Ophthalmoplegic Migraine/Recurrent Painful Ophthalmoplegic Neuropathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Ophthalmoplegic migraine (OM), more recently renamed recurrent ophthalmoplegic neuropathy (RPON) by the International Headache Society, is an uncommon and poorly understood condition with an incidence of 0.7 per million.[1] This rare condition manifests as episodes of ipsilateral headache followed by palsy of 1 or more ocular cranial nerves[2] that begins immediately or up to 14 days after onset of headache.[3] Ophthalmoplegia may persist for weeks to months and is typically reversible.[3] The most commonly affected cranial nerve is the oculomotor nerve, followed by the abducens nerve. Trochlear nerve involvement is rare.[4]
The demographics of OM/RPON differ from that of migraine. While migraine affects women more often than men, and prevalence peaks in middle-aged adults,[5] OM/RPON is primarily a pediatric condition seen in children less than 10 years old and affects males more often than females.[4]
Risk Factors
In cases of reported OM/RPON, there is a high incidence of personal or family history of migraine headaches, suggesting that migraine may be a predisposing factor in the development of the condition.[6] In Nigerian populations, an association between OM/RPON and abnormal hemoglobin has been observed.[7]
Pathophysiology
Changes in the condition’s name reflect the poorly understood pathophysiology of OM/RPON. While the exact pathophysiology has yet to be clarified, current belief is that OM/RPON is unlikely to be a subtype of migraine due to the delay in ophthalmoplegia after the onset of headache.[4] Most theories regarding the pathophysiology underlying OM/RPON are based on case reports and clinical and radiographic observations. Potential etiologic mechanisms include compression, ischemia and demyelination.
Compressive mechanism
Walsh and O’Doherty postulated in 1960 that dilatation or edema of the walls of the internal carotid artery (ICA) or posterior cerebral artery (PCA) during migraine headache might cause direct compression of the oculomotor nerves within the cavernous sinus.[8] However, arguments against this theory point out that in compression of the oculomotor nerve, the first nerve fibers to be affected are usually the parasympathetic fibers, which reside in the periphery of the nerve. In OM/RPON, however, these fibers may be spared[6]; in their review of 39 reported cases of OM/RPON, McMillian et al reported pupillary sparing in 23% of patients.[9] Furthermore, swelling in the walls of the intracavernous carotid artery would cause narrowing of the vessel; however, carotid arteriograms during attacks of OM/RPON have not demonstrated this phenomenon, arguing against the theory of a compressive mechanism.[10] Lastly, compressive symptoms often resolve quickly once the offending lesion is relieved, which is in contrast to OM/RPON where resolution of deficits and symptoms is gradual.[11]
Ischemic mechanism
The vasculature supplying the cisternal parts of the oculomotor, trochlear, and abducens nerves arises from the cavernous section of the ICA and a perforating vessel from the proximal PCA.[12] In 2 patients with OM/RPON, Shin et al used brain single photon emission computed tomography to demonstrate reversible ipsilateral ischemia of the areas supplied by the perforating branches of the PCA.[13] Based on this evidence, Ambrosetto et al proposed that during migraine headache, vasospasm of the vasa nervorum leads to a reversible, ischemic breakdown of the blood-nerve barrier and subsequent vasogenic edema. This mechanism could account for both the contrast enhancement and thickening of the nerve on magnetic resonance imaging (MRI).[14]
Vijayan et al also make a case for an ischemic mechanism, based on numerous similarities between the ophthalmoplegia of diabetes and OM/RPON. First, the third cranial nerve is the most commonly affected nerve in diabetic ophthalmoplegia as well. As in diabetic ischemic neuropathy, the parasympathetic fibers of the oculomotor nerve may be spared in OM/RPON, as both conditions cause the acute onset of ophthalmoplegia and variable pupillary involvement. This phenomenon can likely be explained by the degree of ischemia or the presence or absence of collateral circulation.[11] Migraine-associated edema of the ICA has been proposed as an ischemic mechanism underlying OM/RPON. Vijayan et al suggest that this ischemia is not due to the initial vasoconstrictive phase of migraine, but rather delayed ischemia secondary to edema and inflammation of the ICA or basilar vessel walls in migraine headaches. The edema would theoretically cause occlusion of the ostia of the small vessels of the ICA that supply the involved cranial nerve, causing an injury like the border zone infarction in diabetic third nerve lesions. The fact that resolution of the ophthalmoplegia in both diabetic neuropathy and OM/RPON occurs gradually over weeks is also suggestive of an ischemic process.[11]
