Central Foveal Bouquet Abnormalities
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease
Central foveal bouquet abnormalities are described on optical coherence tomography (OCT) in vitreomacular interface disorders and cystoid macular edema (CME).
They comprise of alterations seen within central bouquet (CB), defined as small circular area in central fovea, approximately of 100 microns and composed of cone photoreceptors and Müller cells. [1] They represent continuous clinical spectrum based on progressive morphological appearances.[1]
These include :
- Stage 1- Cotton ball sign
- Stage 2- Foveolar detachment
- Stage 3- Acquired vitelliform lesion.
Etiology
- CB abnormalities are noted in association with vitreomacular interface disorders.[2] [3][4]These have been often reported in eyes with epiretinal membrane (ERM). [1] [5][6][7] Apart from these, they have also been observed in non-tractional macular condition such as CME.[8]
History and anatomy
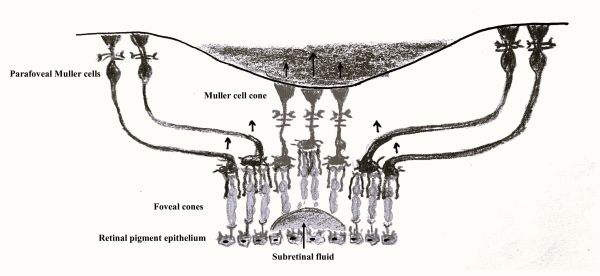
- Rochon Duvigneaud initially described bouquet of central cones as a small circular island, less than 100 mm in diameter and densely composed of the thinnest cone photoreceptors. [9] [10][11]
- Gass had also proposed the presence of a Müller cell cone, an inverted conical plug of Müller cells that binds together the central cone photoreceptors at foveola. [12]
- Since precise cellular architecture of central fovea is debatable, subsequent studies have defined CB to include dense array of both cones and Müller cells. Govetto at al. investigated tractional abnormalities of the CB and proposed that foveal Müller cells play an important role in the transmission of mechanical stress to the central foveal cones. [1]
- A schematic image of presumed Müller cell cone is available here.[13]
Pathophysiology
- CB is a major anatomic landmark within the the central foveal architecture and is highly susceptible to mechanical stress seen in association with vitreomacular interface disorders.[12][15] [16] This may be attributed to foveal Müller cells that intertwine with central foveal cones.

- Müller cells extend from internal limiting membrane (ILM) to external limiting membrane (ELM) and providing architectural support for the various cellular elements. As described by Gass and supported by Yamada’s previous work, Müller cells are more vertically arranged in the central-most region of the fovea (figure 1 and figure 2A). [12][14][15]
- Previous studies have shown that tractional forces are transmitted through retinal tissue to the photoreceptors by the Müller cells.[17] Histopathological studies and staining pattern of the foveal Müller cells also support the hypothesis of Müller cell related traction on CB.[1]
Depending upon the strength and chronicity of traction exerted by Müller cells, following sequential stages of CB abnormalities have been proposed. [1]
- Stage 1 CB alteration (cotton ball sign): Inward traction by Müller cells over CB causes upward displacement of foveal cones without major disturbance of ellipsoid zone (EZ) and ELM (figure 2B).
- Stage 2 CB alteration (foveolar detachment): Progressive inward traction by the Müller cells exceeds the adhesive force between the cones and the retinal pigment epithelium (RPE). This leads to separation of cones from the RPE and subsequent accumulation of central subretinal fluid. This may be associated with EZ disruption (figure 2C).
- Stage 3 CB alteration (acquired vitelliform lesion): Persistent separation of the cone and the underlying RPE leads to disruption of physiology related to the RPE photoreceptor complex. As a result, metabolic cone debris start accumulating in the subretinal space and thereby leading to development of acquired vitelliform lesion (figure 2D). This may be associated with ELM disruption and intraretinal migration of hyperreflective material (figure 2E). This may lead to subsequent collapse of vitelliform lesion.
- In eyes with CME where CB abnormalities have been proposed, there is stretching of Müller cells secondary to fluid accumulation.[8] This leads to transmission of mechanical stress towards CB and leading to foveolar detachment.
Diagnosis
Diagnosis of CB abnormalities is made based on OCT findings.
