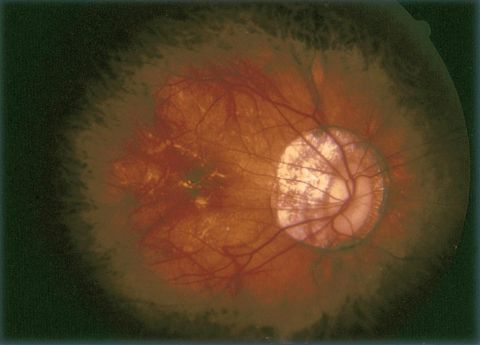
Pathologic Myopia (Myopic Degeneration)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
© 2020 American Academy of Ophthalmology.[1]

Disease Entity
Pathologic Myopia (Myopic Macular Degeneration)
Disease
Pathologic myopia represents a subgroup of myopia and affects up to 3% of the world population.[2] Vision loss related to pathologic myopia is of great clinical significance as it can be progressive and irreversible and affects individuals during their most productive years. High myopia is defined as refractive error of at least -6.00 D or an axial length of 26.5 mm or more.[2] The definition of pathologic myopia in early studies was inconsistent and mostly revolved around a combination of refractive error and axial length, which may simply reflect a high degree of myopia. Additionally, there was no clear evidence for the cutoff values chosen. In recent years, the definition of pathologic myopia has shifted to "the presence of myopic maculopathy equal to or more severe than diffuse chorioretinal atrophy."[3] Myopic maculopathy includes diffuse chorioretinal atrophy, patchy chorioretinal atrophy, lacquer cracks, myopic choroidal neovascularization (myopic CNV), and CNV-related macular atrophy.
Epidemiology
The overall global prevalence is estimated to be 0.2%-3.8% with regional variability, but varying definitions of pathologic myopia used in early epidemiological studies may limit the comparability of findings.[4] The prevalence of pathologic myopia–related visual impairment has been reported as 0.1%-0.5% in European studies and 0.2%-1.4% in Asian studies.[5]
Pathophysiology
The main factors proposed for driving the development of pathologic myopia are elongation of the axial length and posterior staphyloma.[6] Biomechanical forces related to axial elongation of the eye result in stretching of the ocular layers and progressive thinning of the retina, choroid, and sclera.[2]
Risk Factors
Both environmental and genetic factors play a role in the development of myopia, which is further discussed in the corresponding article. Currently, the roles of known myopia-associated genetic variants have not been well established in the development of pathologic myopia.[7] Primary risk factors for pathologic myopia include older age, greater axial length, and higher myopic spherical equivalent.[4] Additional possible risk factors such as female gender, larger optic disc area, and family history of myopia have been suggested.[2][8][5] The role of education level in the development of pathologic myopia is currently unclear.[4][9]
Diagnosis
History
Patients may describe needing to wear thick glasses as a child or slowly progressive vision loss. They may endorse new metamorphopsia or scotoma when vision-limiting macular complications develop.
Physical Examination
Assessment of visual acuity, intraocular pressure, and pupillary reaction and dilated fundus examination are essential. A thorough macular examination and peripheral depressed examination are key to detecting complications related to pathologic myopia: in particular, lacquer cracks, myopic schisis, or choroidal neovascularization in the macular area and holes or tears in the periphery of the retina. Assessment of visual fields and Amsler grid testing may be beneficial.
Symptoms
Patients may be asymptomatic during the slowly progressive attenuations of the retinal pigment epithelium (RPE) and choroid. In cases where central CNV or foveal schisis develops, the patient may note a focal area of blurring, metamorphopsia, or scotoma that can rapidly cause serious decline in central vision. Peripheral CNV may go undetected.
Signs
Progressive RPE thinning and attenuation develops in various clinical stages throughout the fundus. A tessellated appearance corresponding to irregular distribution of RPE atrophy and variable light reflection may be appreciated even in young patients with high myopia. When RPE attenuation surrounds the optic disc, this hypopigmented finding is described as peripapillary atrophy.
