Cyclodialysis Clefts
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
A cyclodialysis cleft is a separation of the ciliary body from the scleral spur, creating a direct connection between the anterior chamber and the suprachoroidal space. Many will spontaneously close, but those that do not can cause chronic hypotony, resulting in hypotony maculopathy, optic disc edema, and decreased visual acuity. Treatment begins with conservative medical therapy, but when this fails, a wide range of laser and surgical procedures have been reported to be effective.
Disease Entity
Etiology/ Pathophysiology
A cyclodialysis cleft is formed when the longitudinal fibers of the ciliary muscle separate from the scleral spur, forming a direct connection between the anterior chamber and the suprachoroidal space. This dramatically increases aqueous outflow and predisposes the eye to hypotony. The most common causes of cyclodialysis clefts are trauma or following various intraocular surgeries including trabeculectomy, trabeculotomy, goniotomy, phacoemulsification extracapsular cataract extraction, secondary intraocular lens (IOL) placement, phakic IOL removal, and displacement of an anterior-chamber IOL.[1] [2][3] In modern surgical practice, creation of a cyclodialysis cleft is rarely intentional; it was once, however, an accepted intervention for open-angle and aphakic glaucoma.[4]
Epidemiology
Cyclodialysis cleft formation is fairly rare, even after blunt trauma. In one retrospective case series of 145 eyes, 6.9% developed hypotony after blunt trauma, but a cyclodialysis cleft was present in only 3 eyes (2%). Other causes of hypotony in the series included traumatic retinal detachment and anterior proliferative vitreoretinopathy. Amongst the 3 eyes with a cyclodialysis cleft, 2 developed clinically significant hypotony.[5] The frequency of cyclodialysis cleft formation after surgery is unknown, but is likely extremely rare for most procedures.
Diagnosis
Presentation
