending into the cavernous and sphenoid sinuses.[3]
Histopathology
Pituitary adenomas have a monomorphic appearance and are arranged in sheets or cords. Adenoma cells stain on immunohistochemistry based on the type of hormone they secrete. The cytoplasm of these cells may be acidophilic, basophilic, or chromophobic, and is generally uniform in its appearance. These tumors also have a poor reticulin network, which distinguishes the tumor from non-neoplastic tissue, which tends to have a heterogenous appearance with an extensive reticulin network.[3]
| Type of adenoma | Secreting Hormone | Staining | Reported Incidence[17] |
|---|---|---|---|
| Lactotrophic (Prolactinoma) | Prolactin | Acidophilic or chromophobic | 29% |
| Somatotrophic | Growth hormone (GH) | Acidophilic | 14% |
| Corticotrophic | Adrenocorticotropic hormone (ACTH) | Basophilic | 13% |
| Gonadotrophic | Luteinizing hormone (LH), Follicle-stimulating hormone (FSH) and their subunits | Basophilic | 13% |
| Thyrotrophic | Thyroid-stimulating hormone (TSH) | Basophilic to chromophobic | Less than 1% |
| Other pituitary adenomas | Some multiple hormones, others are nonsecreting | 30% |
Functional Tumors
Lactotroph Adenomas (Prolactinomas)
See also Prolactinoma
| Condition | Prolactin Level (ng/mL) |
|---|---|
| Normal Male | < 20 |
| Normal Female | < 25 |
| Pregnancy | ~200 |
| Prolactinomas | > 200 (can exceed 10,000) |
Lactotroph adenomas arise from Pit-1 lineage and mainly express prolactin (PRL) and estrogen receptor α (ERα). They make up 30-50% of pituitary adenomas and are the most common clinically functional adenomas. In men, the size of the adenoma is more likely to cause compressive symptoms while in women, there are more hormone imbalances.[6] High prolactin levels cause amenorrhea, galactorrhea, and infertility in women, and hypogonadism and impotence in men. In the setting of a prolactin secreting tumor, galactorrhea-amenorrhea is termed Forbes-Albright syndrome. Modest elevations in prolactin however can occur without prolactin secreting tumor because of the effect of compression of the stalk (removing the inhibition from dopamine) and is called the stalk effect.
Gynecomastia can be seen in both men and women. In adolescents, elevated prolactin levels can delay puberty. Because the symptoms in males are more subtle, pituitary adenomas tend to be detected later in males than in females.[18] Acidophil stem cell adenomas (ASCA) additionally have elevated GH and IGF-1.[6]
Prolactinomas tend to undergo dystrophic calcification, which can appear microscopically as psammoma bodies or encompass the entire mass ("pituitary stone").[3] Most prolactinomas are sparsely granulated; on histology these tumor cells are weakly acidophilic or chromophobic. Rarely these cells will be strongly acidophilic and are classified as densely granulated prolactinomas.[3]
Other conditions can cause elevated prolactin levels, including pregnancy (see Chiari-Frommel Syndrome section), medications that affect dopamine metabolism (e.g., phenothiazines, metochlorpramide, risperidone, verapamil), renal failure, and primary hypothyroidism. Other pituitary tumors can also cause elevated prolactin levels by the decrease in dopaminergic inhibition as these tumors enlarge and compress the pituitary stalk. These other conditions typically do not cause serum prolactin levels as high as those seen in prolactinomas.[5]
Chiari-Frommel Syndrome
Chiari-Frommel Syndrome is a rare disorder characterized by abnormal production of breast milk (persistent lactation), absence of regular menstrual periods (amenorrhea), and uterine-ovarian atrophy lasting for more than 6 months post-partum in otherwise normal women. This condition was first described in 1855 by Chiari, who initially attributed it to poor nutrition and general health. Later in 1882, Frommel reported a similar case, with Schiller eventfully naming the condition “Chiari-Frommel Syndrome” in 1923.[19] Additional symptoms can range from abdominal pain, headache, sensations of active movements in the abdomen, and backache over the entire back extending down both legs.[20]
Pituitary tumors can be associated with Chiari-Frommel Syndrome, leading to secondary ocular symptoms due to compression of nearby structures by an enlarged pituitary gland such as bitemporal hemianopsia, decreased central and/or peripheral visual field loss, or diplopia. The etiology of this syndrome remains unclear. While it often occurs in normal post-partum patients, cases in nulligravid women and those associated with pituitary lesions have been reported.[21][22] Women who experience numerous pregnancies in rapid sequence impose a great demand on genital organs, which may lead to uterine atrophy.[20] In cases where a pituitary tumor is discovered, therapy depends on the size with surgical removal usually only indicated for very large tumors, categorized as macroadenoma. There have been cases where patients have spontaneously stopped lactating or have resumed menstruation. Of those, some were reported to have become pregnant again with normal delivery.[19][23][24]
Somatotroph Adenomas (Growth Hormone Adenomas)
Also arising from Pit-1 lineage, somatotroph adenomas mainly express growth hormone (GH). They are the second most common type of hypersecreting pituitary adenomas. They tend to be macroadenomas at time of clinical diagnosis due to the subtle manifestations of excess growth hormone. They commonly occur in adults around 40-50 years old but can occur in all ages. In children, somatotrophic adenomas have more obvious signs as they may develop physical features that may lead to earlier detection. Many of the features of GH-secreting adenomas are related to the stimulation of insulin-like growth factor I (IGF-I or somatomedin C) by the liver in response to excess growth hormone.[6][12]
GH-secreting adenomas can be either densely-granulated or sparsely granulated, or mammosomatotrophs. Densely granulated somatotroph adenomas (DGSA) respond better to somatostatin analogues rather than sparsely granulated somatotroph adenomas (SGSA). Mammosomatotroph adenomas in addition to expressing GH, also express PRL, though at a lesser extent.[6]
Gigantism
In children who have bony epiphyses that have not yet closed, GH and IGF-I cause gigantism. Gigantism presents as a generalized increase in body size with disproportionately long arms and legs.[3] Gigantism most commonly occurs from mammosomatotroph adneomas.[6]
Acromegaly
If the epiphyses have closed (i.e., late adolescence and adulthood), the result is acromegaly rather than gigantism. The features of acromegaly include characteristic coarsening of facial features (thick lips, exaggerated nasolabial folds, prominent supraorbital ridges, jaw enlargement), enlarged hands and feet, hirsuitism, and joint hypertrophy.[4][5] Skin manifestations include sweating, pigmented skin tags, acanthosis nigricans and cutis verticis gyrata.[25] Acromegaly most commonly occurs from DGSA.[6]
Complications
There are many complications associated with excess growth hormone, including:[3][4][5]
- Carpal tunnel syndrome
- Proximal myopathy
- Gonadal dysfunction
- Respiratory disease
- Diabetes mellitus, which is related to the effect of growth hormone on insulin receptors
- Generalized muscle weakness
- Hypertension
- Arthritis
- Congestive heart failure
- Cardiomyopathy
- Increased risk of gastrointestinal cancers
Corticotroph Adenomas
Corticotroph adenomas derive from Tpit lineage and express adrenocorticotropic hormone (ACTH) plus other hormones from the same precursor, proopiomelanocortin (POMC). ACTH triggers the adrenal glands to secrete cortisol. The densely granulated variant is more common and is usually basophilic, and the sparsely granulated variant is chromophobic. Crooke cell adenomas, another subset of corticotroph adenomas, have characteristic morphology on immunohistology stains with a ring of LMWK and PAS positive granules along the periphery.[6] These tumors stain positively with periodic acid-Schiff (PAS) because of the carbohydrates present in POMC, the precursor molecule to ACTH. Most ACTH-secreting pituitary adenomas are small, with detection of the tumor earlier due to the significant systemic changes. Secondary adrenal hyperplasia often occurs due to the elevated ACTH levels.[26] The elevated levels of serum cortisol lead to a constellation of signs and symptoms that is known as Cushing Syndrome, due to Cushing disease.