Recently Akimoto et al. reported a case of presumed RPON, in which an MRI revealed a Neuromuscular hamartoma (NMH) involving the third (III) cranial nerve at the root exit zone (REZ) presenting as a non-neoplastic cause of RPON. Three such cases of NMH have been reported, all histologically proven, and all with long standing RPON that improved post-surgical intervention. It was therefore felt that dilation of the blood supply to the oculomotor nerve, secondary to trigeminovascular stimulation, could potentially lead to contraction of the musculature of the NMH, resulting in strangulation of the nerve, causing the ophthalmoplegia. [15]
Demyelinating/Inflammatory Mechanism
Based on the MRI findings of the thickening and enhancement of the oculomotor nerve in its root entry zone and cisternal portion, Lance and Zagami proposed that OM/RPON may be a recurring demyelinating neuropathy or neuritis.[16] They noted that the MRI findings of reversible third nerve enhancement could also be seen in other demyelinating post-viral inflammatory conditions, like Miller Fisher syndrome, and in infectious causes, such as HIV.[16][17] Furthermore, while neoplastic, inflammatory, or infiltrative conditions can cause enhancement of the cisternal part of the third nerve, in the majority of these pathologies, the enhancement was not reversible as in OM/RPON.[3] Similar enhancement and thickening have not been seen in the oculomotor nerve ischemia caused by diabetic neuropathy.[18] The recurrent course of some patients with OM/RPON is reminiscent of the relapsing-remitting pattern found in some demyelinating conditions, such as multiple sclerosis[6] or Myelin-Oligodendrocyte-Glycoprotein-associated demyelinating disease. It has been observed that the ophthalmoplegia in OM/RPON persists for longer periods of time with recurrent attacks, a feature that may be explained by cumulative axonal damage from recurrent demyelination.[3] The pathophysiologic mechanism of OM/RPON has also been compared to chronic inflammatory demyelinating polyneuropathy (CIDP) because of similar MRI findings of enhancement involving the nerve roots and plexi.[16] This distinguishing feature on MRI is the globoid appearance of the first part of the third nerve emerging from the midbrain, which is similar to that seen in CIDP.[16] [3] Unlike OM/RPON, however, the enhancement in CIDP persists in periods of remission, with the nerve enlarging with every subsequent relapse.[14]
While demyelination secondary to viral and post-viral inflammatory processes may explain both recurrent ophthalmoplegia and MRI findings,[16][17] no evidence of viral infection (prodromal or concurrent) or immune-mediated neuropathy has been detected in cerebrospinal fluid (CSF) studies, which are typically normal in OM/RPON[14][3] and abnormal in entities like MS and CIDP.
Lastly, due to similar symptoms of recurrent unilateral headache and ophthalmoplegia, it has been proposed that OM/RPON may have a similar inflammatory pathophysiology to Tolosa-Hunt Syndrome (THS). However, MRI and computed tomography (CT) in OM/RPON do not demonstrate mass-like or other enhancement in the cavernous sinus, and marked enhancement of the cisternal portion of the oculomotor nerve has not been described in THS.[4][16]
Diagnosis
Signs and symptoms
Ocular Manifestations
Signs of OM/RPON are dependent upon the cranial nerve involved in the attack. While the oculomotor nerve is most commonly involved, involvement of the trochlear and abducens nerves has been reported.[4]
The typical presentation of OM/RPON is of a child with a history of episodic headaches that presents with ophthalmoplegia involving the distribution of third cranial nerve innervation.[6][19] In oculomotor nerve involvement, ptosis and limited medial, upward, and downward gaze can be expected.[4] Pupillomotor fibers are typically involved, resulting in residual mydriasis and decreased pupillary reflexes that may persist even after resolution of ophthalmoplegia.[4][2] In abducens nerve involvement, there is paralysis of abduction, while in trochlear nerve involvement, the patient will have limited downward and inward gaze and may tilt his or her head toward the contralateral shoulder.[4]
Headache
Lance and Zagami have proposed that the headache in OM/RPON occurs as a result of trigeminal sensory fiber irritation secondary to post-viral inflammation affecting the third nerve. This irritation then activates the trigeminovascular system in patients predisposed to migraines, triggering their headaches.[16]
