Botulinum Toxin Use In Strabismus
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Video demonstration
Introduction
Botulinum toxin represents a group of neurotoxins produced by the bacteria species Clostridium botulinum. Historically known as the causative agent of food borne, wound and infant botulism, injection of botulinum toxin has revolutionized clinical practices. It is currently the standard of care for a broad range of clinical conditions across various organ systems, including dystonia[1], spasticity[2], hyperhidrosis [3], chronic migraine headaches[4] and urinary detrusor dyssynergia.[5]
In Ophthalmology, the therapeutic effects of the neurotoxin were first described for the treatment of strabismus, while its use in other oculomotor disorders has shown promising results.
Historical Perspective
The history of botulinum toxin began with the recognition of the association between food poisoning and most likely emerged over a millennium in Byzantium. Advancements in our understanding of the neurotoxin started in the late 1700s during the Napoleonic War, when a paralytic disease was associated with the consumption of sausages. This association was later documented in 1822 by the German physician and poet Justinus Kerner, who collected information on 230 cases of “sausage poisoning”. However, it was not until 1870 that the disease would be named “botulism”, derived from the word “botulus” which is the Latin word for sausage.[6] [7]
The bacteria Clostridium botulinum was first identified in 1897 by professor Émile van Ermengen. Later, the botulinum toxin type B was isolated in 1910 and the type A in the 1920s. The neurotoxin was studied early on for chemical warfare purposes.[6] [7]
The first attempts at treating strabismus were performed by injecting alcohol or anesthetic solutions, with unfavorable results.[8] The use of botulinum toxin for the treatment of strabismus was first documented in 1973 by Alan B. Scott[8], a San Francisco ophthalmologist who injected botulinum neurotoxin type A on 8 adult rhesus monkeys using a hypodermic injection needle inserted transconjunctivally into either the medial or lateral rectus muscle. The animals were photographed postoperatively and at different follow-up time points to assess eye position and were examined periodically by a veterinarian. The researches were able to produce both transient paralysis of individual rectus muscles which lasted between 2 weeks to 8 months, as well as permanent changes in ocular alignment.
The use of botulinum toxin for the treatment of strabismus in humans was first reported in 1981.[9] Injection of the toxin in 42 participants showed a beneficial effect on horizontal strabismus which lasted up to 411 days since the last documented injection. However, the effects in vertical strabismus and lid retraction were less pronounced. The treatment presented a good safety profile, showing only local effects on adjacent muscles and no systemic effects.
The use of botulinum A exotoxin (BTX-A) was approved on December 29, 1989 [7] by the Food and Drug Administration (FDA) and the National Eye Institute (NEI) for the treatment of strabismus and blepharospasm. By the end of the 1980s, its clinical application was extended to children. The results in the pediatric populations were promising after a single injection as well as after re-injection in a wide spectrum of strabismic entities.[10]
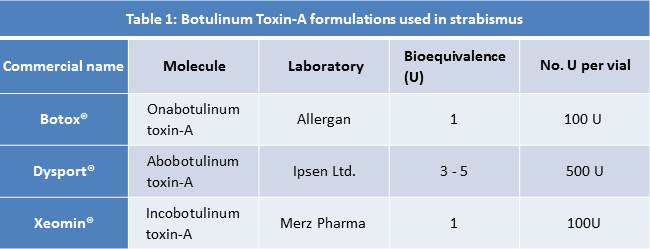
There are different types of botulinum toxin which differ in their antigenic properties (table 1). The first to be produced was the Wisconsin type, created by Professor Shantz from the WS Army Chemical Warfare Department, currently used under the commercial name of Botox® (Allergan). Onabotulinum toxin-A (Botox®) presents equivalent efficacy (1:1) and adverse effects rates as Incobotulinum toxin-A (Xeomin®). Porton laboratories developed the Porton Down toxin or Abobotulinum toxin-A (Dysport®), commercialized by Ipsen Pharma laboratory. I
Mechanism of Action of Botulinum Toxin
There are seven known serotypes of botulinum toxin (A, B, C1-2, D, E, F and G) extracted from the bacteria Clostridium botulinum. Of the aforementioned, type A is the most potent, the first to be commercially available and the most widely used across the globe in multiple formulations for both cosmetic and therapeutic goals.[11]
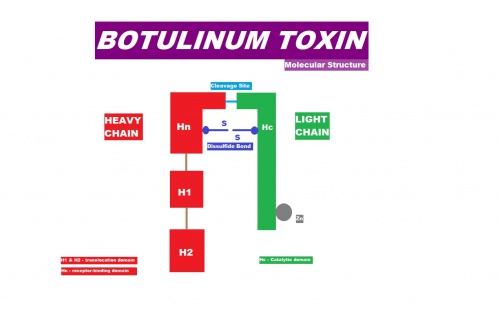
The molecule is formed by a heavy and a light chain, bounded by a disulfide bridge (figure 1). The H1 subunit binds to the neuronal end, while the H2 subunit has antigenic properties, although it lacks toxicity.[11][12]
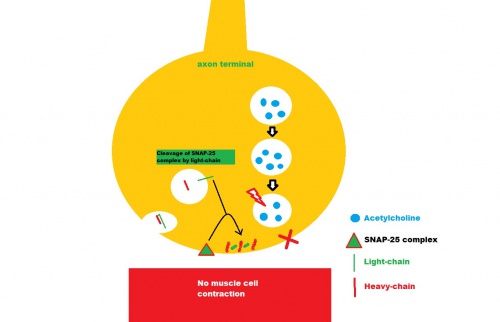
The release of the neurotransmitter acetylcholine at the synaptic cleft requires the participation of the vesicle-associated membrane protein/synaptosomal-associated protein-25 (SNAP-25)/syntaxin protein complex. Once the intramuscular injection has been performed, the molecule undergoes endocytosis at the terminal button. It then acts by binding and clivating the protein complex, thereby inhibiting the release of acetylcholine. Consequently, interruption of the transmission of nerve impulses across the synaptic cleft to the motor end plate is achieved.[11] [12] (figure 2)
Paralysis of the injected EOM begins between 2-4 days of injection and lasts clinically for at least 5-8 weeks. Recovery of muscle function takes between 5 to 14 weeks, depending on the density of innervation, the injection site, amount and concentration of the solution.[13] [14]