Cushing Syndrome (Hypercortisolism)
See also Cushing syndrome
Cushing syndrome describes the assortment of physiologic changes that occurs with elevated serum cortisol levels. Cortisol and other glucocorticoids have many systemic effects, which include immunosuppression, diabetes due to increased gluconeogenesis, hypertension caused by partial mineralocorticoid effects, osteoporosis from inhibition of bone formation and calcium resorption, elevated intraocular pressure (IOP), among many others.[27]
Cushing syndrome can result from both exogenous and/or endogenous sources, which makes the diagnostic workup of a patient presenting with Cushing syndrome very challenging. Hypercortisolism now most commonly results as a result of iatrogenic therapeutic modalities. While a majority of patients with endogenous Cushing syndrome have thyrotrophic adenomas (called Cushing Disease - see below), other tumors should also be considered as part of the endocrinologic workup, including adrenal tumors and especially ACTH-secreting small cell carcinoma of the lung.[5]
Cushing Disease
Cushing disease is a subset of Cushing syndrome that is caused by excess production of ACTH from a corticotroph adenoma. Various studies report that pituitary adenomas account for anywhere from 66% to 80% of endogenous Cushing syndrome cases.[26][27]
Nelson Syndrome
Nelson syndrome describes the enlargement of an ACTH-secreting pituitary adenoma following surgical removal of the adrenal glands for treating Cushing syndrome. The adenoma grows precipitously because of the removal of the feedback inhibition of adrenal corticosteroids on the pituitary tissue. Hypercortisolism is not present because of the absence of the adrenal glands. Patients will complain of symptoms consistent with the mass effects of a pituitary tumor. They may also develop hyperpigmentation, which occurs due to the stimulation of melanocytes by POMC. Nelson syndrome does not occur as often now that neuroimaging techniques are better and more easily available.[5]
Gonadotroph Adenomas
Gonadotrophic adenomas secrete lutenizing hormone (LH) and follicle-stimulating hormone (FSH). They are variable in their secretion, and often do not cause clinical symptoms related to their hormone products. As such, they tend to become symptomatic when they grow large enough to cause the associated neurological symptoms of pituitary tumors. These tumors can also have decreased pituitary hormones, most commonly related to decreased LH. LH deficiency is manifested by decreased energy and libido in men due to decreased testosterone levels, and amenorrhea in premenopausal women. Most gonadotrophic adenomas contain chromophobic cells that react to common gonadotropin α-subunit and β-FSH and β-LH subunits, with FSH being the predominantly secreted hormone.
Thyrotroph Adenomas
Thyrotrophic adenomas are one of the rarest types of secreting pituitary adenomas. They derive from Pit-1 lineage and can express thyroid-stimulating hormone (TSH), which induces the thyroid gland to produce thyroid hormone (thyroxine). Thyrotrophic adenomas will usually induce a hyperthyroid state due to the high levels of TSH, however hypo and euthyroidism is also possible.[6] These tumors tend to be large and invade adjoining structures, which can cause visual field defects. Because this is not an autoimmune process, the hyperthyroid state induced by these adenomas is not associated with thyroid eye disease.
Plurihormonal Adenoma
An adenoma expressing more than one pituitary hormone is considered a plurihormal pituitary adenoma, except in the instances of dual GH and PRL, and FSH and LH expression. Transcription factors are helpful in determining if the tumor is actually two or more separate tumors in the same area, or if the tumor actually co-expresses two different pituitary hormones.[6]
Silent-subtype III is a plurihormonal pituitary adenoma that derives from the Pit-1 lineage and expresses one or more hormones in scattered foci. It commonly presents in a younger patient population and is associated with MEN 1 syndrome. Silent-type III pituitary adenomas can be clinically aggressive but has good treatment response to radiation and temozolomide.[6]
Null Cell Tumors
Tumors that have negative hormone immunohistology, express no pituitary transcription factors, or cannot be linked to a specific cell lineage are termed null cell tumors. They are a diagnosis of exclusion from other tumors in the sella turcica area.[6]
Incidentalomas
Pituitary "incidentalomas" are lesions of any kind found in the pituitary on imaging performed for a different purpose. Most incidentalomas are adenomas, and are not uncommon. Microincidentalomas (<1 cm) have been reported to be more common than macroincidentalomas (4-20% vs. 0.2% on CT, 10-38% vs. 0.16% on MRI, respectively).[28] Most of these tumors tend to remain indolent and only rarely cause pituitary apoplexy, worsening visual fields, or new endocrine dysfunction.[29]
Diagnosis
History
Patients with pituitary adenomas have a variable history, dependent on the functional (secretory) status of the tumor, its size, and any mass effect. As discussed above, a significant portion of pituitary adenomas are asymptomatic or indolent, leading their discovery to be incidental. Adenomas that have subtle features, such as hypotestosteronism, may also lead to a delay in discovery. A detailed review of systems focusing on endocrinological and constitutional symptoms may guide the diagnosis.
