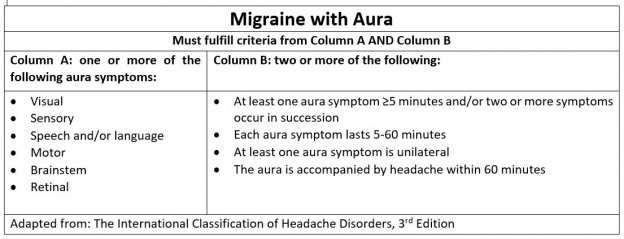
Ophthalmologic Manifestations of Migraines
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
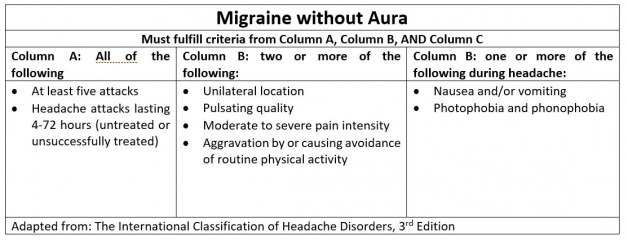
Migraine is a primary headache disorder commonly characterized by severe, unilateral (alternating hemicranias), throbbing pain with associated nausea, photophobia, phonophobia, and preceding vs post-dromal aura. Less commonly, migraines may present bilaterally with a moderate but constant pain.[1] They are typically 4-72 hours in duration and are often debilitating (see HIS criteria below). Migraine headaches can be episodic (occurring <15 days per month) or chronic (occurring >15 days per month), and a number of prodromal or post-dromal symptoms may also occur hours before or after a migraine headache occurs .[1][2]
The most common neuro-ophthalmologic symptoms are:
- Photophobia. Photophobia typically presents as light sensitivity, ocular discomfort, and/or headache exacerbation by light. It may affect visual and color perception and induce visual perceptual distortions. Photophobia is almost always bilateral in patients with migraine. It can be differentiated from photophobia in patients with trigeminal autonomic cephalalgias including hemicrania continua as those conditions more often present unilaterally, ipsilateral to the side of pain.[3]
- Visual aura[See Figure 1 and Figure 4]. The scotoma is a typical flickering, scintillating, crescent-shaped, fortifying, zig-zag scotoma that expands in the visual hemifield with march and build up. The visual aura is often unrelated to the side of head pain and can be heterogenous or pleomorphic.[3][4] The aura is typically bilateral and simultaneous as opposed to retinal vasospasm (“retinal migraine”) which is unilateral.

Visual aura of migraine. A. Starts with a small scotoma usually central that is scintillating, colored, fortifying and expanding towards the periphery (B–C) that eventually breaks up (D). The times shown represent minutes from the onset of the visual aura.
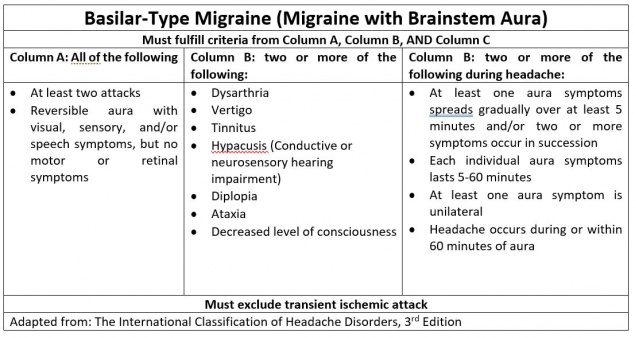
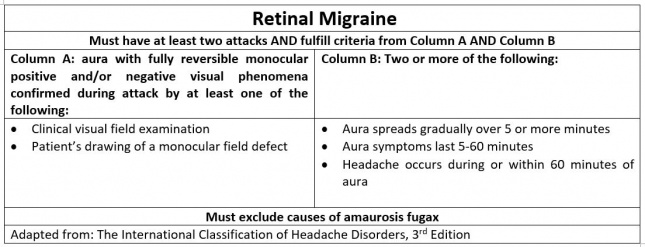
Migraine variants with neuro-ophthalmologic symptoms include aura without headache, basilar-type migraine, retinal migraine, and migraine with binocular blindness.
- Migraine aura without headache may present similarly to transient ischemic attack and occipital lobe seizures, especially when concurrent with negative features (i.e. hemianopia).