Treatment with botulinum toxin produces a pharmacologic recession of the injected EOM, and the muscle lengthens while it is paralyzed while its agonist contracts. Although the pharmacological effect usually resolved by 3 months, improved ocular alignment or reduction in the magnitude of the deviation may remain long-term. Several factors may come into play during the period of muscle paralysis which may contribute to the stabilization and long-term improvement in alignment in strabismus patients, even after the pharmacologic effect has worn off, including mechanical, proprioceptive and binocular effects.[15]
Antibodies against portions of the toxin have been detected but will not attenuate the effect of the neurotoxin on the neuromuscular junction (non-neutralizing antibodies) and have no clinical significance. Only a small percentage of antibodies will neutralize BTX at the functional site of the heavy chain thereby impeding its binding to the neuronal membrane. No cross-reactive antibodies have been identified between the different serotypes.[16]
Indications
Clinical trials using botulinum toxin have suggested this agent to be more effective in the following conditions:[17]
- Esotropia or exotropia presenting with a small-to-moderate angle deviation (<40 PD)
- Acute onset comitant esotropia
- Postoperative residual or consecutive strabismus (2-8 weeks postoperatively or later)
- Acute paralytic strabismus to alleviate diplopia while the palsy resolves (mainly sixth nerve palsies, sometimes fourth nerve palsy)
- Active thyroid eye disease (Graves Disease), inflamed or pre-phthisical eyes, when surgery is not recommended
- Adjunct to surgery for large-angle esotropia or sixth nerve palsy or for large-angle exotropia [18]
- As a muscle sparing option in patients at risk of anterior segment ischemia
Botulinum toxin is least effective when:
- Used alone in large deviations
- Restrictive or mechanical strabismus (e.g. trauma, chronic thyroid eye disease)
- Secondary strabismus due to excessive muscle resection or muscle slippage
- Alphabetical patterns (A, V, X syndromes), dissociated vertical deviation (DVD) and chronic paralytic strabismus
A recent Cochrane Systematic Review[19], which included a total of 4 clinical trials with 242 participants that enrolled adults with esotropia or exotropia and children with acquired and infantile esotropia demonstrating different success rates, has deemed it difficult to ascertain the effectiveness of botulinum toxin (BTX) injections as an independent treatment modality among certain types of strabismus since the authors found only low and very-low certainty evidence in the review.
Contraindications
There are few contraindications to the use of botulinum toxin as the neurotoxin presents a very favorable safety profile:
- Unrealistic expectations
- Neuromuscular disorders: Amyotrophic Lateral Sclerosis (ALS), Myasthenia Gravis and Eaton-Lambert syndrome patients may be especially susceptible to the neuromuscular blockade, with increased risk of dysphagia.[20] Myasthenia gravis, an autoimmune disease affecting the neuromuscular junction, is a commonly reported contraindication, however case reports have described its successful application[21] as well as in patients with chronic progressive external ophthalmoplegia.[22]
- Pregnancy and breastfeeding: BTX is a category C drug which is not recommended during pregnancy and its secretion in breast milk is uncertain.[20] It is not expected to be detected in significant concentrations in the systemic circulation following a proper intramuscular or intradermal injection. Moreover, botulinum toxin does not cross the placenta as it has a high-molecular weight. Several case reports suggest the toxin is not toxic in pregnant patients, including those who had botulinum poisoning during pregnancy.[23]
Technique Description
Forms
The three commercially available forms of Botulinum toxin A used in the treatment of strabismus are onabotulinum toxin A (Botox®/Vistabel®; Allergan, Inc., Irvine, California), abobotulinum toxin A (Dysport®/Azzalure®; Ipsen Ltd., Berkshire, UK) and incobotulinum toxin A (Xeomin®/Bocouture®; Merz Pharmaceuticals, Frankfurt, Germany).
Each one has different unique characteristics and dosage. The respective dose is quantified in units (U). One unit (1U) corresponds to the quantity of toxin that is lethal in 50% of the female rats Swiss-Webster when injected intraperitoneally.[24] The lethal quantity for a 70Kg man is 5000 units, the equivalent of 50 bottles of the botulinum toxin.[25] Therefore, the dose used in strabismus varies between 2.5U and 5U, well below the toxic doses. Nevertheless, an antidote (anti-toxin) is available that blocks the effect of botulinum toxin if injected in under 30 minutes.[25]
It is important to distinguish among formulations, because the number of units used are not interchangeable across all formulations. In clinical practice, 1 unit of Botox® is equivalent to 3 to 5 units of Dysport®.[26] [27]
Doses
There is no consensus regarding the doses to administer.
The most frequently used doses are 2.5U to 5U of Botox®.[16]
Bilateral injections are frequently performed in children. The dose would be adjusted according to patient age, magnitude of deviation, refractive error and the type of strabismus. Some authors suggest: [16][25][28]
- Until 3 years: 2.5U in the dominant eye, and either 2.5 U (if the deviation <30 prismatic diopters, PD) or 5U (if ≥ 30 PD) in the non-dominant eye
- In children from 3 to 10 years: 2.5U in the dominant eye and 5U in the non-dominant eye
- 1,5U for the superior rectus muscle
- 1.5-2.5U for the inferior oblique and inferior rectus muscles
- 3-5U for the medial or lateral rectus muscle
- 10U of Botox in the presence of fibrosis.
To inject Dysport® apply a correction factor of 3 to 5U to Botox® or Xeomin®.
A more detailed description regarding recommended doses and strength of evidence for each specific clinical entity is available below.