This being said, according to the revised ICHD criteria, headache in OM/RPON does not necessarily have to be migranous, nor be accompanied by symptoms classically associated with migraine, such as nausea and vomiting.[20] Similarly, Gelfland et al found that in up to one-third of patients in their systematic review, headaches did not possess these classic characteristics of migraine. They further report that photophobia was present in 65% of cases, nausea in 66%, and vomiting in 69%.[3] Unlike in typical migraine, no published cases of OM/RPON have reported any visual, sensory, and speech, or language auras associated with OM/RPON.[6]
In another review of 84 cases of OM/RPON by Förderreuther and Ruscheweyh, the median age of first attack was 8 years of age. The unilateral headache was often most intense in the periorbital and/or retro-orbital region. Episodes began with a headache that lasted for several days and in some cases up to a week.[6] Their findings suggest that headache duration in OM/RPON is often longer than that of typical migraine, which lasts less than 72 hours.[3]
The interval between onset of headache and onset of ophthalmoplegia may range from immediate to up to 14 days. While headache usually resolves within a week, ophthalmoplegia may persist between 2 weeks and 3 months,[3] usually resolving completely over time. After recurrent bouts of OM/RPON, however, the resolution of symptoms may not be complete,[4] and patients can be left with residual eye movement deficits and ptosis. The time period between a first attack and its recurrence may vary from one week until as long as 5-7 years later.[6]
Physical examination
Physical examination should include
- Thorough ophthalmologic examination
- Thorough neurologic examination to identify the extent of neurological deficits
- Orbital examination to exclude orbital pathologies[4]
- Gonioscopy to rule out intermittent closed angle glaucoma with mydriasis[2]
Clinical diagnosis
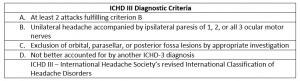
The diagnosis of OM/RPON is typically a clinical one, drawing from a history of past and presenting symptoms[21] and fulfillment of the most recent ICHD criteria (Table).[20] When obtaining history of symptoms, patients must be asked about head pain directly as it may not be mentioned as their chief complaint.[21]
Previously other authors have proposed that it should be classified as a syndrome due to the inconsistencies in presentation; both clinically, and on diagnostic imaging. Friedman has proposed classifying RPON as a syndrome that can be either “primary” or “secondary” Ophthalmoplegic Migraine as defined below. [23]
Primary Ophthalmoplegic Migraine 1.2.7 - Recurrent attacks of ICHD-II-defined migraine headache associated with paresis of one or more ocular cranial nerves (commonly the third nerve).
- At least two attacks fulfilling criteria B and C
- Headache accompanied or followed within 4 days of its onset by paresis of one or more of the third, fourth and/or sixth cranial nerves
- At least one episode of ophthalmoplegia resolves without therapeutic intervention
- The affected cranial nerve does not demonstrate contrast enhancement on neuroimaging studies during the attack
- Not attributed a parasellar, orbital fissure, posterior fossa lesion or other disorder.
Probable Primary Ophthalmoplegic Migraine - Recurrent attacks of ICDH-II-defined probable migraine headache associated with paresis of one or more ocular cranial nerves (commonly the third nerve).
- Diagnostic criteria and comment: same as 1.2.7.
Secondary Ophthalmoplegic Migraine 13.17 - Recurrent attacks of headache with ICHD-II-definedmigraine headache associated with paresis of one or more ocular cranial nerves (commonly the third nerve).
- At least two attacks fulfilling criteria B and C
- Headache accompanied or followed within 4 days of its onset by paresis of one or more of the third, fourth and/or sixth cranial nerves
- The affected nerve may demonstrate contrast enhancement on neuroimaging studies during the attack
- Not attributed a parasellar, orbital fissure, posterior fossa lesion or other disorder.
Probable Secondary Ophthalmoplegic Migraine - Recurrent attacks of ICDH-II-defined probable migraine headaches associated with paresis of one or more ocular cranial nerves (commonly the third nerve). [24]
Differential Diagnosis
The differential diagnosis of OM/RPON includes that of myasthenia gravis and painful ophthalmoplegia.
Myasthenia gravis may be ruled out if there is pupillary involvement and the presence of pain; however, rest or ice pack testing and edrophonium testing may be performed in the office if there is clinical suspicion.[2] Acetylcholine receptor antibodies and single fiber electromyography can be ordered for serological or electrophysiological confirmation of neuromuscular junction dysfunction.
