Punctal Plugs
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Overview
Punctal plugs are a type of occlusion device inserted into the tear duct of an eye to prevent drainage of tears. Tears consist of aqueous, lipid, mucin, proteins and glycoproteins to provide lubrication and protection of the ocular surface[1]. The tear secreting glands of the eye include the lacrimal glands, Meibomian glands and goblet cells which secrete the aqueous, lipid and mucous components of the tear film respectively.
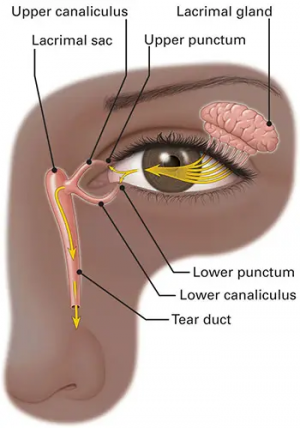
The lacrimal drainage system (figure 1) is responsible for collecting and directing tears from the ocular surface to the punctum, then the canaliculus and into the lacrimal sac which drains into the nasal cavity via the nasolacrimal duct. Punctal and canalicular plugs occlude the lacrimal drainage system and are often used as an adjunct to topical eye medications to treat various ocular diseases and syndromes. There are many different types of punctal and canalicular plugs available on the market, each with its advantages and disadvantages, depending on its shape, material and duration of use.
Classifications
Punctal versus Canalicular Plugs
Lacrimal occluders are classified based on the anatomic location to which they are inserted, either into the punctum or the canaliculus[2]. Puncta are located at the medial margins of the upper and lower eyelids. Previously inserted punctal plugs are easily visible on slit lamp examination and can be removed without difficulty. Because of their superficial nature, there is a lower risk of migration into the lacrimal drainage system but a higher risk for extrusion. Canalicular plugs are deeper within the lacrimal drainage system and carry a lower risk of extrusion but a higher risk of migration[3][4] . Moreover, canalicular plugs are more difficult to locate and retrieve, and may lead to repeated plug placements.
Punctal plugs typically have a conical tip which is the point of insertion, followed by a narrowed neck with a wide cap that rests on the surface of the puncta. The conical tip design allows for easier insertion and prevents extrusion, and the wide cap prevents distal migration. Various medical device manufacturers have developed different design modifications to the core components of punctal plugs including the tapered shaft, hollow collapsible nose, reservoir cap, ribbed shaft, stretched plug and perforated shaft (Table 1). Perforated punctal plugs are used in patients with acquired punctal stenosis to allow for tear flow into an otherwise obstructed lacrimal drainage system[5].
Canalicular plugs are generally cylinders most commonly made of silicone that are inserted into either the vertical or horizontal canaliculus. Herrick plugs are unique in that the posterior segment has a widened end to prevent migration. Other canalicular plugs are made of materials that are thermosensitive or absorptive. (Table 2)
Thermosensitive Plugs
The thermosensitive acrylic canalicular plug, SmartPlug by Medennium, is 6 mm in length and 0.4 mm in diameter prior to insertion. Once inside the canaliculus and the temperature increases, SmartPlug shortens in length to 1.5 mm and widens in diameter to 1 mm.
Absorptive Plugs
Form Fit Canalicular Plugs take advantage of hydrogel’s water-absorbing properties. Once the canalicular plug is inserted and comes into contact with the tear film, the hydrogel plugs expand into a soft and gelatinous material within the canaliculus.
Temporary versus prolonged occlusion
The duration of occlusion provided is variable and dependent on the material used. In general, temporary plugs are initially used to ensure epiphora does not occur and that occlusion provides symptomatic relief before using prolonged occlusion devices. Collagen plugs are inserted into the canaliculi and dissolve within a few weeks. Following a successful trial of temporary occlusion, prolonged occluders lasting months to years may be used[2]. These longer lasting plugs may be made of silicone, Hydrogel, Vicryl or other polymers. An alternative to permanent or long term plugs is punctual cauterization, in which the puncta are scarred closed.