- Basilar-type migraine, or migraine with brainstem aura, is defined as a headache with neurological symptoms localized to the brainstem (i.e. dizziness, ataxia, tinnitus, hearing loss, syncope, etc.). A typical aura in both the temporal and nasal hemifields may be present, accompanied by diplopia.
- Retinal migraine (retinal vasospasm)[Figure 4] presents with typical migraine headache and a reversible monocular visual loss, scintillations, scotoma, or blindness.
- Migraine with binocular blindness that presents with characteristic migraine features with transient binocular visual loss.[3]
A rare but serious complication associated with migraine aura is migrainous infarction. Migrainous infarction is defined by the International Headache Society (IHS) as the presence of migraine aura symptoms associated with ischemic brain lesion(s), typically in the posterior circulation. A visual aura that resolves within minutes to hours is the most common symptom that occurs prior to an acute migrainous infarct.[5] Although a direct causative link has not been identified, an increased frequency of migraine attacks is correlated with an increased risk of ischemic stroke.[6]
A rare but serious complication associated with migraine aura is migrainous infarction. Migrainous infarction is defined by the International Headache Society (IHS) as the presence of migraine aura symptoms associated with ischemic brain lesion(s), typically in the posterior circulation. A visual aura that resolves within minutes to hours is the most common symptom that occurs prior to an acute migrainous infarct.[5] Although a direct causative link has not been identified, an increased frequency of migraine attacks is correlated with an increased risk of ischemic stroke.[6]
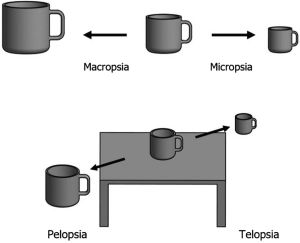
Less common visual manifestations include visual hallucinations, illusions, distortions, and persistent positive visual phenomena. Hallucinations and illusions as a sequela of migraines can present as distorted perception of size, movement, or general characteristics of images, without disturbances in language or motor activity. Specifically, patients may retain an image of an object that is no longer in the field of view, a condition referred to as palinopsia. It is more common in patients who experience migraine with aura compared to migraines without aura.[3] In contrast, a more continuous visual disturbance is persistent positive visual phenomena, or visual snow. Visual snow consists of diffuse small particles, such as TV static, rain, dots, etc. in the total visual field that can persist for years.[1][7] Visual snow is the pathognomonic characteristic of visual snow syndrome (VSS), of which, 60% of patients experience comorbid migraines with or without aura .[8][9] A less prevalent, but possible complication of migraine includes Alice in Wonderland Syndrome (AIWS). Common visual symptoms of Alice in Wonderland Syndrome include pelopsia, telopsia, micropsia, macropsia, and hallucinations. Patients may also experience perceptual disturbances such as microsomatognosia or macrosomatognosia [Figure 2], depersonalization, or auditory hallucinations.
Perfusion scans have shown an association between AIWS and insufficient perfusion of the visual tracts and occipital lobe; however, MRI, CT, and evoked potential tests showed no significant changes.[10] Because this syndrome is widely varied in its presentation, no universally accepted diagnostic criteria exist.[11]
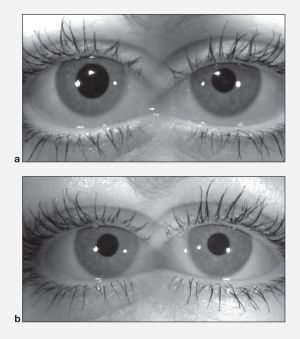
Additional, although rare, Benign Pupillary Dilation (Benign episodic unilateral mydriasis -BEUM , or "bilateral" BE) [Figure 3] can be seen with migraines, causing intermittent blurry vision and symptoms of "mental fogginess" and confusion. These findings are secondary to hyperactivity of the sympathetic nervous system and/or hypoactivity of the parasympathetic system.[12]
Etiology
The etiology for migraine remains under study but some common mechanisms for the migraine headache pain have been proposed including irritation of the trigeminal nerve, meninges, and/or blood vessels. Lifestyle factors may trigger migraine development such as menstrual cycles (catamenial migraines), stress, and irregular sleep patterns.[1] Possible dietary triggers of migraine include alcohol (especially red wine and beer), monosodium glutamate (MSG), caffeine, dairy products (aged cheeses, food preservatives), artificial sweeteners (such as aspartame) and chocolate.[13][14] Medications including oral contraceptives, estrogen hormone therapy, nasal decongestants, opioids, and selective serotonin-reuptake inhibitors can exacerbate migraines.[1] Other factors that may trigger migraine headaches include weather changes and sensory stimuli, including certain noises, smells, and visual disturbances.[15] Light is the most common reported trigger for the development of aura and photophobia, and can worsen acute migraine.[3]
Risk Factors