Reconstitution
Botulinum toxin is commercially distributed as a powder that usually lasts 3 to 4 years but should be used within the next 6 hours after reconstitution with balanced salt solution.[25]
Two mL of balanced salt solution (BSS) are injected into the bottle containing 50 - 100U of botulinum toxin to obtain 5U per 0.1 mL (figure 3).
A dilution in higher volumes is not recommended as it demands the injection of a high volume of solution into the muscle.[25][28]
Anesthesia
The procedure can be performed under:
- Topical anaesthesia (application of one drop of oxybuprocaine 10 minutes before the procedure followed by 3 more drops until the procedure)
- General anesthesia (preferred in children and uncooperative adult patients).
Material
The material required for the procedure includes (fig.4):
- Botulinum toxin bottle
- Blepharostat
- Forceps (e.g. Paufique)
- Two-ml syringe and insulin syringe
- 27 or 30-gauge needle
- Portable electromyography (EMG) device (figure 5; optional in selected cases)
- Topical or general anaesthesia
Technique
The following steps ensure a proper injection technique (see video demonstration):
- EMG: Apply the EMG electrodes to the patient’s forehead. (note: The EMG device is useful for identifying small muscles that are difficult to visualize, but it is usually unnecessary for the internal or external rectus muscles)
- Apply topical anaesthesia to the ocular surface (e.g. oxybuprocaine)
- Ensure assepsia of the surgical field
- Place the blepharostat
- Position yourself on the same side of the eye to be injected
- For a medial rectus injection, ask the patient to abduct the eye, or if under anesthesia, you may plicate the conjunctiva using the forceps in the cantus and rotate the eye towards yourself, allowing introduction of the needle tangentially through the bulbar conjunctiva. The direct approach is a safe option for its simplicity and speed. Dissection the conjunctiva, enabling direct visualization of the muscle prior to the injection is an alternative approach.[29]
- If one is using an EMG device, first ask the patient to look in the opposite direction of the muscle action to introduce the needle. Then ask the patient to look towards the muscle to be injected. The needle is finally advanced to the loudest sound.
- The needle is verticalized and the toxin is slowly injected.
- Slowly withdraw the needle.
- Apply antibiotic ointment at the end of the procedure.
Postoperative Care
In contrast to surgery, no postoperative medication or occlusion is necessary after botulinum toxin injection.
If the patient was receiving occlusion treatment for amblyopia, it may be suspended post-injection and be resumed at one month of follow-up, and only if amblyopia is maintained.[25] [28]
Patients and caregivers should be reminded of the latency of the effects (i.e. 2 to 7 days before becoming noticeable) as well as the potential adverse events associated with the use of botulinum toxin (e.g. ptosis, hypercorrection of the deviation, subconjunctival hemorrhage).
Follow-up is recommended within the first three weeks after the injection to document the early effects of the toxin, to reassure patients and caregivers and later at 4 to 8 months to re-evaluate ocular alignment and need for re-injection.[25] [28]
Complications
Adverse effects from botulinum toxin injection stem from toxin diffusion and inadvertent weakening of adjacent muscles. The effects are usually unilateral and dose-dependent. The dosages used in strabismus, as with other applications of botulinum toxin, are far below those needed to cause systemic toxicity. Patients and parents should be informed of the potential complication prior to injection:
Frequent
- Transient ptosis: The most common adverse event. It is reported to occur in 12% of adults and 25% of children[30], and is usually more frequent with internal rectus muscle injections. It is not a serious adverse effect. Some ophthalmologists ask the patient to rapidly stand after the injection, to decrease the level of exposure of the levator palpebrae superioris and the subsequent ptosis. Deprivation amblyopia from the resulting ptosis is a concern when considering BTX injection in children, however reports suggest that the toxin causes only partial and transient ptosis with no amblyogenic effects observed.
- Conjunctival hemorrhage: Precipitated by the injection technique or inadvertent forceps touch of a vessel. These are usually minor and require no treatment.[25] [28]
- Diplopia and spatial disorientation: In children, these effects last a few days and are well tolerated, but in adults these adverse effects can be very unpleasant and may last up to a month.[25] [28]
- Transient hypercorrection of the strabismus: Usually resolves within weeks but may last up to 6 months.[25] [28]
- Transient vertical deviation: Occurs in 3.3 to 37% of patients [25][31] due to the toxin diffusion to the superior and inferior rectus, almost always transient.
Rare
- Retrobulbar hemorrhage: with a frequency of 0.5 to 2 per 1000 .[25] [28] It is a rare event, usually associated with maneuvers with the electromyogram.[28] Compression, compressive bandage, and ice are recommended measures.
- Ocular perforation: it has an estimated incidence of 0.2 to 1 per 1000.[25] [28] It is recommended an increase in retinal surveillance (and, if necessary, to perform laser or cryotherapy).
- Pupillary abnormalities: Occur in the form of a tonic pupil. They occur either due to direct trauma to the ciliary ganglion, intraconal diffusion of BTX to the ciliary ganglion or intraocular diffusion affecting the pupillary sphincter.[32] [33]
- A decrease in accommodation: transient.
Outcomes
Comitant Strabismus
Comitant esotropia
For comitant esotropia with a small-to-moderate preoperative angle (<30 to 35 PD), botulinum toxin injection success rates seem comparable to surgery but with shorter duration of the general anesthesia.[34] A 2017 systematic review and meta-analysis including a total of nine studies deemed BTX injection into medial recti muscles to be a safe procedure and a valuable alternative to strabismus surgery in congenital esotropia, especially in moderate deviations, with a grouped success rate of 76%.[35] For large-angle comitant esotropia, outcomes are more favorable when surgery is associated with botulinum toxin injection versus surgery alone.[36] [37]
Evidence is mixed regarding the timing of injection: suggests that very early injections (< 6 months of age) may not provide a statistically significant benefit versus later injections (6-24 months of age), while other authors have suggested early treatment to be beneficial .[16]
Children with psychomotor delay may benefit from BTX injection as the angle of deviation is more unstable over time which, in turn, may cause more unstable results with surgical resection. It is important to refract these patients after alignment is achieved as some cases develop accommodative esotropia "de novo".[16]
- Factors associated with a favorable outcome include:[16]
- Small-to-moderate deviations
- Pediatric patients
- Bilateral injections
- High hyperopia
- Potential of binocularity.