Hypotony (IOP ≤ 5 mmHg) after surgery or blunt ocular trauma, especially associated with hyphema or iris sphincter tears, should raise suspicion for a cyclodialysis cleft. In some cases, a previously resolved cleft may reopen during surgery, so even a remote history of ocular trauma obtained preoperatively is noteworthy.[6] Secondary effects of hypotony can include hypotony maculopathy, optic disc edema, and corneal edema, all of which can contribute to loss of visual acuity.[1][7] [8] [9] Blurred vision may also result from irregular astigmatism caused by blinking on a hypotonous globe. Clefts, by definition, result in suprachoroidal fluid as a result of increased aqueous outflow into the suprachoroidal space.[5] But, visible choroidal detachment and anterior chamber shallowing are uncommon findings despite the increased egress of aqueous through the cleft.
Physical Examination
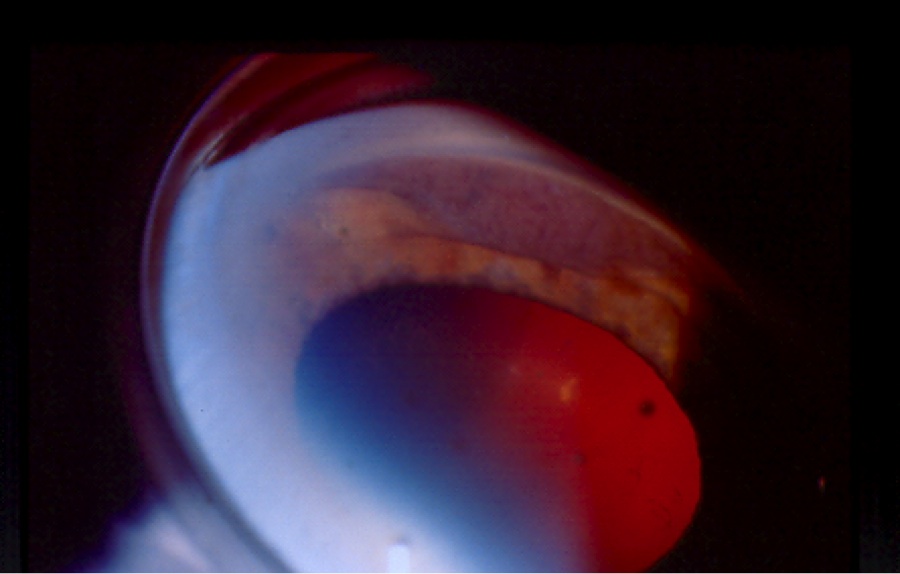
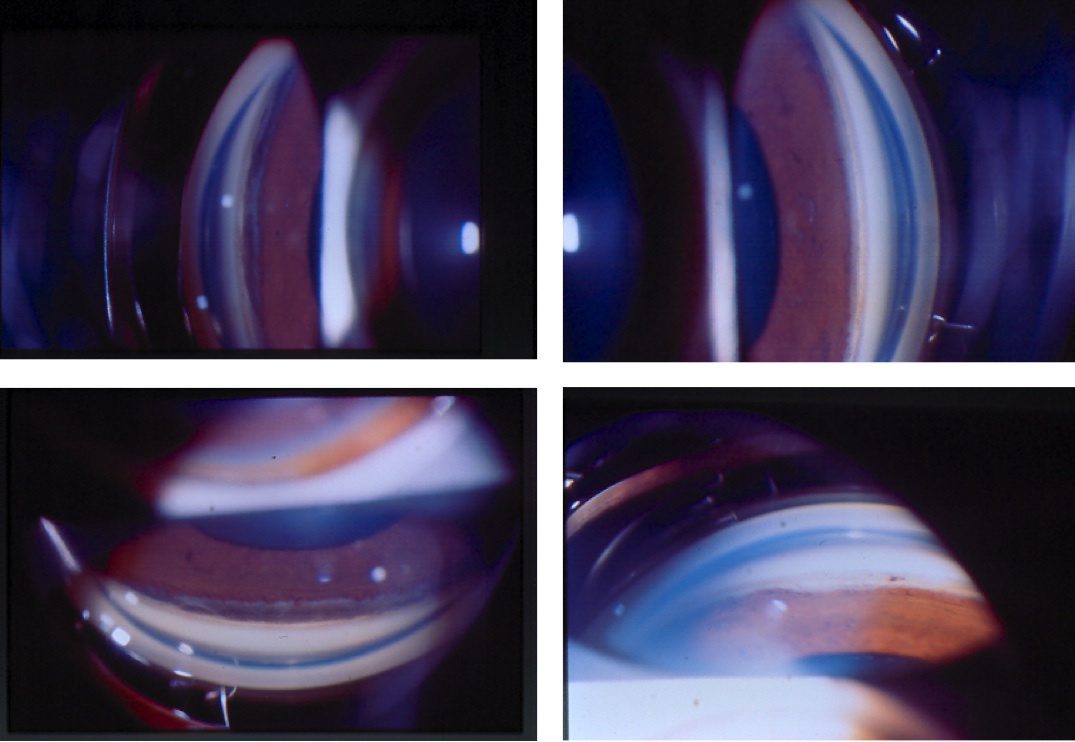
On gonioscopy, a cyclodialysis cleft is visible as an abnormal region posterior to the scleral spur displacing the iris root and ciliary body posteriorly. The appearance of the cleft region can vary considerably, and can look white (like sclera), black, or gray (Figure 1(a) & (b)). Clinical evaluation by gonioscopy is often difficult in a soft eye with a shallow or flat anterior chamber, and can be further impaired by corneal folds/edema or hyphema after trauma. Injection of viscoelastic into the anterior chamber, with or without application of topical pilocarpine to maximally open the angle, can improve the view. Sometimes, a cleft may be hidden adjacent to broad-based peripheral anterior synechia; such areas should be carefully inspected during gonioscopy. In many cases, complementary use of imaging (see section 2.3 Imaging, below) is necessary to confirm the diagnosis and gauge the size of the cleft.[1]
Imaging
Non-invasive imaging techniques that aid in evaluation of cyclodialysis clefts include ultrasound biomicroscopy (UBM) and anterior segment optical coherence tomography (AS-OCT).