Several migraine-associated risk factors have been proposed including female sex, obesity, diabetes, head injury, stress, less than high school education, and lower household income.[16] Risk factors associated with the progression of episodic to chronic migraines include acute migraine medication overuse (>15 days per month of analgesics or >10 days per month of triptans) and ineffective treatment of acute migraine attacks. Proposed mechanisms of the latter association involve sensitization processes due to prolonged exposure to headaches or increased frequency or dosage of migraine medication.[17] Although a Mendelian pattern of inheritance has not been identified in the development of migraines, the importance of genetic factors has been reported in the development of aura.[18]
Although not explicitly defined as risk factors, subtypes of migraines with neuro-ophthalmologic symptoms are associated with certain demographics. Visual aura without headache is more common in older patients with history of migraine or in patients who previously experienced aura with migraine. Basilar-type headache typically occurs in adolescent and young adult women. Retinal migraine is more common in young adult females with a history of aura. Because migraine with binocular blindness is rare, little evidence is available to define risk factors. Proposed risk factors include abnormalities in the coagulation cascade (i.e., polymorphisms in MTHFR C677T) as well as female gender.[3] Patients with migraine with binocular blindness typically do not have a history of aura.[19] Similarly, migrainous infarction is more likely to occur in females under the age of 45 years old, who use oral contraception and tobacco products. Genetic predisposition to hyperactivity of vessels, endothelial dysfunction, and clotting disorders also increase risk for this subtype.[6] In contrast to the typical female predominance, AIWS is more common in young males (5-14 years old) and in adolescent and young adult females (16-18 years old).[11]
Pathophysiology
Migraine has been hypothesized to be caused by irritation of the meninges, blood vessels, or regions supplied by the trigeminal nerve. Substance P, nitrous oxide, and calcitonin gene-related peptide have all been implicated in the possible pathophysiology of migraine as they contribute to plasma extravasation, neurogenic inflammation, and vasodilation. Other structures involved in migraine pathophysiology include the periaqueductal gray (PAG), locus coeruleus (LC), and dorsal raphe nucleus (DRN).[3][20]
The specific pathophysiology has not been elucidated for all subtypes of migraine. Basilar-type headache is presumed to be due to cortical spreading depression in the brainstem. Basilar artery pathology has not been associated with basilar-type headache. Retinal migraine is also hypothesized to involve cortical spreading depression in the retina.[3] Due to the rare occurrence of migraine with binocular blindness, the underlying pathophysiology is unclear. Leading hypotheses to describe this pathology include an underlying clotting disorder, retinal spreading depression, or activation of the retinal-thalamic-visual cortex pathway.[3][19]
Similarly, the mechanisms underlying neuro-ophthalmologic symptoms, such as AIWS, photophobia, aura, hallucinations, and persistent positive visual phenomena, of migraines are still under investigation. AIWS secondary to migraines may involve transient ischemia of the visual tracts.[21] Other proposed mechanisms include hypoperfusion of the frontoparietal and frontal areas, frontal lobe epilepsy seizure, and focal frontal lobe infarct.[11]
In contrast, photophobia in migraine may originate in cone-driven retinal pathways and involve hyperexcitation of light-sensitive thalamic neurons and the cortex.[3][22] It is possible that photophobia is exacerbated when patients are exposed to different wavelengths of visible light (i.e., green light stimulates less than white light).[22] Visual aura is caused by cortical spreading depression characterized by neuronal depolarization, leading to a disruption of iron gradients and loss of membrane resistance. Increased potassium concentrations promote the spread of depolarization across neural tissue and leads to release of excitatory amino acids that potentiate the spread. However, the mechanism of cortical spreading depression initiation has not been fully elucidated.[3]
The pathophysiology of hallucinations in migraines remains uncertain. It has been linked to dysfunction in the parietal lobe coordinate systems and activation of the occipitotemporal region of the nondominant hemisphere.[3]
Lastly, patients with persistent positive visual phenomena (such as visual snow) typically have hypermetabolism in the supplementary visual cortex (lingual gyrus) of Brodmann area 19 demonstrated on imaging.[23] Hypermetabolism of the primary visual cortex was not visualized, therefore it was suggested that processing of higher visual order was affected instead of the upstream visual input.[24]
Diagnosis
Signs & Symptoms
Visual aura without migraine has a variable presentation and may have symptoms similar to transient ischemic attack and occipital lobe seizures. The aura may be of atypical duration, or is accompanied by negative features such as hemianopia.