Painful Ophthalmoplegia
The differential diagnoses of painful ophthalmoplegia include, but are not limited to, cavernous sinus syndromes (i.e., THS, neoplasms, aneurysm, thrombosis, fungal infection, carotid-cavernous fistula, granulomatosis with polyangiitis), brainstem ischemia, ischemic oculomotor nerve palsy, brainstem mass, brainstem multiple sclerosis lesion, oculomotor nerve schwannoma, diabetic oculomotor palsy, traumatic ocular motor nerve palsy, meningeal infection or inflammatory disease, Miller Fisher syndrome, idiopathic intracranial hypertension, thyroid ophthalmopathy, orbital mass, and narrow angle glaucoma.[4]
Many features of OM/RPON distinguish it from migraines associated with motor auras (i.e., complex migraine/hemiplegic migraine, migraine with brainstem aura). First, the onset of motor deficits in migraine with aura occurs up to 60 minutes before the headache and may even occur until the onset of headache itself.[20] In contrast, the order of symptoms is reversed in OM/RPON: headache typically precedes ophthalmoplegia by more than 24 hours.[4] This interval between the initial onset of headache and following ophthalmoplegia has been reported to be as long as 14 days.[3] Second, the duration of deficits in OM/RPON can be significantly longer than that in migraine with aura. The motor deficits of migraine with brainstem aura typically last up to an hour, while those of hemiplegic migraine may last up to 72 hours.[20] In OM/RPON, however, ophthalmoplegia can last from 2 weeks to 3 months.[3] Finally, unlike in migraine with aura, no published cases of OM/RPON have reported any symptoms of aura (visual, sensory, speech, language) within 60 minutes preceding the headache in OM/RPON.[6] Ophthalmoplegia may, however, occur immediately at the onset of headache in OM/RPON.[3]
Diagnostic Procedures
As OM/RPON is a diagnosis of exclusion, it is imperative to first rule out other conditions with similar presentations, such as vascular, neoplastic, infectious, and inflammatory causes.[4]
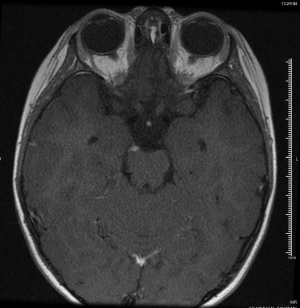
Imaging in the form of MRI or CT at a minimum should be performed to rule out orbital, parasellar, or posterior fossa lesions.[4][6] Mark et al were the first to describe oculomotor nerve enhancement and thickening on MRI in a patient with OM/RPON,[25] and a follow-up study confirmed the finding in 6 patients with OM/RPON.[17] Since then, numerous other studies have confirmed reversible cranial nerve enhancement in cases not just involving the oculomotor nerve, but the abducens and trochlear nerves as well.[19][26][27][28] While reversible focal enhancement and thickening of the cisternal portion of the oculomotor nerve can be seen, 25-81% of cases may have a normal MRI in the acute phase.[6] Enhancement and thickening of the third nerve usually disappear gradually as the patient’s symptoms subside, suggesting that the clinical symptoms may be associated with this radiologic finding.[17] The time it takes for MRI enhancement to disappear is variable: Mark et al reported near complete resolution of enhancement on follow-up studies 7-9 weeks after an attack,[25] while Prats et al reported resolution after a much longer period of 2-4 years.[29]
Magnetic resonance angiography is suggested to rule out vascular pathologies, such as aneurysm, especially when third nerve palsy involving pupillomotor function is concerning for a PCA aneurysm.[2]
Laboratory Studies
CSF studies and blood tests to rule out diabetes, infection, inflammation and other systemic disorders with central nervous system or peripheral nervous system involvement (i.e., myasthenia gravis, Miller Fisher syndrome) should be performed when these entities are suspected.[4][6] They should be negative in the setting of OM/RPON.
Management
Primary Prevention
Prophylaxis may be achieved with calcium channel blockers or beta-blockers[4]; however, the majority of authors assert that the only justification for initiating prophylactic therapy is if patients suffer from typical migraines with frequent attacks.[6] Otherwise, there is no evidence for initiating prophylaxis for OM/RPON.[6]
Medical Therapy
Currently, there are no randomized controlled trials of treatments for OM/RPON. While some patients do improve with corticosteroids, recovery is not as drastic as in other inflammatory conditions, such as THS or giant cell arteritis.[6] Gelfland et al reported that in their systematic review, steroids were used to treat 31% of patients. Although there was discernable improvement from steroid use, treatment and evaluation were unmasked in these cases.[3] In cases where ophthalmoplegia persists, botulinum injection or strabismus surgery may be considered.[30] Botulinum toxin as a beneficial therapy for persistent ophthalmoplegia in the setting of OM/RPON has been described by Manzouri et al. However, most evidence regarding the effectiveness of botulinum toxin in treating acute sixth nerve palsies suggests that while it provides symptomatic relief, long-term outcomes are not improved. Manzouri et al also describe a second patient with OM/RPON whose diplopia was successfully corrected with strabismus surgery.[31] Further studies are needed to specifically determine effectiveness of both botulinum toxin and strabismus surgery on the long-term outcomes of patients with OM/RPON.