Intermittent Exotropia
Outcomes following BTX-A injection are less favorable when compared to those of comitant esotropia. Nevertheless, the neurotoxin may be useful in selected cases, particularly in young patients with poorly controlled intermittent exotropia in order to delay surgery.[16] Success rates for treating childhood exotropia with botulinum toxin range from 50% to 70%, with bilateral, not unilateral, lateral rectus injections. The success rate for exotropia over 35–40 PD is much lower, and, since most infantile exotropias are larger than this, surgery is the preferred treatment.[38] [39] [40]
Convergence insufficiency
It is a common condition in which there is a failure to converge for close activities, with a resultant exotropia at near. Various surgical and nonsurgical treatments have been described, but some patients remain symptomatic despite treatment. A retrospective analysis has demonstrated that, despite early improvement in the majority of patients (80%) who received BTX at 3 weeks postoperatively, most patients experience the return of the symptoms after 3 months to 6 years of follow-up.[41] Botulinum toxin, though not always successful, can be useful in the treatment of convergence insufficiency patients who have failed other treatment options.
Cyclic esotropia
It is a rare form of strabismus in which esotropia alternates with binocular single vision. Classically, it follows a 48-hour cycle with 24 hours of orthotropia and 24 hours of manifest esotropia.[42] BTX-A is an appropriate first-line option for the treatment of cyclic deviations despite its limitations, as surgical treatments have reported a significant proportion of consecutive and recurrent deviations. Case reports suggest that botulinum toxin type A is an effective method to break the cycle in cyclic esotropia long-term.[43] [44] [45][46]
Convergence spasm or spasm of the near reflex
It is characterized by intermittent disjunctive movement of the eyes, excessive accommodation and miosis, often provoked by fixation at near. It is thought to be a form of dystonia and, as with other forms of dystonia it may be responsive to BTX treatment.[47] However, case reports have demonstrated limited efficacy of botulinum toxin for the treatment of convergence spasm, as the deviation and symptoms returned to baseline in most patients when the effect of BTX has worn out [47][48] [49]
Oculomotor paralysis / Incomitant Strabismus
Botulinum toxin is useful in the acute phase of an extraocular muscle paralysis to:[50]
- Increase the chance of recovery
- Reduce the recovery period
- Diminish the action of the antagonist muscle.
BTX-A is more often used for fourth (IV) and sixth (VI) cranial nerve palsies than for third (III) cranial nerve or other supra-nuclear paralysis. The effect of BTX-A remains controversial compared to conservative management.[51] [52]
[53]
In patients with VI cranial nerve palsies secondary to either trauma or tumor associated with a large deviation, injection of BTX within the first month is recommended, while If a microvascular etiology is suspected, injection of the toxin A may be performed if no improvement is observed after one month of follow-up.[54] [55] [56]
For fourth cranial nerve palsy, a study has shown a symptomatic improvement related to superior oblique paresis until recovery, however the study included no control group with a conservative management.[57] Another study suggests that BTX-A injections have limited value as primary treatment in chronic fourth cranial nerve palsy.[58] The superior rectus muscle is rarely injected as a long-term ptosis (albeit transient) may be induced as an adverse effect. Limited evidence is available in its favor.[59]
BTX-A may reduce the symptoms of an acute traumatic III palsy when injected in the lateral rectus muscle.[60]
Botulinum toxin may provide symptomatic relief in internuclear ophthalmoplegia by reducing its diplopia.[61]
Residual and consecutive deviations
For residual deviations after surgery, BTX-A injection is a safe and effective approach.[62] This is true especially for residual esotropia when the remaining deviation is <30 PD, and for exotropia. Results may be better if the injection is made in the first post-surgical week, in a small residual or consecutive exotropia.[63] BTX-A may be useful on residual strabismus in Miller Fisher syndrome, although the evidence is very limited.[64]
Restrictive strabismus
Restrictive strabismus may be less responsive to BTX-A injection. Injection is successful in about a third of cases.[65] In the setting of restrictive strabismus secondary to thyroid-associated ophthalmopathy, BTX-A injection is more effective when performed early to relax the inflammatory spasm that is characteristic of the acute phase of the condition. When compared to paralytic or comitant strabismus, the dosage used in these eyes is higher, while the interval between injections and the duration of effect is shorter for a slighter lower mean change of degree of deviation.[66] However, surgery can be avoided with BTX-A injection in some cases.
Small-angle deviations secondary to retina surgery can be managed with BTX-A injection.[67] [68] [69]
Botulinum toxin injection has a documented success rate in one-third of patients with restrictive strabismus (e.g. high myopia, cataract, orbital fractures, retinal surgery, Graves ophthalmopathy).[70]
As a Diagnostic Tool
Botulinum toxin can be used to evaluate how patients with long-term diplopia will experience their symptoms once the eyes are surgically realigned. The purpose of the procedure is to determine if the patient can compensate for the diplopia. The results may aid the surgeon in deciding the best treatment strategy.[16] In some cases of marked contracture, injection of BTX into the antagonist muscle may help in evaluating the degree of paralysis.
Summary of Recommendations
There is a very sizable interest in clinical guidelines for the treatment of each strabismic entity. The experience with botulinum toxin for strabismus is vast in specific countries.
The Spanish Society of Strabismus and Pediatric Ophthalmology (SEEOP) have elaborated a consensus document in 2017 [71] regarding the indications and use of botulinum toxin across various types of strabismus, as well as recommendations from the American Academy of Ophthalmology and the European Board of Ophthalmology subcommittee.