Patients with secretory tumors may have systemic complaints that are associated with their corresponding hormonal imbalance. Most ophthalmologic complaints are secondary to mass effects caused by macroadenomas. Together many of these symptoms form the so-called "chiasmal syndrome", which applies to any compressive lesion on the chiasm.[30]
Headache
Headaches are one of the most common symptoms reported in pituitary adenomas, with a reported incidence of 45%. These headaches are often localized to the brow or periorbital region.[30]
Decreased Vision
Pituitary adenomas that compress on the chiasm can often produce a slow decrease in visual function. This may present either as decreased central acuity or as a loss in peripheral visual fields.[30]
Loss of Peripheral Vision
Often patients are unaware of peripheral field loss; directed questions towards functional changes such as increased rate of car accidents due to being unaware of cars approaching in the periphery, etc., may alert the clinician to a visual field deficit. The visual loss can be due to a unilateral or bilateral optic neuropathy, bitemporal hemianopsia from chiasmal compression, or rarely homonymous hemianopsia from optic tract involvement. The visual acuity may or may not be involved depending on extension to one or both optic nerves.
Loss of Depth Perception (Stereopsis)
Some patients with complete bitemporal hemianopsia will complain of a complete loss of depth perception. This has been termed "post-fixational blindness", and is caused by the two blind temporal hemifields overlapping during convergence. The result is a completely blind region of the patient's vision at the point of fixation. Patients will often complain that they have trouble with anteroposterior tasks such as cutting fingernails, threading needles, and working with precision tools.[30]
Pulfrich phenomenon is a test for stereopsis, non-specific to chiasmal compression.[31] When a filter is placed in front of one eye and a moving object at a constant distance away is viewed, the person perceives the object as having depth, when in reality it is moving in the same plane. The filter causes the light conduction velocity to be slower, perceived by the brain as the object having false depth.[32] Patients with chiasmal disease will experience Pulfrich phenomenon physiologically. However, this effect is seen in any condition that causes delayed optic nerve conduction such as multiple sclerosis and retinal disorders.[31]
Diplopia
Diplopia may be due to a paretic diplopia from ocular motor cranial nerve (e.g., III, IV, VI) paresis from lateral extension of tumors into the cavernous sinus, or a non-paretic diplopia as a result of "hemifield slide" (described below). The diplopia is binocular (only present when both eyes are open), and can have horizontal, vertical, or torsional components depending on the affected cranial nerve(s). The diplopia may be present constantly or intermittently.[2][30]
Extraocular Muscle Paresis
As described above, extraocular muscle paresis is a rare presentation (~5% of patients with pituitary adenoma) most often associated with a concurrent palsy of the corresponding cranial nerve. Tumor extension into the cavernous sinus can affect cranial nerves III, IV, V, or VI and produce a limited or total cavernous sinus syndrome. Of the cranial nerves, cranial nerve III (oculomotor nerve) is most commonly affected with variable involvement of pupillomotor fibers. Extraocular muscle paresis may also suggest pituitary apoplexy, which must considered in the setting of rapidly progressive vision loss and diplopia.[30]
Hemifield Slide
Non-paretic binocular diplopia may occur as the result of a loss in the physiologic linkage between the two functioning hemifields, termed "hemifield slide phenomenon" by Kirkham in 1972.[33] This binocular diplopia occurs in the presence of intact ocular motility. While the exact pathophysiology is not completely understood, it is hypothesized that when the degree of overlap between one eye's temporal visual field and the contralateral eye's nasal visual field decreases to a certain point, the brain is unable to maintain fusion.[5] This can occur with vertical hemifield defects such as those seen in pituitary adenomas, as well as altitudinal hemifield defects from optic nerve disease.[34] Patients may experience overlapping images or divergent images corresponding to esodeviation, exodeviation, or hyperdeviation.[33]
Photophobia
A few case reports have reported photophobia as the main presenting symptom in some patients with chiasmal disease.[35]
Physical Examination
Findings associated with endocrine disturbance
While there may be many subtle or obvious signs of endocrine disturbance, description of these signs is beyond the scope of this discussion. For further information, please see the additional resources section at the end of this article.
Visual Field Deficits
Visual field defects are caused by tumor compression on the optic nerve or chiasm leading to axonal damage. Depending on the size and location of the tumor, as well as the anatomical relationship of the chiasm to the pituitary stalk, the severity and symmetry of the visual field defect may vary. Testing strategies for visual fields are discussed below.
| Visual field deficit | Description | Mechanism | Compression side |
|---|---|---|---|
| Monocular visual field deficits or junctional scotoma of Traquair | Temporal (more common) or nasal monocular hemianopsia | Postfixed chiasm or anterior asymmetrical tumor spread | Ipsilateral optic nerve before chiasm |
| Junctional scotoma | Ipsilateral central scotoma and contralateral superior temporal visual field defect | Postfixed chiasm or anterior asymmetrical tumor spread | Ipsilateral optic nerve and Wilbrand's knee of the chiasm |
| Bitemporal hemianopsia (most common) | Progress from superior to complete bitemporal hemianopsia (can be asymmetrical initially) | Normal chiasm compressed by tumor from below | Chiasm |
| Cecocentral scotoma | Paracentral bitemporal hemianopsia | Prefixed chiasm or posterior tumor spread | Macular fibers on the posterior of the chiasm |
| Homonymous hemianopsia | Contralateral visual field loss, RAPD, and band or "bow-tie" optic atrophy | Prefixed chiasm or posterior asymmetrical tumor spread | Optic tract |
Monocular Visual Field Deficits
Asymmetric tumors may preferentially involve one side of the chiasm or an optic nerve, and most commonly presents as a superotemporal quadrantanopsia. The incidence of pituitary adenomas presenting as monocular visual field defects was reported to be 9% (in a series of 1,000 patients). If the presenting symptom is sudden monocular visual loss, a pituitary adenoma may be acutely missed, as other more common etiologies are evaluated. Another consideration for sudden monocular visual loss is pituitary apoplexy, though visual field evaluation is often deferred due to the emergent nature of this condition. [5]
The patient may have a unilateral or bilateral optic neuropathy or develop an optic neuropathy in one eye and a contralateral superotemporal defect in the fellow eye (i.e., the junctional scotoma). Interestingly, monocular temporal (uncrossed fibers) or nasal (crossed fibers) hemianopic visual field loss can also occur from a lesion at the junction of the optic nerve and chiasm. This monocular but hemianopic visual field loss is referred to as the junctional scotoma of Traquair in order to differentiate it from the junctional scotoma (i.e., ipsilateral optic neuropathy with contralateral superotemporal visual field loss).[5]
Chiasmal Field Deficits
Characteristically lesions at the level of the optic chiasm produce a bitemporal hemianopia. Pituitary adenomas, which grow upward from the pituitary stalk, compress the chiasm from below, which preferentially involves the inferior, nasal, and macular nerve fibers. This corresponds to superior, bitemporal, and central vision loss. While these field defects typically respect the vertical midline, pituitary adenomas large enough to cause compression tend to also reduce visual acuity and cause diffuse central depression on automated and Goldmann perimetry.[5] Vison loss due to compression first affects the superotemporal visual fields, then inferotemporal, inferonasal and finally superonasal fields.[31]
In prefixed chiasms or tumors that preferentially grow posteriorly, selective compression of macular fibers may cause a bitemporal hemianopsia involving the central visual field while sparing the peripheral field.[37]
Optic Tract
Rarely, pituitary adenomas can compress the optic tract and produce a homonymous hemianopsia. In an optic tract lesion, there may be a relative afferent pupillary defect (typically in the eye with greater visual field loss), or a special type of optic atrophy called “band” or “bow-tie” optic atrophy representing nasal fiber loss in the eye with the temporal visual field defect.