Table 1. Punctal Plug Features and Retention Rates
| Design | Brand name | Features | Retention rate | Material |
| Tapered shaft | EaglePlug | Designed to fit snugly in the punctal opening | 50% at 60 days[6] | Silicone |
| Collapsible nose | Parasol plug | Hollow nose collapses on insertion and re-expands once inserted. Reduces need for dilators | 68% at 6 months[7] | Silicone |
| Reservoir | AquaFlo, LacriPro | Indentations capture tears and reduce foreign body sensation | N/A | Silicone |
| Ribbed shaft | SuperFlex plug | Flexible designs provides easy insertion and improved retention | 30-32% at 6 months[7][8] | Silicone |
| Stretched plug | Painless Punctal Plug | Preloaded in stretched position and returns to natural shape after insertion. One-size-fits all | 70-93% at 6 months[8][9] | Silicone |
| Perforated shaft | PVP Perforated Plug, Soft Plug Flow Control, EaglePlug TearFLow and Flow Controller, BVI Micro Flow | Patency allows for tear drainage | 97% at 1 month[5] | Silicone (and PVP coating |
Table 2. Canalicular Plug Features and Retention Rates
| Design | Brand name | Features | Retention rate | Material |
| Temporary short-term | Soft Plug collagen, UltraPlug, Eagle collagen | Dissolves in 1-2 weeks after insertion | N/A | Collagen |
| Temporary long-term | Soft Plug Extended, Vera90/180, BVI Extend 180 | Dissolves in 3-6 months after insertion | N/A | Polydioxanone |
| Thermosensitive permanent material | SmartPLUG | Shortens and widens when temperature increases within canaliculus | 98% at 6 months[10] | Thermodynamic hydrophilic acrylic |
| Absorptive permanent material | FORM FIT | Expands into soft and gelatinous material upon contact with tear film | N/A | Hydrogel |
| Permanent | Herrick Plug | Opaque-design allows for post-insertion identification by transillumination | N/A | Silicone |
Insertion
Punctal and canalicular plugs may be inserted into the upper and lower punctum or canaliculus, though they are typically inserted into the lower eyelids first. Topical anesthetics such as proparacaine drops are sometimes used prior to insertion of lacrimal plugs but are not required. There are three general steps to insertion of punctal and canalicular plugs:
Gauging
Before insertion of a plug, the diameter of the punctum or canaliculus must be measured using a punctal gauge to determine the appropriate size of the plug required. The punctal gauge is initially inserted vertically to the puncta and if needed, deeper into the vertical canaliculus. To measure the horizontal canaliculus, the punctal gauge is then rotated horizontally towards the midline.
Dilation
After appropriate gauging of the punctum or canaliculus, some punctal plug designs require dilation using a lacrimal dilator for ease of insertion. In preloaded punctal plugs, the dilator is on the opposite end of a plug inserter.
Insertion
Most punctal and canalicular plugs are inserted utilizing an inserter which help guide and release the plug when both sides of the inserter are squeezed. Some plugs come preloaded in the inserter while others require manual loading into the inserter using forceps. Unlike other canalicular plugs, the Smartplug is inserted into the canaliculus utilizing specialized forceps with one-third left protruding from the punctum.
Removal
Non-dissolving punctal and canalicular plugs are generally not removed unless a complication arises. Forceps are used to easily remove punctal plugs that rest at the punctal opening. Pressured saline irrigation is often used to remove punctal plugs that have migrated deeper into the lacrimal drainage system or canalicular plugs which are not easily localized. Surgical intervention by canaliculotomy and dacryocystorhinostomy have been described to successfully remove canalicular plugs complicated by canaliculitis and pyogenic granulomas[11].