Below is a summary of recommendations derived from the document:
Congenital Esotropia (onset < 6 months age)
- Recommendation: Strong in favor of BTX use with a moderate evidence level (GRADE classification: B1)
- Dose recommended: 2,5 - 5U diluted in 0,1 mL of BTX-A in each internal rectus muscle in patients up to 2 years of age. One injection will suffice in most cases, a second injection may be necessary if the desired effect is not attained.
- Comments: large-angle esotropia (surgery should be preferred), cerebral palsy patients.
Acquired Esotropia of Infancy (onset > 6 months of age)
- Recommendation: Moderate in favor of BTX use with a moderate evidence level (GRADE classification: B/C1).
- Dose recommended: 2,5 - 5 U diluted in 0,1 mL of BTX-A in each internal rectus muscle in patients up to 3 to 4 years of age. Doses should be adjusted according to the age of onset, angle of deviation and duration of the esotropia. One injection will suffice in most cases; a second injection may be performed exceptionally.
- Comments: large-angle esotropia (BTX should be avoided in deviations > 40 PD). The American Academy of Ophthalmology and European Board of Ophthalmology subcommittee state that BTX is a valid therapeutic option and early injection is recommended.
Decompensated congenital esotropia in the adult
- Recommendation: Weak in favor of BTX in small-angle (up to 10-14 PD) esotropia with a weak evidence level (GRADE classification: C2).
- Dose recommended: Doses range from 2,5-5U. 2,5U is the most used dose in the internal rectus muscle and 5U in the external rectus muscle.
- Comments: Consider BTX on an individual basis. Surgery may be a more suitable option.
Infantile Exotropia
- Recommendation: Weak in favor of BTX in infantile intermittent exotropia with a weak evidence level (GRADE classification: C/D2).
- Dose recommended: Doses range from 1,5-5U. Lower doses are recommended (1,5-2,5U) to avoid hypercorrection of the deviation; however, 5U may be opted in all cases. Re-injection may be performed up to three times if clinical improvement is documented, in an attempt to delay surgical correction.
- Comments: Surgery provides better outcomes than BTX. Consider BTX on an individual basis. The American Academy of Ophthalmology and European Board of Ophthalmology subcommittee state that BTX is recommended in small-to-moderate angle deviations (<40PD), although multiple injections may be needed. BTX is recommended in combination with surgical resection in large-angle exotropia.
Infantile third, fourth and sixth cranial nerve paralysis
- Recommendation: Weak in favor of the use of BTX in infantile sixth cranial nerve paralysis with a weak evidence level (GRADE classification: D2).
- Dose recommended: Doses range from 2,5 - 5U. One to two injections are advocated, while some authors prefer to inject more frequently if the deviation persists.
- Comments: No evidence available regarding the use of BTX for third and fourth cranial nerve palsies.
Acute cranial nerve paralysis of the adult
- Recommendation: Strong in favor of the use of BTX in acute sixth cranial nerve paralysis presenting in the adult patient with a moderate evidence level (GRADE classification: B1) Weak in favor of the use of BTX in acute third and fourth cranial nerve paralysis with a weak level of evidence (C/D2).
- Dose recommended: 2,5-5U, larger doses may be considered for tumoral or traumatic etiologies or large deviations. The injection should be performed 1-2 times, preferable within 6-12 weeks of paralysis onset.
- Comments: BTX should be avoided in chronic cranial nerve paralysis.
Congenital decompensated fourth cranial nerve paralysis of the adult
- Recommendation: Weak against the use of BTX in congenital fourth cranial nerve paralysis presenting in the adult patient with a weak evidence level (GRADE classification: C/D2).
- Dose recommended: 5U of BTX-A may be injected in the inferior rectus for small vertical deviation. On the other hand, some authors recommend 2-3 U injection in the inferior oblique if a torsional component is documented. The injection should be performed only once.
- Comments: BTX may be considered in small or residual deviations, or in mild cases with a significant torsional component which may make prism adaptation more challenging. The American Academy of Ophthalmology and European Board of Ophthalmology subcommittee have no information regarding this indication.
Congenital nystagmus
- Recommendation: No recommendations exist regarding the use of BTX (GRADE classification: D)
- Dose recommended: 5-10 U in the retrobulbar space by the sub-tenon route if no null point is present 2,5 U intramuscularly if a strabismus or compensatory torticolis is present, the null point should be identified on exam.
- Comments: The American Academy of Ophthalmology and European Board of Ophthalmology subcommittee have no information regarding this indication.
Residual or consecutive deviations
- Recommendations: The American Academy of Ophthalmology and European Board of Ophthalmology subcommittee recommend the use of BTX preferably between 2 to 8 weeks after surgery.
- Comments: Results are similar to surgery in postsurgical residual or consecutive deviations
Alphabetical syndromes (A, V, X syndromes) and Dissociated Vertical Deviation (DVD)
- Recommendation: BTX is not effective for these indications (American Academy of Ophthalmology and European Board of Ophthalmology subcommittee).
Conclusions
The concept of a minimally invasive procedure for strabismus has long been an ambition of strabismus surgeons. There is vast experience regarding the use of botulinum toxin and, in the setting of strabismus, it is a treatment modality with favorable efficacy and safety profiles.
Potential benefits of BTXA injection for strabismus over eye muscle surgery include preservation of eye muscle and pulley anatomy with reduced scarring, shorter anesthesia time, reduced risk of anterior segment ischemia due to the preservation of anterior ciliary arteries, low risk of globe perforation, and potentially reduced risk of consecutive exotropia. Potential disadvantages include delayed orthotropia, multiple injections required to achieve the desired long-term effect, transient consecutive strabismus, and adverse short- or long-term effects of local spread of the toxin.[72]
More extensive trials are needed to further clarify the efficacy of the exotoxin across strabismic entities and different patient populations.