Pareja et al presented 2 cases in with Indomethacin was used and saw symptomatic improvement within 24 hrs after starting the medication. [32]
Ghosh presented a case in which a child with RPON was given IVIG co-treatment w/ steroids and showed a complete resolution in symptoms. [33]
Zamproni et al presented a case in which typical migraine prophylaxis (Beta Blockers, Topiramate, and TCAs) had failed to show symptomatic improvement. Pregabalin was then used for treatment of RPON symptoms. The reasoning being that migraine prophylactics such as Valproate and Topiramate increase GABA, and Pregabalin, being a structural analog of GABA, might have an effect on the relieving the symptoms. The patient showed resolution of symptoms within 7 days and the pregabalin was continued for a year before being withdrawn. The patient has remained pain free for 2 years. 8 Patient fail prophylaxis with beta blockers, CCB, Topiramate, and TCAs was then started on 150mg pregabalin daily and showed remission of pain within 7 days. Pregabalin was continued for one year and patient has remained pain free for two years. [34]
Prognosis
Prognosis of OM/RPON is generally favorable due to the usually transient and self-resolving nature of the ophthalmoplegia. However, after multiple attacks, deficits may become persistent.[4] In Gelfand et al’s metanalysis, permanent deficits such as persistent mydriasis, ptosis, or tropias were found in 54% of patients on follow-up examinations.[3]
References
- ↑ Hansen SL, Borelli-Moller L, Strange P, Nielsen BM, Olsen J. Ophthalmoplegic migraine: diagnostic criteria, incidence of hospitalization and possible etiology. Acta Neurol Scand. 1990; 81:54–60.
- ↑ 2.0 2.1 2.2 2.3 2.4 Troost BT. Ophthalmoplegic migraine. Biomed Pharmacother. 1996;50(2):49-51.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Gelfand AA, Gelfand JM, Prabakhar P, Goadsby PJ. Ophthalmoplegic “migraine” or recurrent ophthalmoplegic cranial neuropathy: new cases and a systematic review. J Child Neurol. 2012;27(6):759-66.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 Levin M, Ward TN. Ophthalmoplegic migraine. Curr Pain Headache Rep. 2004;8(4):306-9.
- ↑ Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 Förderreuther S, Ruscheweyh R. From ophthalmoplegic migraine to cranial neuropathy. Curr Pain Headache Rep. 2015;19(6):21.
- ↑ Osuntoken O, Osuntoken BO. Ophthalmoplegic migraine and hemoglobinopathy in Nigerians. Am J Ophthalmol. 1972; 74(3):451-455.
- ↑ Walsh JP, O'Doherty DS. A possible explanation of the mechanism of ophthalmoplegic migraine. Neurology. 1960;10:1079-1084
- ↑ McMillian HJ, Keene DL, Jacob P, Humphreys P. Ophthalmoplegic migraine: Inflammatory neuropathy with secondary migraine? Can J Neurol Sci. 2007;34(3):349-355.
- ↑ Friedman AP, Harter DH, Merritt HH. Ophthalmoplegic migraine. Arch Neurol. 1962;7:320-327.
- ↑ 11.0 11.1 11.2 Vijayan N. Ophthalmoplegic migraine: Ischemic or compressive neuropathy? Headache. 1980;20(6):300-304.
- ↑ Milisavljević M, Marinković S, Lolić-Draganić V, Kovačević M. Oculomotor, trochlear, and abducens nerves penetrated by cerebral vessels: Microanatomy and possible clinical significance. Arch Neurol. 1986;43(1):58-61.
- ↑ Shin DJ, Kim JH, Kang SS. Ophthalmoplegic migraine with reversible thalamic ischemia shown by brain SPECT. Headache. 2002;42(2):132-135.