Indications
Dry eye syndrome
Dry eye syndrome, also known as keratoconjunctivitis sicca, affects an estimated 6.8% of the adult population in the United States[12]. Dysfunctions in tear film production and tear retention result in symptoms of dry eyes. There are numerous causes of dry eye syndrome, including systemic autoimmune diseases (Sjögren’s syndrome, graft-versus-host disease, lupus, rheumatoid arthritis), contact lens use, diabetes mellitus, dry weather, hormonal changes, medications and refractive corneal surgeries. Treatment of the underlying disease followed by artificial tears or lubricating eye drops are considered first line for dry eye syndrome. In some studies, use of punctal plugs as an adjunct have been demonstrated to improve signs and symptoms of dry eye syndrome and reduce the need for artificial lubrication use[13][14][15]. Likewise, punctal plugs have provided relief in dry eye symptoms not improved with artificial lubrication[15]. However, a systematic review of 18 studies by the Cochran group concludes that improvement on symptoms and signs was inconclusive [16].
Increasing topical medication availability
By occluding the lacrimal drainage system with punctal plugs, topical pharmacologic and nonpharmacologic agents can remain on the ocular surface for longer periods of time. In patients with open-angle glaucoma and ocular hypertension, silicone punctal plugs used in conjunction with a prostaglandin analogue have been shown to provide a significant decrease in intraocular pressures compared to the control contralateral eye group[17][18]. Non-pharmacologic agents such as artificial tears are typically used four to six times per day for dry eye syndrome[19] and with punctal plugs, use of artificial tears was significantly decreased[14].
Medication delivery system
Non-compliance with medication eye drop treatment have been reported to be about 40%[20]. To address this issue, sustained drug delivery systems for treatment of ocular hypertension and glaucoma are an active area of research. Prostaglandin punctal plugs, as well as prostaglandin contact lens and intraocular implants, are being investigated as an alternative to medication eye drop therapy[21].
Additional indications for punctal plugs include acquired punctal stenosis, protecting the lacrimal system from developing stenosis due to topical medications (mitomycin-c),[22] superior limbic keratoconjunctivitis and other ocular conditions that affect the tear film.
Complications
While punctal and canalicular plugs are generally well-tolerated, there are a number of complications that exist and include:
- Plug loss
- Foreign body sensation
- Epiphora
- Extrusion
- Migration
- Pyogenic granuloma [23]
- Canaliculitis
- Dacryocystitis
- Corneal injury [24]
References
- ↑ Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 08 2020;197:108115.
- ↑ Jump up to: 2.0 2.1 Salmon J. Kanski's Clinical Ophthalmology. 9th ed: Elsevier; 2019. p. 100-101, 163-165.
- ↑ Best AL, Labetoulle M, Legrand M, M'garrech M, Barreau E, Rousseau A. Punctal and canalicular plugs: Indications, efficacy and safety. J Fr Ophtalmol. Mar 2019;42(3):e95-e104.
- ↑ Jehangir N, Bever G, Mahmood SM, Moshirfar M. Comprehensive Review of the Literature on Existing Punctal Plugs for the Management of Dry Eye Disease. J Ophthalmol. 2016;2016:9312340.
- ↑ Jump up to: 5.0 5.1 Konuk O, Urgancioglu B, Unal M. Long-term success rate of perforated punctal plugs in the management of acquired punctal stenosis. Ophthalmic Plast Reconstr Surg. 2008 Sep-Oct 2008;24(5):399-402.
- ↑ Parikh NB, Francis JH, Latkany RA. Retention rate of silicone punctal plugs placed by residents in a general clinic setting. Ophthalmic Plast Reconstr Surg. 2010 Nov-Dec 2010;26(6):400-2.