Optic Atrophy
Chronic tumor compression can lead to optic atrophy. Some atrophy is reported to be present in approximately 50% of pituitary lesions with visual field defects. If only the fibers nasal to the macula are damaged, only the nasal and temporal optic disc may be atrophic, often described as "bow-tie" or "band-shaped" pattern in both eyes in a bitemporal hemianopsia and only in the eye with the temporal visual field loss (nasal fiber loss) in a homonymous hemianopsia from an optic tract lesion.[4]
Papilledema
Papilledema is a rare finding in pituitary adenomas. The slow-growing nature of these tumors tends to cause optic atrophy before the tumor enlarges sufficiently to compress the third ventricle or produce sufficient mass effect to increase intracranial pressure (ICP). Cortisol (i.e., Cushing disease) or growth hormone-secreting adenomas can increase ICP in a fashion similar to those in children taking growth hormone for short stature or patients with Turner syndrome who develop idiopathic intracranial hypertension. Bilateral hypertensive optic disc edema in the setting of Cushing's disease may mimic papilledema.[5]
Nystagmus
Chiasmal lesions of any kind can be associated with seesaw nystagmus, an extremely rare form of nystagmus in which one eye elevates and intorts while the other eye synchronously depresses and extorts, then alternates. The exact pathophysiology of this phenomenon is unknown; however, as most of these lesions involve the chiasm and/or diencephalon, many have proposed that disruption of visual and vestibular input contribute to this pathology. Several case studies have shown that children born without a chiasm and lesions that do not affect the chiasm or diencephalon can also cause see-saw nystagmus; tumor resection can sometimes result in resolution of nystagmus.[1][5][38][39]
Diagnostic procedures
Visual Field Testing
Automated static perimetry or kinetic perimetry are used to formally assess visual fields. In an undiagnosed patient with a history suspicious for pituitary disease, this often confirms the presence of a compressive chiasmal lesion. Visual fields are also commonly documented prior and following neurosurgical intervention.
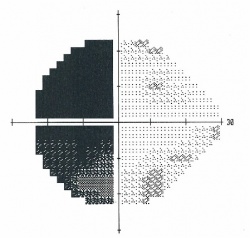
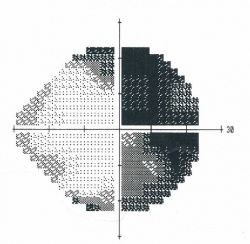 Figure 1. Humphrey visual field 24-2 showing a bitemporal hemianopsia.
Figure 1. Humphrey visual field 24-2 showing a bitemporal hemianopsia.
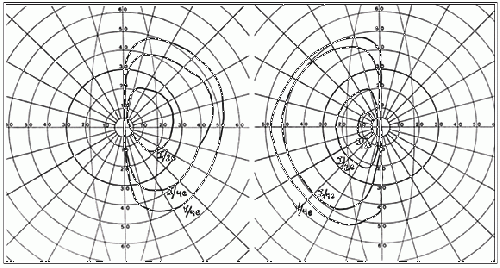 Figure 2. Goldmann visual field showing a bitemporal hemianopsia.[38]
Figure 2. Goldmann visual field showing a bitemporal hemianopsia.[38]
Optical Coherence Tomography
Optical Coherence Tomography (OCT) has shown significant promise in the early detection of chiasmal compression by providing high-resolution, quantitative imaging of retinal structures. Studies have demonstrated that OCT can detect thinning of the retinal nerve fiber layer (RNFL) and reductions in the ganglion cell layer (GCL) thickness, even before visual field defects become clinically evident . Optical Coherence Tomography (OCT) has been instrumental in identifying specific patterns of retinal nerve fiber layer (RNFL) loss associated with chiasmal compression and other optic neuropathies. In cases of chiasmal compression, often caused by pituitary adenomas and other parasellar tumors, OCT typically reveals bitemporal or binasal peripapillary RNFL thinning due to the crossing fibers at the optic chiasm being affected. This bitemporal loss is a key indicator of chiasmal compression, distinguishing it from other conditions. Additionally, in conditions causing intracranial hypertension, OCT can detect papilledema, characterized by optic disc swelling and increased RNFL thickness. The ability of OCT to discern these patterns — bitemporal RNFL thinning indicative of chiasmal compression and RNFL thickening associated with papilledema — underscores its value in early diagnosis and management of these potentially vision-threatening conditions.[36]
Neuroimaging
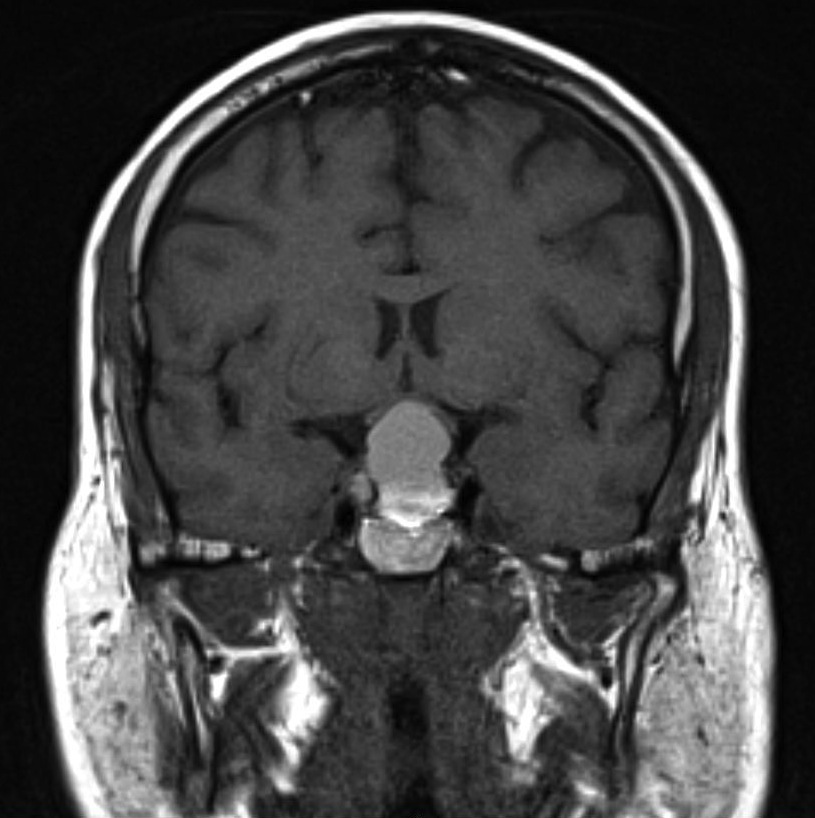