References
- ↑ Garcia-Ruiz PJ, Sanz-Cartagena P, Martinez-Castrillo JC, et al. Mitos y evidencias en el empleo de la toxina botulinica: neurofarmacologia y distonias [Myths and evidence on the use of botulinum toxin: neuropharmacology and dystonia]. Rev Neurol. 2018;66(5):163‐172.
- ↑ Gupta AD, Chu WH, Howell S, et al. A systematic review: efficacy of botulinum toxin in walking and quality of life in post-stroke lower limb spasticity. Syst Rev. 2018;7(1):1. Published 2018 Jan 5. doi:10.1186/s13643-017-0670-9
- ↑ Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Therapeutic options. J Am Acad Dermatol. 2019;81(3):669‐680. doi:10.1016/j.jaad.2018.11.066
- ↑ Tassorelli C, Sances G, Avenali M, et al. Botulinum toxin for chronic migraine: Clinical trials and technical aspects. Toxicon. 2018;147:111‐115. doi:10.1016/j.toxicon.2017.08.026
- ↑ Malde S, Fry C, Schurch B, et al. What is the exact working mechanism of botulinum toxin A and sacral nerve stimulation in the treatment of overactive bladder/detrusor overactivity? ICI-RS 2017. Neurourol Urodyn. 2018;37(S4):S108‐S116. doi:10.1002/nau.23552
- ↑ Jump up to: 6.0 6.1 Escuder AG, Hunter DG. The Role of Botulinum Toxin in the Treatment of Strabismus. Semin Ophthalmol. 2019;34(4):198‐204. doi:10.1080/08820538.2019.1620795
- ↑ Jump up to: 7.0 7.1 7.2 Whitcup SM. The History of Botulinum Toxins in Medicine: A Thousand Year Journey [published online ahead of print, 2019 Aug 27]. Handb Exp Pharmacol. 2019;10.1007/164_2019_271. doi:10.1007/164_2019_271
- ↑ Jump up to: 8.0 8.1 Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12(12):924‐927.
- ↑ Scott AB. Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc. 1981;79:734‐770.
- ↑ Solebo AL, Austin AM, Theodorou M, Timms C, Hancox J, Adams GGW. Botulinum toxin chemodenervation for childhood strabismus in England: National and local patterns of practice. PLoS One. 2018;13(6):e0199074. Published 2018 Jun 14. doi:10.1371/journal.pone.0199074
- ↑ Jump up to: 11.0 11.1 11.2 Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol Rev. 2017;69(2):200‐235. doi:10.1124/pr.116.012658
- ↑ Jump up to: 12.0 12.1 Kumar R, Dhaliwal HP, Kukreja RV, Singh BR. The Botulinum Toxin as a Therapeutic Agent: Molecular Structure and Mechanism of Action in Motor and Sensory Systems. Semin Neurol. 2016;36(1):10‐19. doi:10.1055/s-0035-1571215
- ↑ Vissenberg I, Dieleman-Smet H, Tassignon MJ. Botulinum A toxin therapy in ophthalmology: a report of patients with facial dystonias. Bull Soc Belge Ophtalmol. 1993;248:77‐82.
- ↑ Dutton JJ, Buckley EG. Long-term results and complications of botulinum A toxin in the treatment of blepharospasm. Ophthalmology. 1988;95(11):1529‐1534. doi:10.1016/s0161-6420(88)32977-5
- ↑ Gómez de Liaño R. The Use of Botulinum Toxin in Strabismus Treatment. J Binocul Vis Ocul Motil. 2019;69(2):51‐60. doi:10.1080/2576117X.2019.1601973
- ↑ Jump up to: 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 - Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord. 2004;19 Suppl 8:S92‐S100. doi:10.1002/mds.20022
- ↑ Hered R. Chapter 14: Surgery for extraocular muscles. In: 2018-2019 Basic and Clinical Science Course, Section 06: Pediatric Ophthalmology and Strabismus. PA: American Academy Ophthalmology. 2018: 241-242
- ↑ Ozkan SB, Topaloğlu A, Aydin S. The role of botulinum toxin A in augmentation of the effect of recession and/or resection surgery. J AAPOS. 2006;10(2):124‐127. doi:10.1016/j.jaapos.2005.11.011
- ↑ Bort-Martí AR, Rowe FJ, Ruiz Sifre L, Ng SM, Bort-Martí S, Ruiz Garcia V. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2023;3(3):CD006499. Published 2023 Mar 14. doi:10.1002/14651858.CD006499.pub5
- ↑ Jump up to: 20.0 20.1 Quereshy FA, Marechek A. Chapter 24: Botulinum Toxin and Facial Chemical Peels for Skin Enhancement. In Fonseca, RJ, ed. Oral and Maxillofacial Surgery, 3rd edition. PA: Saunders, Inc; 2017: 439-445
- ↑ Bentley CR, Dawson E, Lee JP. Active management in patients with ocular manifestations of myasthenia gravis. Eye (Lond). 2001;15(Pt 1):18‐22. doi:10.1038/eye.2001.6
- ↑ Tinley C, Dawson E, Lee J. The management of strabismus in patients with chronic progressive external ophthalmoplegia. Strabismus. 2010;18(2):41‐47. doi:10.3109/09273971003758388
- ↑ Tan M, Kim E, Koren G, Bozzo P. Botulinum toxin type A in pregnancy. Can Fam Physician. 2013;59(11):1183‐1184.
- ↑ Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52(1):13‐31. doi:10.1016/j.survophthal.2006.10.003
- ↑ Jump up to: 25.00 25.01 25.02 25.03 25.04 25.05 25.06 25.07 25.08 25.09 25.10 25.11 25.12 Rui, C., 2006. Estrabismo. 1st ed. Lisbon: Lidel, pp.140-150.