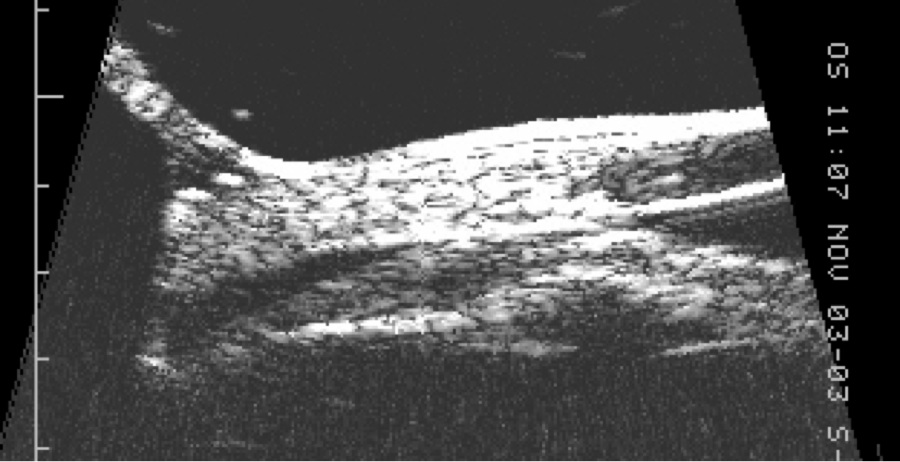
UBM makes use of a high frequency B-scan transducer (50-100 MHz) to image anterior segment structures with very high (25 to 50 micron) resolution (Figure 2). It produces extremely detailed images of the anterior chamber, angle and ciliary body, and is thus the best device for determining the presence and size of cyclodialysis clefts and any associated anterior suprachoroidal fluid. In a series of six eyes with post-traumatic hypotony and suspected cyclodialysis, gonioscopy revealed a cleft in only one while UBM identified clefts in all six.[10] UBM also facilitates detection of clefts in the presence of a cloudy cornea where gonioscopy is not helpful. However, UBM technology is somewhat inconvenient for the patient; older models required immersion of the globe in a water bath with the patient supine, while newer models incorporate the water bath into the probe but still require direct ocular contact with coupling gel.
AS-OCT images, on the other hand, are quickly and easily acquired without ocular contact.[11] OCT technology, which uses 830nm light for retinal imaging, is modified for anterior segment visualization by using a longer wavelength (1310nm) light, and produces 18 to 100 micron resolution. It is well-suited for analyzing the angle, but unfortunately pigmentation of the posterior iris prevents light transmission in most cases, so the ciliary body and suprachoroidal space are not well-visualized.[12] Moreover, a cloudy cornea degrades the quality of AS-OCT images. These limitations restrict its utility for evaluating cyclodialysis clefts.
Finally, an endoscope may be used intraoperatively to help visualize cyclodialysis clefts and has the potential advantage of not being affected by a cloudy cornea.
Differential diagnosis
Many mechanisms may cause or contribute to hypotony following ocular trauma. Decreased aqueous production by a damaged ciliary body may be a factor, but this often resolves with time. Other sequelae of trauma which can produce hypotony include chronic retinal detachment and anterior proliferative vitreoretinopathy (e.g. formation of a cyclitic membrane).[5]
Management
Medical therapy
Cycloplegic drugs are the mainstay of medical therapy. These medications cause relaxation of the ciliary muscle tone and dilatation of the ciliary body ring and help appose the detached muscle fibers to the sclera. Topical atropine sulfate 1% is typically applied twice daily for as long as 6 – 8 weeks.[2][7] The role of topical steroids is less clear: some providers increase steroid use, while others advocate reducing steroids to deliberately promote inflammation and cilioscleral adhesion.[1]
Laser Therapy
A number of laser techniques have been suggested when medical therapy fails to close a cyclodialysis cleft. Laser therapy functions by inducing local inflammation and seals the cleft by promoting adhesion between the choroid and sclera.
Argon laser, typically used for trabeculoplasty, was described first, and is applied deep in the cleft, first to the sclera and then to exposed ciliary muscle as well as to peripheral iris.[13] Early reports described settings as follows: power 1.0-1.2W to sclera, 0.3-0.7W to choroid and ciliary body, spot size 200μm, duration 0.5 seconds, applied under topical anesthesia. Ormerod et al. reported better success with higher power, 1-3W, applied in the operating room after retrobulbar anesthesia, using viscoelastic to maintain the anterior chamber and removing it at the end.[7] More recently, successful use of transscleral diode photocoagulation has been reported, using the G-probe designed for therapeutic cyclophotocoagulation (CPC/ TDC) to apply 1500-2500mW for 1500-2000ms in two parallel rows over the cleft, 1.5mm behind the limbus.[14] [15] A similar non-contact technique using defocused Nd:YAG laser has been published, though the authors noted a risk of scleral injury due to high laser energy.[16] Finally, two groups have reported single cases in which endolaser photocoagulation resulted in cleft closure. The first used an argon laser endophotocoagulator to treat both walls of the cleft; the second used an 810nm diode laser microendoscope, typically used in vitreoretinal surgery (power 3 W, duration 1 second), in a similar fashion.[17] [18] A similar technique might be considered in pediatric patients with relatively small clefts (<3 clock hours) who cannot tolerate a slit-lamp laser procedure. Of note, use of the endocyclophotocoagulation probe for this purpose has not been described.