The basilar-type headache often presents with dizziness, dysarthria, ataxia, tinnitus, hearing loss, bilateral paresthesia, altered consciousness, syncope, which are indicative of symptoms involving the brainstem.[3] These headaches may also be associated with bilateral visual impairment.[19]
Retinal migraine presents with reversible monocular visual loss, "C" shape, colored, scintillating and expanding scotomas, and typically presents in patients with a history of migraine with aura.[3]
Similarly, migraine with binocular blindness presents with transient vision loss that may be irreversible, usually in patients with no history of aura .[3] There are case reports illustrating that during the migranous aura the patients can get an abnormal automated visual field defect that resolves after cessation of migraine[25].
Photophobia presents with abnormal sensitivity to light, ocular discomfort, and exacerbation of headache by light. It is typically bilateral. Patients experiencing aura will describe a bright crescent shape in the visual hemifield. It may be of a flickering quality. Hallucination is the perception of viewing objects that are not present in the visual field, which differs from persistent positive visual phenomena. The latter can present as continuous, white and black dots in the entire visual field that can persist for years. Patients may also complain of seeing floaters or halos, and symptoms may be accompanied by palinopsia, photophobia, and phosphenes.[3] Palinopsia specifically may be triggered by medications such as trazodone, topiramate, mirtazapine, zosuquidar, and sumatriptan.[26][27][28]
Physical Examination
A general physical examination of an adult presenting with headache includes obtaining vital signs, auscultating bruits for signs of arteriovenous malformation, palpating the head and neck, examining temporal and neck arteries and veins, and examining the neck and spine muscles. A neurological examination is also warranted if patients present with focal deficits.
Patients who additionally present with ocular manifestations could undergo a complete ocular examination. Physicians could initially inspect the orbit and surrounding structures and assess visual acuity and visual fields, extraocular movement, and pupillary response to light. A fundoscopic examination and a slit lamp examination is also recommended. The eye exam in typical migraine however should be normal including visual field testing.
Clinical Diagnosis
The clinical diagnosis of migraine is based on patient history, physical examination, and fulfillment of specific ICHD-3 diagnostic criteria (see below).
Clear diagnostic criteria for AIWS and migraine with binocular blindness do not currently exist.[3][11] Proposed criteria for AIWS include: one or more episodes of self-experienced body schema illusion or metamorphopsia, duration lasting longer than 30 minutes, symptoms of headache or a history of migraine, normal MRI, CSF, and EEG (although visual evoked potentials may be abnormal).[29]
Diagnostic Procedures & Laboratory Tests
Diagnosis of migraine is based on patient history and physical exam findings (clinical signs) as specific diagnostic tests do not exist. Imaging is not typically indicated in patients with typical migraine symptoms. It may be considered in patients who present with unexplained abnormal neurological exam findings or with atypical headache features (onset of headache after 40 years of age, progressive worsening of symptoms, suspicion of migrainous infarction, etc.). A sudden, severe headache necessitates imaging to rule out subarachnoid hemorrhage. The preferred imaging study for an acute intracranial hemorrhage is a CT (computed tomography) scan without contrast when indicated. If posterior fossa lesions or CSF leaks are suspected, an MRI (magnetic resonance image) is warranted.[3]
Differential Diagnosis
The differential diagnosis of migraine includes other primary causes of headache such as tension headache and cluster headache. It also includes headaches secondary to other conditions such as trauma, infection, congenital disorders, or cerebrovascular disorders. Specifically, the differential diagnosis for migraine with visual aura includes TIA (transient ischemic attack) and seizure. Migraine with visual aura may be differentiated from TIA or seizure due to the more characteristic gradual onset, duration and quality of the aura and associated nausea, vomiting, phonophobia and photophobia.