- ↑ 14.0 14.1 14.2 Ambrosetto P, Nicolini F, Zoli M, Cirillo L, Feraco P, Bacci A. Ophthalmoplegic migraine: from questions to answers. Cephalalgia. 2014;34(11):914-919.
- ↑ Akimoto J, Fukami S, Hashimoto R, Haraoka J. Neuromuscular hamartoma is a possible primary pathology of oculomotor ophthalmoplegic migraine. Cephalgia. 2012;32(2):171-174. doi:10.1177/0333102411431331
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 Lance JW, Zagami AS. Ophthalmoplegic migraine: a recurrent demyelinating neuropathy? Cephalalgia. 2001;21(2):84-89.
- ↑ 17.0 17.1 17.2 17.3 Mark AS, Casselman J, Brown D, et al. Ophthalmoplegic migraine: reversible enhancement and thickening of the cisternal segment of the oculomotor nerve on contrast-enhanced MR images. AJNR Am J Neuroradiol. 1998;19(10):1887-1891.
- ↑ Blake PY, Mark AS, Kattah J, Kolsky M. MR of oculomotor nerve palsy. AJNR Am J Neuroradiol. 1995;16(8):1665-1672.
- ↑ 19.0 19.1 O'Hara MA, Anderson RT, Brown D. Magnetic resonance imaging in ophthalmoplegic migraine of children. J AAPOS. 2001;5(5):307-310.
- ↑ 20.0 20.1 20.2 20.3 Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2013;33(9):629-808.
- ↑ 21.0 21.1 De Silva DA, Siow HC. A case report of ophthalmoplegic migraine: a differential diagnosis of third nerve palsy. Cephalalgia. 2005;25(10):827-830.
- ↑ Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2013;33(9):629-808.
- ↑ Recurrent Painful Ophthalmoplegic Neuropathy | MedLink Neurology. https://www.medlink.com/article/recurrent_painful_ophthalmoplegic_neuropathy. Accessed Februaruy 16, 2020.
- ↑ Friedman D. The ophthalmoplegic migraines: A proposed classification. Cephalalgia. 2010;30(6):646-647. doi: 10.1111/j.1468-2982.2009.02004.x
- ↑ 25.0 25.1 Mark AS, Blake P, Atlas SW, Ross M, Brown D,Kolsky M. Gd-DTPA enhancement of the cisternal portion of the oculomotor nerve on MR imaging. AJNR Am J Neuroradiol. 1992;13(5):1463-1470.
- ↑ Lee TG, Choi WS, Chung KC. Ophthalmoplegic migraine with reversible enhancement of intraparenchymal abducens nerve on MRI. Headache. 2002;42(2):140-141.
- ↑ Van der Dussen DH, Bloem BR, Liauw L, Ferrari MD. Ophthalmoplegic migraine: migrainous or inflammatory? Cephalalgia. 2004;24(4):312-315.
- ↑ Bharucha DX, Campbell TB, Valencia I, Hardison HH, Kothare SV. MRI findings in pediatric ophthalmoplegic migraine: a case report and literature review. Pediatr Neurol. 2007;37(1):59-63.
- ↑ Prats JM, Mateos B, Garaizar C. Resolution of MRI abnormalities of the oculomotor nerve in childhood ophthalmoplegic migraine. Cephalalgia. 1999;19(7):655-9.
- ↑ Granado LI, Guillen G. Treatment options for ophthalmoplegic migraine. J Postgrad Med. 2009;55(3):231.
- ↑ Manzouri B, Sainani A, Plant G, Lee J, Sloper J. The aetiology and management of long-lasting sixth nerve palsy in ophthalmoplegic migraine. Cephalalgia. 2007;27:275-278.
- ↑ Pareja J, Churruca J, de la Casa Fages B, de Silanes CL, Sanchez C, Barriga F. Ophthalmoplegic migraine. Two patients with an absolute response to indomethacin. Cephalalgia. 2010;30(6):757-760. doi: 10.1111/j. 1468-2982.2009.02003.x
- ↑ Ghosh PS. Recurrent Right-Sided Ptosis in a Child. JAMA Pediatr. 2015:169(7):693-694. doi: 10.001/jamapediatrics.2015.0587
- ↑ Zamproni LN, Ribeiro RT, Cardeal M. Treatment of Recurrent Painful Ophthalmoplegic Neuropathy: A Case Where Pregabalin Was Successfully Employed. Case Rep Neurol Med. 2019;2019:1-5. doi: 10.1155/2019/9185603