The pituitary gland is best seen on coronal sections and normally enhances with contrast. As with any radiographic study, detailed communication with the radiologist/neuroradiologist will help guide the appropriate study as well as careful examination of the images. Pituitary or sella sequence magnetic resonance imaging (MRI) with and without contrast is the study of choice for most sellar and parasellar lesions, but high-resolution CTs with fine cuts (1.5-3 mm) have also been shown to be very helpful in the differential diagnosis especially if calcification is seen (e.g., craniopharyngioma or meningioma).[2]
Computed Tomography (CT)
Microadenomas may present as less-enhancing lesions within the substance of the pituitary gland, whereas macroadenomas are more variable in their appearance. Bone window scans help assess erosions and extensions into the sphenoid sinus.[40]
Magnetic Resonance Imaging (MRI)
Microadenomas are best seen on unenhanced T1-weighted coronal or sagittal scans. They are seen as hypointense areas within the substance of the relatively hyperintense pituitary gland. With gadolinium contrast these lesions will remain less intense compared to the normal gland and will enhance late. On T2-weighted images, the adenoma is more indistinct, as it appears isointense or only slightly hyperintense in comparison with the surrounding gland.[40]
Macroadenomas are more obviously seen, as they extend beyond the anatomical borders of the sella. The homogeneity of the images suggests the relative solidity of the tumor. Nonsolid tumors (macrocystic and macrohemorrhagic) have been suggested to be more effectively managed with transsphenoidal hypophysectomy in comparison with solid tumors.[41]
Laboratory testing
An endocrine workup is important for determining the functional status of a pituitary adenoma. This is often performed with the guidance of an endocrinologist. The laboratory tests may vary depending on the clinical presentation.
Differential diagnosis
Of all conditions causing the chiasmal syndrome, pituitary adenomas are the most common (50-55%). Some other causes of the chiasmal syndrome are listed below:[30]
Compressive Lesions
- Craniopharyngioma (20-25%)
- Meningioma (10%)
- Glioma (7%)
- Dysgerminoma
- Aneurysms (internal carotid artery, middle or anterior cerebral artery, or anterior communicating artery)
- Mucocele
- Abscess from an intracranial infection
- Lymphocytic hypophysitis
- Metastatic disease
- Arachnoidal and epithelial cysts
Ischemic Lesions
- Pituitary apoplexy
- Radionecrosis
- Arteritis
- Arteriosclerosis
Inflammatory Lesions
- Multiple sclerosis
- Sarcoidosis
- Tuberculosis
Toxic Lesions
- Pheniprazine (Catron)
- Muslin gauze
- Ethchlorvynol (Placidyl)
Management
Management of pituitary adenomas depends on the size of the tumor, its secretory function (as well as what type of hormone is secreted), and its impact on the patient's vision. Goals of treatment include visual recovery/preservation, reversal of hypersecretory syndromes, and control of tumor growth.[5] While some forms of pituitary adenoma can be managed medically, most pituitary adenomas that have demonstrable visual field loss are treated surgically. Radiation therapy (external beam or gamma-knife) is considered a second-line treatment.
General Approach
Medical and surgical management of pituitary adenomas require a multidisciplinary team. Ophthalmology is typically consulted to evaluate the patient's visual fields. Less commonly, ophthalmologists may be the first to detect evidence of a pituitary adenoma. Standard automated perimetry is often utilized by ophthalmologists and pituitary surgeons to document indication and follow progression of visual involvement. Endocrinology is often involved to help evaluate hormonal levels and guide medical management. Surgical debulking of pituitary tumors is often performed by otorhinolaryngology (ENT) or neurosurgery.[42]
Medical therapy
Prolactinomas
Medical treatment is considered the primary treatment for prolactinomas. Dopamine agonists (e.g., bromocriptine or cabergoline) have been shown to normalize hyperprolactinemia as well as shrink tumors. Management of prolactinomas is often in conjunction with an endocrinologist in regards to medication administration and decisions regarding indication for surgical resection.[5] While both medications have similar mechanisms of action, the other medication may be tried if there is a minimal response from the first drug.