- Stage 1 (cotton ball sign) is characterized by small, fuzzy subfoveal hyperreflective area between the inner segment ellipsoid zone (EZ) and the interdigitation zone (IZ) (figure 3).
- In stage 2 (foveolar detachment), there is central subfoveal hyporeflective pocket corresponding to subretinal fluid accumulation under interdigitation zone (figure 4).
- In stage 3 (acquired vitelliform lesion), there is presence of thick dome-shaped subfoveal hyperreflective material between ellipsoid zone and retinal pigment epithelium (figure 5).
- Additionally, OCT can also demonstrate findings related to vitreomacular interface disorders. In eyes having ERM, OCT will show ERM as a hyperreflective line above ILM along with intraretinal findings specific to the stages of ERM. These may include foveal herniation and of ectopic inner foveal layers (EIFL).[1] [18]
- EIFL has been observed in stage 3 and 4 ERM and was found to be associated with lower incidence of CB abnormalities.[1] This has been attributed to EIFL causing reduced displacement and stretching of outer retina at fovea.
Symptoms
- Symptoms are related to the underlying macular pathologies and may include blurred vision and metamorphopsia.
- Previous studies have observed correlation between stages of CB abnormalities and visual acuity.
- Govetto et al. studied CB abnormalities in eyes with ERM and observed that visual acuity was better in presence of cotton ball sign and worsened with progression to subsequent stages.[1] This may be related to EZ disruption seen in advanced stages of CB abnormalities.
- In eyes with CME, presence of CB abnormalities was found to be associated with increased macular thickness, horizontal extent of edema and worse visual acuity at baseline.[8]
Differential diagnosis
CB abnormalities need to be differentiated from conditions such as:
- Acute retinal pigment epitheliitis (Krill disease) - It has an acute onset with painless loss of vision or central scotoma. OCT shows dome-shaped hyperreflective lesion at the level of photoreceptors layer disrupting the ellipsoid zone and interdigitation zone. There may be initial involvement of outer nuclear layer. The disease is known to have rapid resolution in 6 to 12 weeks.
- Central serous chorioretinopathy - Patient usually presents with complaints of unilateral central vision loss with or without distortion or a possible central scotoma. However, presentation can be bilateral. CSCR is usually self-limiting resolves spontaneously within 2 to 3 months. However, recurrent episodes are known to occur. OCT may show serous PED, hyper-reflective, fibrinous sub-retinal fluid, RPE defect corresponding to potential leakage site and choroidal thickening.
- Best Disease and Bestrophinopathies - Best vitelliform macular dystrophy generally presents first decade of life and is usually bilateral. Early stages are associated with minimal visual symptoms. Progression of stages is associated with gradual decrease in visual acuity. Adult-onset foveomacular vitelliform dystrophy is typically seen between 30-50 years of age with minimal visual symptoms. OCT may show nonhomogenous hyper reflectivity at the level of RPE and thickening of the cone outer segments. OCT can also pick up development of CNV in later stage.
- Solar retinopathy - Patient has history of direct sun-gazing or eclipse viewing. Usually, there is bilateral foveal involvement with OCT showing disruption of the outer retinal layers.
- Acute idiopathic maculopathy - Patient presents with acute unilateral visual loss. OCT shows subfoveal hyperreflective thickening at the level of the outer retina and RPE and hyporeflective fluid in subretinal space. It is generally a self-limiting condition.
Management
- Management includes treatment of underlying associations. These include treating ERM and CME due to various etiologies.
- While CME can be treated with topical non-steroidal anti-inflammatory (NSAID) eyedrops / intravitreal injections / oral therapy, ERM and vitreomacular traction (VMT) needs pars plana vitrectomy (PPV) for removal.
Clinical significance and Prognosis
- Govetto et al. showed that most of the eyes having cotton ball sign maintained visual and morphologic stability over follow up.[1] They also observed spontaneous resolution of the same with relief of traction over CB.
- In eye which developed acquired vitelliform lesion, spontaneous collapse of same was observed with intraretinal migration of hyperreflective material and persistent discontinuity of EZ and ELM.