- ↑ Jump up to: 7.0 7.1 Brissette AR, Mednick ZD, Schweitzer KD, Bona MD, Baxter SA. Punctal Plug Retention Rates for the Treatment of Moderate to Severe Dry Eye: A Randomized, Double-Masked, Controlled Clinical Trial. Am J Ophthalmol. Aug
- ↑ Jump up to: 8.0 8.1 Kaido M, Ishida R, Dogru M, Tsubota K. Comparison of retention rates and complications of 2 different types of silicon lacrimal punctal plugs in the treatment of dry eye disease. Am J Ophthalmol. Apr 2013;155(4):648-653, 653.e1.
- ↑ Javate RM, Dy IE, Buyucan KF, Ma Guerrero EE. Retention rates and benefits of Painless Punctal Plug F(TM) in dry eye patients. Orbit. Jun 2016;35(3):126-31.
- ↑ Chen SX, Lee GA. SmartPlug in the management of severe dry eye syndrome. Cornea. Jun 2007;26(5):534-8.
- ↑ Huang CJ, Lu CJ, Tu WH, et al. Analysis of SmartPlug Insertion-Related Complications. Eye Contact Lens. Nov 2018;44 Suppl 2:S333-S337.
- ↑ Farrand KF, Fridman M, Stillman I, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. Oct 2017;182:90-98.
- ↑ Yen MT, Pflugfelder SC, Feuer WJ. The effect of punctal occlusion on tear production, tear clearance, and ocular surface sensation in normal subjects. Am J Ophthalmol. 2001 Mar;131(3):314-23. doi: 10.1016/s0002-9394(00)00822-9. PMID: 11239863.
- ↑ Jump up to: 14.0 14.1 Balaram M, Schaumberg DA, Dana MR. Efficacy and tolerability outcomes after punctal occlusion with silicone plugs in dry eye syndrome. Am J Ophthalmol. Jan 2001;131(1):30-6.
- ↑ Jump up to: 15.0 15.1 Marcet MM, Shtein RM, Bradley EA, et al. Safety and Efficacy of Lacrimal Drainage System Plugs for Dry Eye Syndrome: A Report by the American Academy of Ophthalmology. Ophthalmology. Aug 2015;122(8):1681-7.
- ↑ Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev. 2017;6(6):CD006775. Published 2017 Jun 26. doi:10.1002/14651858.CD006775.pub3
- ↑ Opitz DL, Tung S, Jang US, Park JJ. Silicone punctal plugs as an adjunctive therapy for open-angle glaucoma and ocular hypertension. Clin Exp Optom. Sep 2011;94(5):438-42.
- ↑ Sherwin JC, Ratnarajan G, Elahi B, Bilkiewicz-Pawelec A, Salmon JF. Effect of a punctal plug on ocular surface disease in patients using topical prostaglandin analogues: a randomized controlled trial. Clin Exp Ophthalmol. 11 2018;46(8):888-894.
- ↑ Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 07 2017;15(3):575-628.
- ↑ Vandenbroeck S, De Geest S, Dobbels F, Fieuws S, Stalmans I, Zeyen T. Prevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian Compliance Study in Ophthalmology (BCSO). J Glaucoma. Sep 2011;20(7):414-21.
- ↑ Aref AA. Sustained drug delivery for glaucoma: current data and future trends. Curr Opin Ophthalmol. Mar 2017;28(2):169-174.
- ↑ Kopp ED, Seregard S. Epiphora as a side effect of topical mitomycin C. Br J Ophthalmol. 2004 Nov;88(11):1422-4. doi: 10.1136/bjo.2004.048033. PMID: 15489486; PMCID: PMC1772395.
- ↑ Kim BM, Osmanovic SS, Edward DP. Pyogenic granulomas after silicone punctal plugs: a clinical and histopathologAref AA. Sustained drug delivery for glaucoma: current data and future trends. Curr Opin Ophthalmol. Mar 2017;28(2):169-174. ic study. Am J Ophthalmol. Apr 2005;139(4):678-84.
- ↑ Barnett BP, Akpek EK, Jun AS. Punctal eversion with silicone plug resulting in ocular surface trauma. BMJ Case Rep. 05 2020;13(5)