Bromocriptine is the first-line agent used in treating prolactinomas and suppresses prolactin secretion by binding to D2 dopamine receptors. Because it has been used for over 25 years, it is the recommended medication for use in treatment of pituitary adenomas in pregnancy. Side effects are three times more frequent in bromocriptine compared with cabergoline, which may include gastrointestinal, cardiovascular, or neurological symptoms.[43]
Cabergoline is a newer medication that also binds to D2 dopamine receptors. It has a smaller incidence of side effects when compared to bromocriptine, and various studies suggest that it has a comparable effect of normalizing hyperprolactinemia and shrinking tumor size.[44]
Acromegaly
Somatostatin analogs (octreotide, lanreotide), bromocriptine, and growth hormone receptor agonists (pegvisomant) have been used to treat growth hormone-secreting tumors causing acromegaly. Octreotide has the most effect on tumor shrinkage, and pegvisomant has no effect on tumor size or growth hormone secretion but may have better effect on the overall systemic effects of elevated growth hormone.[5]
Thyrotrophic adenomas
Thyrotrophic tumors can be treated with octreotide due to the presence of somatostatin receptors on the tumor.[5] It is successful at decreasing levels of thyroid-stimulating hormone (TSH), and normalizes hyperthyroidism in around 75%. Octreotide can shrink thyrotropin-secreting tumors in a third of reported cases.[45]
Surgery
Pituitary adenoma debulking, or partial excision, is often carried out via transfrontal, transsphenoidal, or transpterional approaches.[46] Endoscopic techniques have improved visualization of the tumor and allowed for smaller incisions and faster healing.[47][48]
Surgical Indications
The following are some of the indications for surgical debulking of a pituitary adenoma:[49][50][51][52]
- Patients with prolactinomas who cannot tolerate the side effects from dopaminergic agonist therapy
- Patients with prolactinomas who had no tumor shrinkage with dopaminergic agonist therapy
- Patients with prolactinomas who decided on primary surgical excision
- Patients with prolactinomas who had cystic features on MRI (80% of tumor volume on T2 sequences) that were unlikely to shrink sufficiently with medical management alone
- Patients with cavernous sinus involvement
- Patients with pituitary apoplexy
- Patients with vision-related symptoms from compression of the optic apparatus
Surgical Outcomes
Surgical debulking in a timely fashion (within 6 months of onset of visual symptoms) has been shown to normalize or improve visual acuity as well as improve visual field with rare worsening or severe surgical complication.[50] The outcome is affected by the severity of symptoms - for example, sudden ophthalmoplegia due to pituitary apoplexy would have a different result than a slow-growing prolactinoma.[53]
Moreover, the visual outcome is also affected by the preoperative peripapillary retinal nerve fiber layer thickness- for example, preoperative temporal peripapillary retinal nerve fiber layer thickness ≥ 60 µm and inferior peripapillary retinal nerve fiber layer thickness ≥ 105 µm are associated with postoperative visual field index > 90% and a postoperative visual acuity of at least 20/25, respectively.[54]
Follow up
No Ophthalmologic Involvement/No Surgery/Medical Therapy
Patients with pituitary adenomas without compression can be followed annually by serial exam and imaging. Patients with visual loss may require more frequent follow up however including formal visual field testing.
Postoperative Follow Up
Because pituitary adenomas are sometimes incompletely excised, recurrence is an important consideration and reason for continued screening following surgical treatment. The first sign of recurrence may be visual loss; therefore, baseline visual fields and visual acuity testing are recommended 2-3 months after treatment and at intervals of 6-12 months afterwards depending on the course, completeness of the resection, and serial neuroimaging studies.[1] Periodic neuroimaging is also essential, as recurrence may occur even as late as 10 years post-surgery.[55]
Complications
Pituitary Apoplexy
See also pituitary apoplexy on wikipedia
Pituitary apoplexy is a life-threatening complication of pituitary adenoma caused by a sudden hemorrhage or infarction of the tumor. It is characterized by the presentation of acute, severe headache, nausea, and altered consciousness. Sudden expansion of the pituitary mass into the cavernous sinus can cause sudden ophthalmoplegia and facial hypoesthesia due to compression of cranial nerves III, IV, V, and VI, with cranial nerve III being affected most commonly. Superior extension of the pituitary mass can cause sudden vision loss or visual field loss. Patients can have decreased consciousness or strokes from extravasation of blood into the subarachnoid space. The life-threatening emergency associated with pituitary apoplexy is adrenal crisis, as well as other acute endocrine abnormalities. The diagnosis is confirmed by CT or MRI. Because this condition is a true emergency, corticosteroids have to be started immediately and severe cases have to be taken to the operating room for emergent surgical decompression of the sella.[1]
Tumor Recurrence
Pituitary adenomas often recur following resection. Nonfunctioning adenomas are less likely to enter remission following surgical treatment than functioning adenomas. Recurrence of tumor is most likely to occur between 1-5 years following surgery, with prolactinomas having the highest incidence of recurrence. Having a high basal hormonal level postoperatively was predictive of recurrence in functional adenomas, but there are no known predictive factors for recurrence in nonfunctioning adenomas.[56] It was found that recurrence rates can be decreased with a combination treatment of surgery and radiotherapy (from 7-35% to 7-13%).[5]
Secondary Empty Sella
Following surgical excision of a pituitary adenoma, the sella can remain radiographically "empty." The chiasm can herniate into this space, rarely causing secondary visual loss. Proposed treatments are often geared towards decreasing the traction on the chiasm surgically or by decreasing medical therapy and allowing the tumor to regrow.[5]
Other Complications
Other complications associated with pituitary adenomas include delayed radionecrosis if radiotherapy was performed, chiasmal traction from adhesions, and chiasmal compression from expansion of fat.[1]
Prognosis
Visual recovery following treatment of pituitary adenomas is largely dependent on pre-treatment visual acuity/visual fields/etc., age of patient, duration of symptoms, severity of visual defects, presence of optic atrophy, and size of the tumor. The timeframe of recovery ranges from early (within 24 hours after surgery, between 24-72 hours after starting medical therapy) to late (6 months to 3 years). Most improvement takes place between 1-4 months. Most patients show improvement of visual acuity, visual fields, or both following surgical treatment. Patients undergoing radiation therapy tend to have a slower recovery and time to improvement.[5]
Additional Resources
- Neuro-Ophthalmology. Basic and Clinical Science Course, Section 5. American Academy of Ophthalmology; 2010:159-165.
- Gittinger JW. "Tumors of the Pituitary Gland." In: Miller NR, Newman NJ, eds. Walsh and Hoyt's Clinical Neuro-Ophthalmology, 6th Ed. Volume Two. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:1531-1546.
- Yanoff M, Duker JS. Ophthalmology, 3rd Ed. Elsevier; 2009:986-994.
- Kanski JJ, Bowling B. Clinical Ophthalmology: A Systematic Approach, 7th Ed. Elsevier; 2011:816-827.
- Pituitary adenoma article on Wikipedia
- Patient Information Brochure. North American Neuro Ophthalmology Society (NANOS). https://www.nanosweb.org/Pituitary_mass_information_brochure. Accessed March 3, 2025
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 Neuro-Ophthalmology. Basic and Clinical Science Course, Section 5. American Academy of Ophthalmology; 2010:159-165. ISBN 978-1-61525-133-9.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 Yanoff M, Duker JS. Ophthalmology, 3rd Ed. Elsevier; 2009:986-994.
- ↑ Jump up to: 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease, 7th Ed. Elsevier; 2005:1156-1164. ISBN 978-0-8089-2302-2.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Kanski JJ, Bowling B. Clinical Ophthalmology: A Systematic Approach, 7th Ed. Elsevier; 2011:816-827.
- ↑ Jump up to: 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 Gittinger JW. "Tumors of the Pituitary Gland." In: Miller NR, Newman NJ, eds. Walsh and Hoyt's Clinical Neuro-Ophthalmology, 6th Ed. Volume Two. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:1531-1546. ISBN 978-0-7817-4812-4
- ↑ Jump up to: 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 Mete O, Lopes MB. Overview of the 2017 WHO Classification of Pituitary Tumors. Endocr Pathol. 2017;28:228-243. https://doi.org/10.1007/s12022-017-9498-z
- ↑ Jump up to: 7.0 7.1 7.2 Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The Prevalence of Pituitary Adenomas: A Systematic Review. Cancer. 2004;101(3):613-9. doi:10.1002/cncr.20412. PMID 15274075.