- ↑ Scaglione F. Conversion Ratio between Botox®, Dysport®, and Xeomin® in Clinical Practice. Toxins (Basel). 2016;8(3):65. Published 2016 Mar 4. doi:10.3390/toxins8030065
- ↑ Ravenni R, De Grandis D, Mazza A. Conversion ratio between Dysport and Botox in clinical practice: an overview of available evidence. Neurol Sci. 2013;34(7):1043‐1048. doi:10.1007/s10072-013-1357-1
- ↑ Jump up to: 28.0 28.1 28.2 28.3 28.4 28.5 28.6 28.7 28.8 28.9 Lavenant, F., 2004. La toxine botulinique. In: M. Espinasse-Berrod, ed., Strabologie: approches diagnostique et thérapeutique, 2nd ed. Paris: Elsevier-Masson.
- ↑ Campos EC, Schiavi C, Bellusci C. Critical age of botulinum toxin treatment in essential infantile esotropia. J Pediatr Ophthalmol Strabismus. 2000 Nov-Dec;37(6):328-32; quiz 354-5.
- ↑ Vieira M et al. Terapêutica do estrabismo com toxina botulínica. Rev Soc Port Oftamol, Vol XX, nr 2: 27-33.
- ↑ Binenbaum G, Chang MY, Heidary G, et al. Botulinum Toxin Injection for the Treatment of Strabismus: A Report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(12):1766-1776. doi:10.1016/j.ophtha.2021.05.009
- ↑ Levy N, Beylerian M, Dambricourt L, Esposito F, Denis D. Mydriase persistante après injection de toxine botulique dans le cadre d’une ésotropie précoce [Persistent mydriasis after botulinum toxin injection for infantile early onset esotropia]. J Fr Ophtalmol. 2019;42(10):e473‐e474. doi:10.1016/j.jfo.2019.05.027
- ↑ Speeg-Schatz C. Persistent mydriasis after botulinum toxin injection for congenital esotropia. J AAPOS. 2008;12(3):307‐308. doi:10.1016/j.jaapos.2007.12.012
- ↑ Campomanes AG de A, Binenbaum G, Eguiarte GC. Comparison of botulinum toxin with surgery as primary treatment for infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus {JAAPOS}. 1 avr 2010;14(2):111‑6.
- ↑ Issaho DC, Carvalho FRS, Tabuse MKU, Carrijo-Carvalho LC, de Freitas D. The Use of Botulinum Toxin to Treat Infantile Esotropia: A Systematic Review With Meta-Analysis. Invest Ophthalmol Vis Sci. 2017;58(12):5468‐5476. doi:10.1167/iovs.17-22576
- ↑ Wan MJ, Chiu H, Shah AS, Hunter DG. Long-term Surgical Outcomes for Large-angle Infantile Esotropia. American Journal of Ophthalmology. 1 mai 2018;189:155‑9.
- ↑ Wan MJ, Gilbert A, Kazlas M, Wu C, Mantagos IS, Hunter DG, et al. The Effect of Botulinum Toxin Augmentation on Strabismus Surgery for Large-Angle Infantile Esotropia. American Journal of Ophthalmology. 1 mai 2018;189:160‑5
- ↑ Razavi ME, Sharifi M, Armanfar F. Efficacy of Botulinum Toxin in the treatment of Intermittent Exotropia. Strabismus. 1 déc 2014;22(4):176‑81.
- ↑ Spencer RF, Tucker MG, Choi RY, McNeer KW. Botulinum toxin management of childhood intermittent exotropia. Ophthalmology. nov 1997;104(11):1762‑7.
- ↑ Kraft SP: Selected exotropia entities and principles of management. In Rosenbaum AL, and Santiago AP (eds): Clinical Strabismus Management. Philadelphia, PA: Saunders, 1999. pp. 176-201
- ↑ Dawson E, Child C, Lee JP & Adams GG (2012): The use of botulinum toxin in the management of convergence insufficiency exotropia. J Am Assoc Pediatr Ophthalmol Strabismus 16: e13.
- ↑ Paciuc-Beja M, Galicia-Alfaro VH, Retchkiman-Bret M. Unusual Presentation of an Uncommon Disease: 24-Hour Cyclic Esotropia. Case Rep Ophthalmol Med. 2019;2019:8602987. Published 2019 Mar 5. doi:10.1155/2019/8602987
- ↑ Akyuz Unsal AI, Özkan SB, Ziylan S. Role of Botulinum Toxin Type A in Cyclic Esotropia: A Long-Term Follow-up. J Pediatr Ophthalmol Strabismus. 2019;56(6):360‐364. doi:10.3928/01913913-20190909-01
- ↑ Wipf M, Bok-Beaube C, Palmowski-Wolfe A. Botulinum Toxin for the Treatment of Cyclic Strabismus in Children: Three Case Reports. Botulinumtoxin in der Therapie des zyklischen Innenschielens bei Kindern: 3 Fallberichte. Klin Monbl Augenheilkd. 2018;235(4):465‐468. doi:10.1055/s-0043-125060
- ↑ Jones A, Jain S. Botulinum toxin: a novel treatment for pediatric cyclic esotropia. J AAPOS. 2014;18(6):614‐615. doi:10.1016/j.jaapos.2014.07.155
- ↑ Abdelaal AM, Alhemidan A, Alabdulqader RA, Jeddawi LH. Cyclic Esotropia Managed With Botulinum A Toxin Injections: A Report of Four Cases and Literature Review. Cureus. 2023;15(9):e46266. Published 2023 Sep 30. doi:10.7759/cureus.46266
- ↑ Jump up to: 47.0 47.1 Kaczmarek BB, Dawson E, Lee JP. Convergence spasm treated with botulinum toxin. Strabismus. 2009;17(1):49‐51. doi:10.1080/09273970802678511
- ↑ Gupta S, Gan J, Jain S. Efficacy of Botulinum Toxin in the Treatment of Convergence Spasm. Strabismus. 2018;26(3):122‐125. doi:10.1080/09273972.2018.1483952
- ↑ Merino P, Rojas P, Gómez de Liaño P, Franco Iglesias G. Espasmo del reflejo de cerca. Tratamiento con toxina botulínica [Spasm of the near reflex. Treatment with botulinum toxin]. Arch Soc Esp Oftalmol. 2015;90(5):244‐246. doi:10.1016/j.oftal.2014.03.003
- ↑ Crouch ER. Use of botulinum toxin in strabismus. Curr Opin Ophthalmol. oct 2006;17(5):435‑40.