- Several studies have analyzed the presence of CB abnormalities and outcomes of ERM surgery However, the results have been found to be mixed. Damasceno et al observed favorable outcome with increase in visual acuity and resolution of CB abnormalities.[7] Brinkmann et al. observed that presence of CB abnormalities correlated with worse visual outcomes after PPV for ERM removal.[5]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Govetto A, Bhavsar KV, Virgili G, Gerber MJ, Freund KB, Curcio CA, et al. Tractional Abnormalities of the Central Foveal Bouquet in Epiretinal Membranes: Clinical Spectrum and Pathophysiological Perspectives. Am J Ophthalmol. 2017;184:167-180.
- ↑ Tsunoda K, Watanabe K, Akiyama K, Usui T, Noda T. Highly reflective foveal region in optical coherence tomography in eyes with vitreomacular traction or epiretinal membrane. Ophthalmology 2012;119:581–587.
- ↑ Pison A, Dupas B, Couturier A, Rothschild PR, Tadayoni R. Evolution of subfoveal detachments secondary to idiopathic epiretinal membranes after surgery. Ophthalmology 2016; 123:583–589.
- ↑ Dupas B, Tadayoni R, Erginay A, et al. Subfoveal deposits secondary to idiopathic epiretinal membranes. Ophthalmology 2009;116:1794–1798.
- ↑ 5.0 5.1 Brinkmann MP, Michels S, Brinkmann C, Rommel F, Ranjbar M, Graf Johansen N, Becker M. Epiretinal membrane surgery outcome in eyes with abnormalities of the central bouquet. Int J Retina Vitreous. 2021;7:7.
- ↑ Brinkmann MP, Michels S, Brinkmann C, Toro MD, Johansen NG, Rommel F, Ranjbar M, Becker M. Influences of Central Bouquet Alterations on the Visual Outcome in Eyes Receiving Epiretinal Membrane Surgery. Applied Sciences. 2021; 11:926.
- ↑ 7.0 7.1 Damasceno NA, Damasceno EF, Yannuzzi NA, Crane AM, Relhan N, Smiddy WE, Flynn HW Jr. The Central Subfoveal Bouquet in Idiopathic Epiretinal Membranes. Clin Ophthalmol. 2020;14:2353-2359.
- ↑ 8.0 8.1 8.2 Lenis TL, Au A, Hou K, Govetto A, Sarraf D. Alterations of the foveal central bouquet associated with cystoid macular edema. Can J Ophthalmol. 2020;55:301-309.
- ↑ Polyak SL. The Retina. Chicago: University Press; 1941:455–489.
- ↑ Polyak SL. The Vertebrate Visual System. Chicago: University Press; 1957:259–278.
- ↑ Rochon-Duvigneaud A. Recherches sur la fovea de la retine humaine et particulie`rement sur le bouquet des coˆnes centraux. Arch Anat Microsc 1907;9:315–342.
- ↑ 12.0 12.1 12.2 Gass JDM. Müller Cell Cone, an Overlooked Part of the Anatomy of the Fovea Centralis: Hypotheses Concerning Its Role in the Pathogenesis of Macular Hole and Foveomacular Retinoschisis. Arch Ophthalmol. 1999;117:821–823.
- ↑ Ho TC, Ho AY, Chen MS. Reconstructing Foveola by Foveolar Internal Limiting Membrane Non-Peeling and Tissue Repositioning for Lamellar Hole-Related Epiretinal Proliferation. Sci Rep. 2019;9(1):16030. Published 2019 Nov 5. doi:10.1038/s41598-019-52447-4
- ↑ 14.0 14.1 14.2 Salman A, McClements ME, MacLaren RE. Insights on the Regeneration Potential of Müller Glia in the Mammalian Retina. Cells. 2021; 10:1957.
- ↑ 15.0 15.1 Yamada E. Some structural features of the fovea central in the human retina. Arch Ophthalmol 1969;82:151–159.
- ↑ Matet A, Savastano MC, Rispoli M, et al. En face optical coherence tomography of foveal microstructure in full-thickness macular hole: a model to study perifoveal Muller cells. Am J Ophthalmol 2015;159:1142–1151.
- ↑ Spaide RF. Closure of an outer lamellar macular hole by vitrectomy: hypothesis for one mechanism of macular hole formation. Retina 2000;20:587–590.
- ↑ Uzel MM, Gelisken F, Konrad E, Neubauer J. Clinical and morphological characteristics of patients with idiopathic epiretinal membrane with foveal herniation. Eye (Lond). 2023;37:1357-1360.