- ↑ Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High Prevalence of Pituitary Adenomas: A Cross-Sectional Study in the Province of Liege, Belgium. J Clin Endocrinol Metab. 2006 Dec;91(12):4769-75. Epub 2006 Sep 12. PMID 16968795.
- ↑ Marini F, Falchetti A, Luzi E, Tonelli F, Luisa BM. Multiple Endocrine Neoplasia Type 1 (MEN1) Syndrome. Cancer Syndromes [Internet]. Bethesda (MD): National Center for Biotechnology Information (US). Riegert-Johnson DL, Boardman LA, Hefferon T, et al., editors. NCBI Bookshelf. 9 Aug 2008. PMID 21249756.
- ↑ Jump up to: 10.0 10.1 Asa SL. Practical Pituitary Pathology: what does the pathologist need to know? Arch. Pathol. Lab. Med. 2008 Aug;132(8):1231–40. PMID 18684022.
- ↑ Zhang X, Horwitz GA, Heaney AP, Nakashima M, Prezant TR, Bronstein MD, Melmed S. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999 Feb;84(2):761–767. doi:10.1210/jc.84.2.761. PMID 10022450.
- ↑ Jump up to: 12.0 12.1 Personnier C, Cazabat L, Bertherat J, Gaillard S, Souberbielle JC, Habrand JL, Dufour C, Clauser E, SainteRose C, Polak M. Clinical features and treatment of pediatric somatotropinoma: case study of an aggressive tumor due to a new AIP mutation and extensive literature review. Horm Res Paediatr. 2011;75(6):392-402. Epub 2011 May 6. doi:10.1159/000327831. Available online. PMID 21546764.
- ↑ Florio T. Adult Pituitary Stem Cells: From Pituitary Plasticity to Adenoma Development. Neuroendocrinology 2011;94:265–277. doi:10.1159/000330857. PMID 22116388.
- ↑ Fideleff HL, Boquete HR, Suárez MG, Azaretzky M. Prolactinoma in children and adolescents. Horm Res. 2009;72(4):197-205. Epub 2009 Sep 29. doi:10.1159/000236081. Available online. PMID 19786791.
- ↑ Righi A, Agati P, Sisto A, Frank G, Faustini-Fustini M, Agati R, Mazzatenta D, Farnedi A, Menetti F, Marucci G, Foschini MP. A classification tree approach for pituitary adenomas. Hum Pathol. 2012 Mar 23. [Epub ahead of print]. doi:10.1016/j.humpath.2011.12.003. PMID 22446019.
- ↑ Adapted from Pituitary Adenoma. Wikipedia. Accessed 04-28-2012.
- ↑ Ironside JW. Best Practice No 172: Pituitary Gland Pathology. J Clin Pathol. 2003 Aug;56(8):561-8. PMID 12890801
- ↑ Spark RF, Wills CA, O'Reilly G, Ransil BJ, Bergland R. Hyperprolactinaemia in males with and without pituitary macroadenomas. Lancet. 1982 Jul 17;2(8290):129-32. PMID 6123841.
- ↑ Jump up to: 19.0 19.1 LIPPARD CH. The Chiari-Frommel syndrome. Am J Obstet Gynecol. 1961;82:724-726. doi:10.1016/s0002-9378(16)36134-8
- ↑ Jump up to: 20.0 20.1 MENDEL EB. Chiari-Frommel syndrome; an historical review with case report. Am J Obstet Gynecol. 1946;51:889-892. doi:10.1016/s0002-9378(16)39969-0
- ↑ FORBES AP, HENNEMAN PH, GRISWOLD GC, ALBRIGHT F. Syndrome characterized by galactorrhea, amenorrhea and low urinary FSH: comparison with acromegaly and normal lactation. J Clin Endocrinol Metab. 1954;14(3):265-271. doi:10.1210/jcem-14-3-265
- ↑ CHRISTIANSEN EG. A case of Chiari-Frommel’s syndrome. Acta Endocrinol (Copenh). 1957;24(4):407-410. doi:10.1530/acta.0.0240407
- ↑ ASHKAR PA. Chiari’s syndrome; report of a case. J Obstet Gynaecol Br Emp. 1950;57(1):78. doi:10.1111/j.1471-0528.1950.tb05218.x
- ↑ Jaszmann L, van Lith ND. The prognosis of the Chiari-Frommel syndrome: a case report. J Obstet Gynaecol Br Commonw. 1970;77(1):72-73. doi:10.1111/j.1471-0528.1970.tb03411.x
- ↑ Nabarro JD. Acromegaly. Clin Endocrinol (Oxf). 1987 Apr;26(4):481-512. PMID 3308190.
- ↑ Jump up to: 26.0 26.1 Kirk LF Jr, Hash RB, Katner HP, Jones T. Cushing's Disease: Clinical Manifestations and Diagnostic Evaluation. Am Fam Physician. 2000 Sep 1;62(5):1119-27, 1133-4. Available online. PMID 10997535.
- ↑ Jump up to: 27.0 27.1 Guaraldi F, Salvatori R. Cushing syndrome: maybe not so uncommon of an endocrine disease. J Am Board Fam Med. 2012 Mar;25(2):199-208. doi:10.3122/jabfm.2012.02.110227. Available online. PMID 22403201.
- ↑ Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, Post KD, Vance ML. Pituitary Incidentaloma: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011 Apr;96(4):894-904. doi:10.1210/jc.2010-1048. Available online. PMID 21474686.
- ↑ Fernández-Balsells MM, Murad MH, Barwise A, Gallegos-Orozco JF, Paul A, Lane MA, Lampropulos JF, Natividad I, Perestelo-Pérez L, Ponce de León-Lovatón PG, Erwin PJ, Carey J, Montori VM. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2011 Apr;96(4):905-12.doi:10.1210/jc.2010-1054. Available online. PMID 21474687.
- ↑ Jump up to: 30.0 30.1 30.2 30.3 30.4 30.5 30.6 Trevino R. Chiasmal Syndrome. J Am Optom Assoc. 1995 Sep;66(9):559-75. PMID 7490416.
- ↑ Jump up to: 31.0 31.1 31.2 Foroozan, R. Visual findings in chiasmal syndromes. International Ophthalmology Clinics. 2016;56(1):1-27.