- ↑ Holmes JM, Beck RW, Kip KE, Droste PJ, Leske DA. Botulinum toxin treatment versus conservative management in acute traumatic sixth nerve palsy or paresis. J AAPOS. juin 2000;4(3):145‑9.
- ↑ Lee J, Harris S, Cohen J, Cooper K, MacEwen C, Jones S. Results of a Prospective Randomized Trial of Botulinum Toxin Therapy in Acute Unilateral Sixth Nerve Palsy. J Pediatr Ophthalmol Strabismus. 1 sept 1994;31(5):283‑6.
- ↑ Holmes JM, Droste PJ, Beck RW. The natural history of acute traumatic sixth nerve palsy or paresis. J AAPOS. oct 1998;2(5):265‑8.
- ↑ Wong ES, Lam CPS, Lau FHS, Lau WWY, Yam JCS. Botulinum toxin as an initial therapy for management of sixth nerve palsies caused by nasopharyngeal carcinomas. Eye (Lond). 2018;32(4):768‑74.
- ↑ Merino P, Gómez de Liaño P, Villalobo JMC, Franco G, Gómez de Liaño R. Etiology and treatment of pediatric sixth nerve palsy. J AAPOS. déc 2010;14(6):502‑5.
- ↑ Wagner RS, Frohman LP. Long-term results: botulinum for sixth nerve palsy. J Pediatr Ophthalmol Strabismus. 1989;26(3):106‐108.
- ↑ Bagheri A, Eshaghi M. Botulinum Toxin Injection of the Inferior Oblique Muscle for the Treatment of Superior Oblique Muscle Palsy. Journal of American Association for Pediatric Ophthalmology and Strabismus {JAAPOS}. 1 oct 2006;10(5):385‑8.
- ↑ Garnham L, Lawson JM, O’Neill D, Lee JP. Botulinum toxin in fourth nerve palsies. Australian and New Zealand Journal of Ophthalmology. 1997;25(1):31‑5.
- ↑ Dawson E, Ali N, Lee JP. Botulinum Toxin Injection into the Superior Rectus for Treatment of Strabismus. Strabismus. 1 mars 2012;20(1):24‑5.
- ↑ Talebnejad MR, Sharifi M, Nowroozzadeh MH. The role of Botulinum toxin in management of acute traumatic third-nerve palsy. Journal of American Association for Pediatric Ophthalmology and Strabismus {JAAPOS}. 1 oct 2008;12(5):510‑3.
- ↑ Murthy R, Dawson E, Khan S, Adams GG, Lee J. Botulinum toxin in the management of internuclear ophthalmoplegia. Journal of American Association for Pediatric Ophthalmology and Strabismus {JAAPOS}. 1 oct 2007;11(5):456‑9.
- ↑ Wutthiphan S. Botulinum toxin A in surgically overcorrected and undercorrected strabismus. J Med Assoc Thai. 2008;91 Suppl 1:S86-91.
- ↑ Couser NL, Lambert SR. Botulinum Toxin A Treatment of Consecutive Esotropia in Children. Strabismus. 1 déc 2012;20(4):158‑61.
- ↑ Wagner SK, Uddin N, Jain S. The Role of Botulinum Toxin in the Management of Ophthalmoplegia Secondary to Miller Fisher Syndrome. Neuroophthalmology. 18 août 2017;42(3):153‑5.
- ↑ Merino PS, Vera RE, Mariñas LG, Gómez de Liaño PS, Escribano JV. Botulinum toxin for treatment of restrictive strabismus. Journal of Optometry. 1 juill 2017;10(3):189‑93.
- ↑ X W, N L, Lk A, Jh W, Lj Y. [The application of botulinum toxin A in the treatment of restrictive strabismus in thyroid associated ophthalmopathy]. Zhonghua Yan Ke Za Zhi. 1 déc 2006;42(12):1063‑7.
- ↑ Scott AB. Botulinum Treatment of Strabismus Following Retinal Detachment Surgery. Arch Ophthalmol. 1 avr 1990;108(4):509‑10.
- ↑ Farr AK, Guyton DL. Strabismus after retinal detachment surgery. Curr Opin Ophthalmol. juin 2000;11(3):207‑10.
- ↑ Seaber JH, Buckley EG. Strabismus after retinal detachment surgery: etiology, diagnosis, and treatment. Semin Ophthalmol. mars 1995;10(1):61‑73.
- ↑ Merino PS, Vera RE, Mariñas LG, Gómez de Liaño PS, Escribano JV. Botulinum toxin for treatment of restrictive strabismus. Journal of Optometry. 1 juill 2017;10(3):189‑93.
- ↑ Martin SN et al. Guía Prático-Clínica Sobre La Indicacíon Y Administración De La Inyección De Toxina Botulínica En El Tratamiento De Los Estrabismos. Primera Edición. Prodiko; 2017.
- ↑ Binenbaum G, Chang MY, Heidary G, et al. Botulinum Toxin Injection for the Treatment of Strabismus: A Report by the American Academy of Ophthalmology. Ophthalmology. 2021;128(12):1766-1776. doi:10.1016/j.ophtha.2021.05.009


![Figure 4: Material required for the injection technique. [1] Blepharostat [2] Insulin syringe and 27 or 30G needle [3] Forceps [4] Botulinum toxin bottle](/w/images/thumb/d/d3/Botox_material.jpg/400px-Botox_material.jpg)