Surgical Approaches - Extraocular
Transconjunctival cryotherapy is another noninvasive, ab externo approach for smaller cyclodialysis clefts, and has been employed both alone and in combination with other surgical techniques (see section 3.4. Surgical Approaches – Intraocular). After injection of viscoelastic to maintain the anterior chamber, a gonioprism (typically a direct one, such as a Koeppe or Swan-Jacobs) is used to define the borders of the cleft. The cryotherapy probe is then used 3mm behind the limbus to manually indent and close the cleft while simultaneously freezing the tissue in either a double or triple freeze-thaw sequence (temperature -85 degrees Celsius, duration 30 seconds). In a recently-published retrospective case series of 18 eyes, 1/3 were initially approached with cryopexy. None of these had more than 3 continuous or 4 total clock hours of cyclodialysis. In a separate case series, 50% achieved surgical success after 1 procedure.[19] A more recent series of 17 eyes found 36% of initial cryopexies successful in eyes with no more than 3 clock hours of cyclodialysis.[20] However, one case has been published in which a 360 degree cleft was successfully closed with 270 degrees of cryotherapy, improving intraocular pressure from 0 mmHg to 9 mmHg and visual acuity from 20/200 to 20/20.[21]
Transscleral diathermy has been in use since the 1950s and may help reduce the size of surgically created cyclodialysis clefts causing hypotony when other techniques fail.[4] The most recent iteration of this technique involves creation of a partial thickness scleral flap beneath which the diathermy pin is applied. Scleral ectasia and lens damage have been reported with this technique, and it is recommended that treatment not exceed 4 clock hours.[7][22]
Surgical Approaches - Intraocular
Direct cyclopexy is the best-studied intraocular approach to refractory cyclodialysis clefts. Although a complex technique requiring good knowledge of angle anatomy, this procedure is anatomically precise. Several techniques exist, employing full-thickness, partial-thickness (with slit entry) or double-lamellar scleral flaps to gain access to the ciliary body and suture it to the undersurface of the sclera.[19] [20] The flaps are typically extended ½ clock hour beyond the cleft margins and open 2-3mm behind the limbus, beneath a conjunctival peritomy. Various suture techniques are described, including placement of 8-0 or 9-0 nylon (some prefer Prolene) mattress,[19] [20] overlapping running[23], or interrupted 10-0[24] sutures under direct visualization. Some add ciliary body cautery to reinforce sutured closure.
The simpler cross-chamber cyclopexy technique, described in a case report by Metrikin et al, may be employed in eyes that are pseudophakic or aphakic.[25] This flap-less procedure is loosely based on principles of suturing posterior chamber intraocular lenses. Briefly, both ends of a double-armed 10-0 polypropylene suture on an STC-6 needle are passed 1mm posterior to the surgical limbus, 3mm apart, guided into the ciliary sulcus using a 25-gauge curved cannula. The ciliary body is apposed to the sclera with a series of suture loops created in this way. In the case described, Argon laser was applied to the iris at post-op week 1 week to incite a uveitic response; the patient’s hypotony, chronic for 4-5 months, resolved by post-op week 2.