Management
Primary Prevention
General prophylactic measures for migraine include lifestyle modification (diet, physical activity), avoidance of triggers, and medications including tricyclic antidepressants (TCAs), certain anti-epileptics agents, beta blockers, calcium channel blockers (CCBs).[3] Botulinum toxin injections have recently been approved for the prophylactic treatment of chronic migraine as the toxin leads to reduced headache days and is generally well tolerated.[30] While not the standard, magnesium administration and riboflavin administration also may be effective.[31][32][33] A randomized control trial evaluated the efficacy of magnesium as a prophylactic agent. The trial demonstrated a reduction in attack frequency by 41.6% in the experimental group (treated with high dose oral magnesium) versus a 15.8% reduction in the placebo group compared to baseline.[33] Riboflavin may also aid in the reduction in migraine headache frequency.[33] A systematic review of 11 clinical trials, suggested a therapeutic benefit whereas the rest showed mixed results or no benefit.[33] Although patients have reported prophylactic success with magnesium or riboflavin, more robust data are needed. Prophylaxis for migraine is primarily dependent on medication side effect profiles and specific patient co-morbidities. Selection of prophylactic agents may be particularly difficult in preventing symptoms such as palinopsia that has a multifaceted etiology.
Medical Therapy
In the acute setting, migraines are managed with analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs). These medications work by blocking inflammation via the arachidonic pathway. Caffeine can be used as an adjuvant to enhance the effects of analgesics for acute treatment of migraine headaches if used in appropriate doses.[34] If symptoms are not relieved, treatment with triptans may be used. Triptans act as agonists to the 5-hydroxytryptamine-1B/1D (5-HT-1B/1D) receptors and cause vasoconstriction of the intracranial blood vessels and inhibition of the pain pathways involving the trigeminal nerve. Given their vasoconstriction properties, triptans are contraindicated in the setting of coronary artery disease and ischemic stroke.[35][36] Ergotamine compounds work similarly and have been indicated, but are used less frequently due to their side effect profile.[3] Because triptans and ergotamines both stimulate serotonergic receptors in the cerebral and coronary vasculature, it is contraindicated to administer them within 24 hours of each other.[36] Data for this recommendation are limited; however, cardiac events in patients using these agents (i.e. myocardial infarction, stroke) have been reported.[36]
In select patients, corticosteroid therapy has demonstrated efficacy as abortive therapy in acute migraine attacks. Patients experiencing refractory headaches, severe baseline pain intensity, and status migrainosus had the most beneficial response.[37]
Although not a standard treatment, magnesium sulfate has demonstrated efficacy as a prophylactic agent (see ‘Primary Prevention’) and an abortive therapy in the setting of migraine. A randomized control trial with n=30 was conducted to evaluate the efficacy of IV magnesium sulfate in the treatment of migraines. The experimental group (n=15) was treated with IV magnesium sulfate. N=13 patients (86.6%) experienced complete resolution of pain and the remaining 2 patients (13.4%) experienced a reduction in pain intensity. Accompanying symptoms (nausea, irritability, photophobia, etc.) in the experimental group resolved completely in all patients. In the control group, administration of IV saline (placebo) resulted in a reduction of pain intensity in n=1 patient (6.7%), while the remaining n=14 (93.3%) patients remained at their baseline pain intensity. All patients in the control group were subsequently treated with IV magnesium sulfate and n=14 patients (93.3%) had a complete resolution of pain, while the remaining n=1 patient reported reduced pain.[31] While this study provides convincing evidence of the therapeutic benefit of magnesium administration, larger-scale trials are indicated.
Medical Follow Up
Patients who experience recurrent attacks of migraines are advised to follow up with their physicians for prophylactic therapy. Doses of these preventative medications can be titrated at follow-up visits as necessary. It is also recommended that patients follow up if they continue to experience migraine attacks despite pharmacologic intervention. It is important to closely follow patients who present with retinal migraine to prevent irreversible blindness.[3]
Surgery
Surgery is not typically indicated for migraine. A proposed prophylactic surgical intervention is removal of nerve tissue or muscle in patients with moderate to severe and frequent migraines. This proposal followed a randomized control trial that involved removal of the glabellar muscles from patients with frontal trigger sites, removal of the zygomaticotemporal branch for temporal trigger sites, and partial removal of the semispinalis capitus muscle for occipital trigger sites in the experimental group (n=49). The control group (n=26) involved a sham surgery. This study demonstrated improvement in the experimental group, with complete cessation of migraines in 57% of experimental patients compared to 4% of control patients.[38]
Additionally, surgery involving resection of the corrugator supercilia muscle has been reported for long-term relief. The result is decompression of supra-trochlear and supra-orbital nerves with avulsion of zygomaticotemporal branch of the trigeminal nerve. The study involved n=30 volunteers. There were no controls used in this study. Migraine reduction was assessed via pre- and post-surgery questionnaires. The average number of migraines decreased from 15.2±6.3 episodes per month to 1.9±2.4 episodes per month. Two patients had no significant improvement after surgery.[39]
Although both studies endorse improvement of migraine symptoms, these techniques have not been universally accepted as an effective prophylactic measure as more rigorous trials are necessary.