- ↑ O’Doherty M, Flitcroft DI. An unusual presentation of optic neuritis and the Pulfrich phenomenon. J neurol Neurosurg Psychiatry. 2007 Aug;78(8):906-907.
- ↑ Jump up to: 33.0 33.1 Kirkham TH. The ocular symptomatology of pituitary tumours. Proc R Soc Med. 1972 Jun;65(6):517-8. PMID 5035898.
- ↑ Borchert MS, Lessell S, Hoyt WF. Hemifield slide diplopia from altitudinal visual field defects. J Neuroophthalmol. 1996 Jun;16(2):107-9. PMID 8797166.
- ↑ Kawasaki A, Purvin VA. Photophobia as the Presenting Visual Symptom of Chiasmal Compression. Journal of Neuro-Ophthalmology. 2002 March;22(1):3-8.
- ↑ Jump up to: 36.0 36.1 Khadamy J. The Role of Optical Coherence Tomography in the Early Detection of Chiasmal Compression: Hypophyseal Adenoma Presenting With Bitemporal Nerve Fiber Layer Thinning. Cureus. 2024 Mar 2;16(3):e55371. doi: 10.7759/cureus.55371. PMID: 38562328; PMCID: PMC10982832.
- ↑ Chiu EK, Nichols JW. Sellar lesions and visual loss: key concepts in neuro-ophthalmology. Expert Rev Anticancer Ther. 2006 Sep;6(Suppl 9):S23-8. PMID 17004853.
- ↑ Jump up to: 38.0 38.1 Moura FC, Gonçalves AC, Monteiro ML. Seesaw nystagmus caused by giant pituitary adenoma: case report. Arq Neuropsiquiatr. 2006 Mar;64(1):139-41. Epub 2006 Apr 5. doi:10.1590/S0004-282X2006000100030. PMID 16622572.
- ↑ Drachman DA. See-saw nystagmus. J Neurol Neurosurg Psychiatry. 1966 Aug;29(4):356-61. Available online. PMID 5298192.
- ↑ Jump up to: 40.0 40.1 Dutton, JJ. Radiology of the Orbit and Visual Pathways. Elsevier; 2010:340-341. ISBN 978-1-4377-1151-6.
- ↑ Boxerman JL, Rogg JM, Donahue JE, Machan JT, Goldman MA, Doberstein CE. Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. AJR Am J Roentgenol. 2010 Sep;195(3):720-8. doi:10.2214/AJR.09.4128. Available online. PMID 20729452.
- ↑ Malenković V, Gvozdenović L, Milaković B, Sabljak V, Ladjević N, Zivaljević V. Preoperative preparation of patients with pituitary gland disorders. Acta Chir Iugosl. 2011;58(2):91-6. PMID 21879656.
- ↑ Colao A, Di Sarno A, Guerra E, De Leo M, Mentone A, Lombardi G. Drug insight: Cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat Clin Pract Endocrinol Metab. 2006 Apr;2(4):200-10. PMID 16932285.
- ↑ Ono M, Miki N, Kawamata T, Makino R, Amano K, Seki T, Kubo O, Hori T, Takano K. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab. 2008 Dec;93(12):4721-7. Epub 2008 Sep 23. Available online. PMID 18812485.
- ↑ Chanson P, Weintraub BD, Harris AG. Octreotide therapy for thyroid-stimulating hormone-secreting pituitary adenomas. A follow-up of 52 patients. Ann Intern Med. 1993 Aug 1;119(3):236-40. PMID 8323093.
- ↑ Liu L, Liu ZX, Liu YS, Liu JF, Zeng Y, Zeng ZC, Wang M, Wang H, Zeng CM, Jiang XJ, Chen X, Yang SG. Applied anatomy for pituitary adenoma resection. Chin Med J (Engl). 2011 Aug;124(15):2269-74. PMID 21933555.
- ↑ Rosseau GL. Pituitary tumors and transsphenoidal surgery. Dis Mon. 2011 Oct;57(10):607-14. PMID 22036116.
- ↑ DeKlotz TR, Chia SH, Lu W, Makambi KH, Aulisi E, Deeb Z. Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope. 2012 Mar;122(3):511-8. Epub 2012 Jan 17. doi:10.1002/lary.22479. Available online. PMID 22252670.
- ↑ Kreutzer J, Buslei R, Wallaschofski H, Hofmann B, Nimsky C, Fahlbusch R, Buchfelder M. Operative treatment of prolactinomas: indications and results in a current consecutive series of 212 patients. Eur J Endocrinol. 2008 Jan;158(1):11-8. Available online. PMID 18166812.
- ↑ Jump up to: 50.0 50.1 Müslüman AM, Cansever T, Yılmaz A, Kanat A, Oba E, Çavuşoğlu H, Sirinoğlu D, Aydın Y. Surgical results of large and giant pituitary adenomas with special consideration of ophthalmologic outcomes. World Neurosurg. 2011 Jul-Aug;76(1-2):141-8; discussion 63-6. PMID 21839965.
- ↑ Ceylan S, Anik I, Koc K. A new endoscopic surgical classification and invasion criteria for pituitary adenomas involving the cavernous sinus. Turk Neurosurg. 2011;21(3):330-9. doi:10.5137/1019-5149.JTN.4149-11.0. Available online. PMID 21845568.
- ↑ Wolf A, Coros A, Bierer J, et al. Quantitative evaluation of vision-related and health-related quality of life after endoscopic transsphenoidal surgery for pituitary adenoma. J Neruosurg. Published online first Oct 7, 2016. 2017;127:409-416. doi:10.3171/2016.7.JNS16200.
- ↑ Chuang CC, Chen E, Huang YC, Tu PH, Chen YL, Pai PC. Surgical outcome of oculomotor nerve palsy in pituitary adenoma. J Clin Neurosci. 2011 Nov;18(11):1463-8. Epub 2011 Sep 14. PMID 21920756.
- ↑ Thammakumpee K, Buddawong J, Vanikieti K, et al. Preoperative Peripapillary Retinal Nerve Fiber Layer Thickness as the Prognostic Factor of Postoperative Visual Functions After Endoscopic Transsphenoidal Surgery for Pituitary Adenoma. Clin Ophthalmol. 2022;16:4191-4198.
- ↑ Reddy R, Cudlip S, Byrne JV, Karavitaki N, Wass JA. Can we ever stop imaging in surgically treated and radiotherapy-naive patients with non-functioning pituitary adenoma? Eur J Endocrinol. 2011 Nov;165(5):739-44. Epub 2011 Sep 7. Available online. PMID 21900406.
- ↑ Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012 Mar;15(1):71-83. Available online. PMID 21918830.