Success rates for direct cyclopexy are good, ranging from 67% to 96% for first procedures and improving to nearly 100% with two procedures.[20][26] One method proposed for ensuring cleft closure intraoperatively involves injection of fluorescein into the anterior chamber: egress through the sclerostomy demonstrates a still-patent cleft. An alternative is to inject intravenous fluorescein, which stains the sclera overlying a patent cleft.[27]
A few other successful, novel techniques have been described in the literature. These include: 1) 20% SF6 gas tamponade combined with cryotherapy, with or 2) without pars plana vitrectomy[9][28], 3) temporary anterior scleral buckling with short sponge segments 3mm behind the limbus, also 4) combined with cryotherapy.[29] Others have reported using the haptics of 3-piece IOLs in the sulcus[30] [31] or a sulcus-sutured capsular tension ring[32] (See video from Dr. Shareef Shakeel, MD here for a minimally invasive repair of a cyclodialysis cleft, 3 clock hours.[33])
Timing of Cyclodialysis Cleft Repair
While there are documented cases of restored vision after months to years of persistent hypotony, it is generally recommended that intervention take place within 3 months. A series of 9 patients with hypotony maculopathy after trabeculectomy was published in 1995. In 6 of the 9, post-op vision was restored to within 1 line of pre-op vision with IOP elevation. Of the 3 eyes that did not return to preoperative vision, the shortest duration of hypotony was 3.5 months.[34]
Complications
More than half of eyes (12/18 and 13/17 in two case series) experience a period of significant, often painful IOP elevation following successful closure of a cyclodialysis cleft.[19] [20] This is typically self-limited and can be managed with topical IOP-lowering drops with or without oral CAIs, systemic osmotics, and oral analgesics. Rarely, a patient will require traditional filtering surgery (trabeculectomy or tube shunt).[19] There is evidence that larger clefts require more time for elevated IOP to normalize after repair.[24] One group has also described a trend towards increased duration of hypotony correlating with greater likelihood of an IOP spike.[19]
Outcomes
Ultrasound biomicroscopic evaluation of eyes before and after cyclodialysis cleft repair indicates that after closure of a cleft, anterior chamber depth and axial length increase while crystalline lens thickness decreases, reversing changes in refractive error that may have occurred.[24] While some eyes continue to have poor vision due to sequelae of trauma or failure to reverse underlying hypotony maculopathy (presumably due to fibrosis of a chronically distorted retina and choroid), data show that vision may improve even in cases of protracted hypotony (19, 20, 24).[19] [20] [24] Therefore, while repair should be pursued efficiently where possible, providers should not shy away from addressing long-standing cyclodialysis clefts. Finally, eyes with resolved cyclodialysis clefts are certain to have at least some degree of angle recession, and should be followed for angle recession glaucoma.
Figures
Figures 1(a) & (b). Gonioscopic views of cyclodialysis clefts after paintball injuries to two different eyes
Figure 2. UBM demonstrating cyclodialysis cleft in patient (a) above
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Ioannidis A, Barton K. Cyclodialysis cleft: Causes and repair. Current Opinion in Ophthalmology. 2010;21:150.
- ↑ Jump up to: 2.0 2.1 Aminlari A, Callahan C. Medical, laser, and surgical management of inadvertent cyclodialysis cleft with hypotony. Archives of Ophthalmology. 2004;122:399.
- ↑ Demeler U. Surgical management of ocular hypotony. Eye. 1988;2:77.
- ↑ Jump up to: 4.0 4.1 Shaffer R, Weiss D. Concerning cyclodialysis and hypotony. Archives of Ophthalmology. 1962;68:55.
- ↑ Jump up to: 5.0 5.1 5.2 Ding C, Zeng J. Clinical study on hypotony following blunt ocular trauma. International Journal of Ophthalmology. 2012;5(6):771.
- ↑ Mushtaq B, Chiang M, Kumar V, Ramanathan U, Shah P. Phacoemulsification, persistent hypotony, and cyclodialysis clefts. Journal of Cataract and Refractive Surgery. 2005;31:1428.
- ↑ Jump up to: 7.0 7.1 7.2 7.3 Ormerod L, Baerveldt G, Sunalp M, Riekhof F. Management of the hypotonous cyclodialysis cleft. Ophthalmology. 1991;98:1384.
- ↑ Ormerod L, Baerveldt G, Green R. Cyclodialysis clefts: Natural history, assessment, and management. In: Weinstein G, editor. Open-Angle Glaucoma. New York: Churchill Livingstone; 1986. p. 201.
- ↑ Jump up to: 9.0 9.1 Ceruti P, Tosi R, Marchini G. Gas tamponade and cyclocryotherapy of a chronic cyclodialysis cleft. British Journal of Ophthalmology. 2009;93:414.
- ↑ Gentile R, Pavlin C, Liebmann J, Easterbrook M, Tello C, Foster F, et al. Diagnosis of traumatic cyclodialysis by ultrasound biomicroscopy. Ophthalmic Surgery and Lasers. 1996;27(2):97.
- ↑ Mateo-Montoya A, Dreifuss S. Research letter: Anterior segment optical coherence tomography as a diagnostic tool for cyclodialysis clefts. Archives of Ophthalmology. 2009;127(1):109.
- ↑ Nolan W. Anterior segment imaging: Ultrasound biomicroscopy and anterior segment optical coherence tomography. Current Opinion in Ophthalmology. 2008;19:115.
- ↑ Harbin T. Treatment of cyclodialysis clefts with argon laser photocoagulation. Ophthalmology. 1982;89:1082.