Complications
General migraine complications include attacks with prolonged symptoms, infarction, or seizure. Prolonged symptoms may include status migrainosus or persistent aura without infarction. Status migrainosus lasts greater than 72 hours and is characterized by a debilitating migraine attack, and has shown high likelihood of progressing to chronic migraine.[40] Persistent aura without infarction involves aura symptoms that last for more than one week without evidence of infarction on neuroimaging. Migraine with aura may also trigger a seizure, a rare but possible complication; however, recurrence rates of migraine with aura may be lower after migrainous infarction.[41] For migraine attacks that last for greater than one hour, migrainous infarction is a feared complication. Neuroimaging reveals infarction of the involved area of the brain and physical exam findings are consistent with focal deficits.[3]
Specific migraine subtypes are associated with unique long-term sequelae. Basilar-type migraine symptoms are usually fully reversible when they present as individual attacks. Coma and death are possible but are very rare occurrences. Focal and generalized seizures may result, especially when basilar-type migraines are present in children.[42] In contrast, retinal migraine can result in irreversible visual loss and/or persistent cutaneous allodynia. Possible etiologies include migrainous infarction or vasospasm and ischemia. Predictors of this long-term complication are unknown.[3][43] Although migraine with binocular blindness presents with vision loss, it most often occurs transiently without recurrence. Studies have indicated visual loss to last between one to ten minutes. Long-term complications are unknown due to the rarity of this migraine variant.[19] Lastly, AIWS may result in perceptual disturbances that are typically benign. However, symptoms may recur with onset of migraine in chronic cases.[44]
Prognosis
Relapse has been implicated in chronic migraine. Certain factors have been proposed as predictors of poor prognosis in migraine. These include psychological contributors (such as depression, stress, and sleep) and migraine characteristics (such as duration and lack of response to prophylaxis and medical intervention).[3][45] The prognosis of migraine variants has not been fully elucidated due to limited data. Typically, migraines are managed with prophylactic therapy and individual migraine attacks can be controlled with pharmacologic intervention. Rarely, migraine variants, such as the basilar-type, may transform into other types.[3]
Additional Resources
- Boyd K, DeAngelis KD. Migraine. American Academy of Ophthalmology. EyeSmart/Eye health. https://www.aao.org/eye-health/diseases/migraine. Accessed March 18, 2019.
- Dr Kathleen Digre discusses why all ophthalmologists should take a closer look at migraines. Available at AAO website, posted Nov 22, 2017 https://www.aao.org/interview/migraines-more-than-meets-eye-2, last accessed Aug 11, 2022.
- Visual snow simulator [1]
- Patient Information Brochure. North American Neuro Ophthalmology Society (NANOS) https://www.nanosweb.org/migraine_information_brochure accessed March 3, 2025
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Charles A. Migraine. N Engl J Med. 2017 Aug; 377:553-561. doi: 10.1056/NEJMcp1605502.
- ↑ Katsarava Z, Buse DC, Manack AN, Lipton RB. Defining the Differences Between Episodic Migraine and Chronic Migraine. Current Pain and Headache Reports. 2012;16(1):86-92. doi:10.1007/s11916-011-0233-z.
- ↑ Jump up to: 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 Schwartz DP, Robbins MS. Primary headache disorders and neuro-ophthalmologic manifestations. Eye and Brain. 2012; 4:49-61. doi:10.2147/EB.S21841.
- ↑ Cutrer FM, Huerter K. Migraine aura. Neurologist. 2007 May;13(3):118-25. Review. PubMed PMID: 17495755.
- ↑ Jump up to: 5.0 5.1 Campagna G, Vickers A, Ponce CMP, Lee AG. Homonymous hemianopsia as the presenting sign of migrainous infarction. Canadian Journal of Ophthalmology. 2018.
- ↑ Jump up to: 6.0 6.1 6.2 Kreling GAD, de Almeida NR, dos Santos PJ. Migrainous infarction: a rare and often overlooked diagnosis. Autopsy & Case Reports. 2017; 7(2):61-68. doi:10.4322/acr.2017.018.
- ↑ Schankin CJ, Goadsby PJ. Visual snow, persistent positive visual phenomenon distinct from migraine aura. Curr Pain Headache Rep. 2015 Jun;19(6):23. doi: 10.1007/s11916-015-0497-9. Review.
- ↑ Puledda F, Schankin C, Digre K, Goadsby PJ. Visual snow syndrome: what we know so far. Current opinion in neurology. 2018;2017;31:52.
- ↑ White, O. B., Clough, M., McKendrick, A. M., & Fielding, J. Visual snow: Visual misperception. Journal of Neuro-Ophthalmology : The Official Journal of the North American Neuro-Ophthalmology Society (2018)1. doi:10.1097/WNO.0000000000000702
- ↑ Asensio-Sánchez VM. Alice in Wonderland syndrome. Archives of the Spanish Society for Ophthalmology (Archivos de la Sociedad Española de Oftalmología, English Edition). 2011;2014;89:77-78.
- ↑ Jump up to: 11.0 11.1 11.2 11.3 Mastria G, Mancini V, Viganò A, Di Piero V. Alice in Wonderland Syndrome: A Clinical and Pathophysiological Review. BioMed Research International. 2016; 2016:8243145. doi:10.1155/2016/8243145.
- ↑ Skeik N, Jabr FI. Migraine with benign episodic unilateral mydriasis. Int J Gen Med. 2011;4:501-503. doi:10.2147/IJGM.S18613
- ↑ Panconesi A. Alcohol and migraine: trigger factor, consumption, mechanisms. A review. J Headache Pain. 2008;9(1):19-27. doi:10.1007/s10194-008-0006-1.
- ↑ Zaeem Z, Zhou L, Dilli E. Headaches: a Review of the Role of Dietary Factors. Curr Neurol Neurosci Rep. 2016 Nov;16(11):101. Review.
- ↑ Martin PR. Behavioral Management of Migraine Headache Triggers: Learning to Cope with Triggers. Current Pain and Headache Reports. 2010;14:221-227.
- ↑ Lipton RB, Bigal ME. Migraine: Epidemiology, Impact, and Risk Factors for Progression. Headache. 2005 Apr; 45 Suppl 1:S3-S13. doi: 10.1111/j.1526-4610.2005.4501001.x.
- ↑ May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016 Aug;12(8):455-64. doi: 10.1038/nrneurol.2016.93. Epub 2016 Jul 8. Review.
- ↑ Pisanu C, Preisig M, Castelao E, et al. A genetic risk score is differentially associated with migraine with and without aura. Human Genetics. 2017; 136(8):999-1008. doi:10.1007/s00439-017-1816-5.
- ↑ Jump up to: 19.0 19.1 19.2 19.3 Rozen TD. Migraine with binocular blindness: a clinic-based study. Headache. 2011 Nov-Dec; 51(10):1529-36. doi: 10.1111/j.1526-4610.2011.01968.x. Epub 2011 Aug 3.
- ↑ Costa C, Tozzi A, Rainero I, et al. Cortical spreading depression as a target for anti-migraine agents. J Headache Pain. 2013;14(1):62. doi:10.1186/1129-2377-14-62.
- ↑ 21 Camacho Velasquez JL, Rivero Sanz E, Tejero Juste C, Suller Marti A. Alice in Wonderland syndrome in cerebrovascular disease. Neurologia. 2016 Jul-Aug; 31(6):418-20. doi: 10.1016/j.nrl.2014.09.009. Epub 2014 Nov 20. English, Spanish.
- ↑ Jump up to: 22.0 22.1 Noseda R, Bernstein CA, Nir R-R, et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 2016; 139(7):1971-1986. doi:10.1093/brain/aww119.
- ↑ Schankin CJ, Maniyar FH, Sprenger T, Chou DE, Eller M, Goadsby PJ. The relation between migraine, typical migraine aura and "visual snow". Headache. 2014 Jun; 54(6):957-66. doi: 10.1111/head.12378. Epub 2014 May 9. Erratum in: Headache. 2015 Apr;55(4):592.
- ↑ Schankin CJ, Viana M, Goadsby PJ. Persistent and Repetitive Visual Disturbances in Migraine: A Review. Headache. 2017 Jan; 57(1):1-16. doi: 10.1111/head.12946. Epub 2016 Oct 7. Review.
- ↑ Morris, James et al. Case Illustrating the Visual Field Effects of a Migraine Aura. Ophthalmology, Oct 2024 DOI: 10.1016/j.ophtha.2024.08.010
- ↑ Hughes MS, Lessell S. Trazodone-induced palinopsia. Arch Ophthalmol. 1990 Mar;108(3):399-400.