- ↑ Brown S, Mizen T. Transscleral diode laser therapy for traumatic cyclodialysis cleft. Ophthalmic Surgery and Lasers. 1997;28(4):313.
- ↑ Amini H, Razeghinejad M. Transscleral diode laser therapy for cyclodialysis cleft induced hypotony. Clinical and Experimental Ophthalmology. 2005;33:348.
- ↑ Brooks A, Troski M, Gillies W. Noninvasive closure of a persistent cyclodialysis cleft. Ophthalmology. 1996;103:1943.
- ↑ Alward W, Hodapp E, Parel J, Anderson D. Argon laser endophotocoagulator closure of cyclodialysis clefts. American Journal of Ophthalmology. 1988;106:748.
- ↑ Caronia R, Sturm R, Marmor M, Berke S. Treatment of a cyclodialysis cleft by means of ophthalmic laser microendoscope endophotocoagulation. American Journal of Ophthalmology. 1999;128(6):760
- ↑ Jump up to: 19.0 19.1 19.2 19.3 19.4 19.5 19.6 Ioannidis A, Bunce C, Barton K. The evaluation and surgical management of cyclodialysis clefts that have failed to respond to conservative management. British Journal of Ophthalmology. 2014;0:1.
- ↑ Jump up to: 20.0 20.1 20.2 20.3 20.4 20.5 Agrawal P, Shah P. Long-term outcomes following the surgical repair of traumatic cyclodialysis clefts. Eye. 2013;27:1347.
- ↑ Trikha S, Turnbull A, Agrawal S, Amerasinghe N, Kirwan J. Management challenges arising from a traumatic 360 degree cyclodialysis cleft. Clinical Ophthalmology. 2012;6:257.
- ↑ Maumenee A, Stark W. Management of persistent hypotony after planned or inadvertent cyclodialysis. American Journal of Ophthalmology. 1971;71(1):320.
- ↑ Wang M, Hu S, Zhao Z, Xiao T. A novel method for the localization and management of traumatic cyclodialysis cleft. Journal of Ophthalmology. 2014.
- ↑ Jump up to: 24.0 24.1 24.2 24.3 Hwang J, Ahh K, Kim C, Park K, Kee C. Ultrasonic biomicroscopic evaluation of cyclodialysis before and after direct cyclopexy. Archives of Ophthalmology. 2008;126(9):1222.
- ↑ Metrikin DC, Allinson RW, Snyder RW. Transscleral repair of recalcitrant, inadvertent, postoperative cyclodialysis cleft. Ophthalmic Surg 1994; 25: 406-408.
- ↑ Kuchle M, Naumann G. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony: Report in 29 consecutive patients. Ophthalmology. 1995;102:322.
- ↑ Elder M, Hitchings R, Dart J. Diagnosis and management of an occult cyclodialysis cleft. British Journal of Ophthalmology. 1995;79(6):619.
- ↑ Hoerauf H, Roider J, Laqua H. Treatment of traumatic cyclodialysis with vitrectomy, cryotherapy, and gas endotamponade. Journal of Cataract and Refractive Surgery. 1999;25:1299.
- ↑ Mandava N, Kahook M, Mackenzie D, Olson J. Anterior scleral buckling procedure for cyclodialysis cleft with chronic hypotony. Ophthalmic Surgery, Lasers & Imaging. 2006;37:151.
- ↑ Mardelli P. Closure of persistent cyclodialysis cleft using the haptics of the intraocular lens. American Journal of Ophthalmology. 2006;142:676.
- ↑ Malandrini A, Balestrazzi A, Martona G, Tosi G, Caporossi A. Diagnosis and management of traumatic cyclodialysis cleft. Journal of Cataract and Refractive Surgery. 2008;34:1213.
- ↑ Yuen N, Hui S, Woo D. New method of surgical repair for 360-degree cyclodialysis. Journal of Cataract and Refractive Surgery. 2006;32:13.
- ↑ Shareef S, DiLoreto, D. Surgical Repair of Traumatic Cyclodialysis Cleft. AGS 2013 Annual Meeting. American Academy of Ophthalmology. https://www.aao.org/education/clinical-video/surgical-repair-of-traumatic-cyclodialysis-cleft Accessed May 23, 2024.
- ↑ Cohen S, Flynn H, Palmberg P, Gass D, Grajewski A. Treatment of hypotony maculopathy after trabeculectomy. Ophthalmic Surgery, Lasers & Imaging. 1995;26:435.