- ↑ Yun SH, Lavin PJ, Schatz MP, Lesser RL. Topiramate-induced palinopsia: a case series and review of the literature. J Neuroophthalmol. 2015 Jun;35(2):148-51. doi: 10.1097/WNO.0000000000000216. Review.
- ↑ Abert B, Ilsen PF. Palinopsia. Optometry. 2010 Aug;81(8):394-404. doi:10.1016/j.optm.2009.12.010.
- ↑ Valença MM, de Oliveira DA, Martins HA. Alice in Wonderland Syndrome, Burning Mouth Syndrome, Cold Stimulus Headache, and HaNDL: Narrative Review. Headache. 2015 Oct; 55(9):1233-48. Review.
- ↑ 28 Escher CM, Paracka L, Dressler D, Kollewe K. Botulinum toxin in the management of chronic migraine: clinical evidence and experience. Therapeutic Advances in Neurological Disorders. 2017;10(2):127-135. doi:10.1177/1756285616677005.
- ↑ Jump up to: 31.0 31.1 Demirkaya S, Vural O, Dora B, Topçuoğlu MA. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache. 2001 Feb;41(2):171-7.
- ↑ Peikert A, Wilimzig C, Köhne-Volland R. Prophylaxis of migraine with oral magnesium: results from a prospective, multi-center, placebo-controlled and double-blind randomized study. Cephalalgia. 1996 Jun;16(4):257-63.
- ↑ Jump up to: 33.0 33.1 33.2 33.3 Thompson DF, Saluja HS. Prophylaxis of migraine headaches with riboflavin: A systematic review. J Clin Pharm Ther. 2017 Aug;42(4):394-403. doi:10.1111/jcpt.12548. Epub 2017 May 8. Review.
- ↑ Lipton RB, Diener H-C, Robbins MS, Garas SY, Patel K. Caffeine in the management of patients with headache. The Journal of Headache and Pain. 2017;18(1):107. doi:10.1186/s10194-017-0806-2.
- ↑ Capi M, Curto M, Lionetto L, de Andrés F, Gentile G, Negro A, Martelletti P. Eletriptan in the management of acute migraine: an update on the evidence for efficacy, safety, and consistent response. Ther Adv Neurol Disord. 2016 Sep;9(5):414-23. doi: 10.1177/1756285616650619. Epub 2016 Jun 3. Review.
- ↑ Jump up to: 36.0 36.1 36.2 Jamieson DG. The safety of triptans in the treatment of patients with migraine. Am J Med. 2002 Feb 1;112(2):135-40. Review.
- ↑ Woldeamanuel YW, Rapoport AM, Cowan RP. What is the evidence for the use of corticosteroids in migraine? Curr Pain Headache Rep. 2014 Dec;18(12):464. doi:10.1007/s11916-014-0464-x. Review.
- ↑ Guyuron B, Reed D, Kriegler JS, Davis J, Pashmini N, Amini S. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009 Aug; 124(2):461-8. doi: 10.1097/PRS.0b013e3181adcf6a.
- ↑ Jose A, Nagori SA, Roychoudhury A. Surgical Management of Migraine Headache. J Craniofac Surg. 2017 Oct 24. doi: 10.1097/SCS.0000000000004078.
- ↑ Singh TD, Cutrer FM, Smith JH. Episodic status migrainosus: A novel migraine subtype. Cephalalgia : an international journal of headache. 2018;38:304-311.
- ↑ Serrano F, Arauz A, Uribe R, Becerra LC, Mantilla K, Zermeño F. Long-term follow-up of patients with migrainous infarction. Clinical Neurology and Neurosurgery. 2018;165:7-9.
- ↑ Blumenfeld AE, Victorio MC, Berenson FR. Complicated Migraines. Semin Pediatr Neurol. 2016 Feb; 23(1):18-22. doi: 10.1016/j.spen.2016.01.007. Epub 2016 Jan 22. Review.
- ↑ Grosberg BM, Solomon S, Friedman DI, Lipton RB. Retinal migraine reappraised. Cephalalgia. 2006 Nov; 26(11):1275-86.
- ↑ Blom JD. Alice in Wonderland syndrome: A systematic review. Neurology: Clinical Practice. 2016;6(3):259-270. doi:10.1212/CPJ.0000000000000251.
- ↑ Probyn K, Bowers H, Caldwell F, et al. Prognostic factors for chronic headache: A systematic review. Neurology. 2017;89(3):291-301. doi:10.1212/WNL.0000000000004112.