Commonly, the optic disc has an oval appearance en face and is referred to as a tilted disc. The optic nerve appears to insert into the elongated globe at an angle. The tilted appearance is characterized by temporal flattening of the disc due in part to peripapillary scleral expansion. As a result, a hypopigmented myopic crescent or myopic cone is seen where sclera is directly visible. In intermediate disease, choroidal vessels will be seen more prominently beneath the atrophic RPE. However, with progressive disease the choroid itself also atrophies and the choroidal vessels may become less noticeable.
Lacquer cracks are irregular yellow-appearing bands often seen in the posterior pole and are present in 4.2% of eyes with an axial length of at least 26.5 mm.[2] These represent breaks in Bruch membrane and may be foci of future choroidal neovascularization. It has been reported that among patients with lacquer cracks, 29.4% eventually develop CNV.[10] Over time these breaks can expand and stretch, and in late stages they may resemble the appearance of geographic atrophy similar to that seen in advanced nonneovascular age-related macular degeneration (AMD).
Fuchs spot (also referred to as Forster-Fuchs spot) is an area of RPE hyperplasia suspected to be the response of the RPE to previous regressed CNV. Myopic CNV is the most common cause of vision loss in high myopia and has been reported in 5% to 10% of cases of pathologic myopia.[10][11]
Staphyloma development, characterized by outpouching of scleral tissue typically involving the optic disc or macula, is a common occurrence, estimated in 35% of eyes with high myopia.[12] This can be difficult to appreciate with biomicroscopy but is evident on optical coherence tomography (OCT) or B-scan ophthalmologic ultrasound. Staphylomata are commonly associated with lacquer cracks, RPE attenuation, epiretinal membrane, and macular or foveal schisis.
Classification
Given the lack of a centralized definition and terminology for pathologic myopia, an international group of experts in high myopia developed a simplified, systematic classification based on a meta-analysis of pathologic myopia (META-PM).[13] Myopic maculopathy was classified into 5 different categories based on atrophic change:
- Category 0: no macular degenerative lesions
- Category 1: tessellated fundus only
- Category 2: diffuse chorioretinal atrophy
- Category 3: patchy chorioretinal atrophy
- Category 4: macular atrophy
Recently, it has been noted that many patients with macular changes from pathologic myopia are not sufficiently represented by an atrophy-centered classification system. A newly proposed ATN classification system for myopic maculopathy includes atrophic (A), tractional (T), and neovascular (N) components.[6]
Clinical Diagnosis
Diagnosis is based on fundus examination with identification of characteristic features, lack of more plausible cause for the degeneration, and diagnostic testing as described below.
Diagnostic Procedures
Fluorescein Angiography
Fluorescein angiography (FA) is useful for evaluating myopic patients for development of CNV. Early images may show transmission defects in patches or areas of RPE atrophy in the macula and/or around the optic disc. Angiography can identify lacquer cracks in early and transit phases by linear distribution of transmission defect. In pathologic myopia, the development of CNV tends to be smaller and less exudative compared to CNV seen in AMD. Myopic CNV may appear as a focus of hyperfluorescence with a rim of hypofluorescence corresponding to hyperpigmentation at the border of the lesion. Any associated hemorrhage will result in blocked fluorescence. Leakage is seen in late images with or without blurring of the pigmented rim. The leakage present with myopic CNV is more subtle than with CNV related to AMD, and it is common that the CNV leakage may be partially or completely obscured by overlying subretinal hemorrhage.
Indocyanine Green Angiography
Indocyanine green (ICG) angiography may be more sensitive for detecting CNV as the vascular leakage in pathologic myopia is typically less prominent than for AMD-related pathology and can be more easily missed on fluorescein angiography. Despite subtler findings on imaging studies with myopic CNV compared to those with AMD-related CNV, patients often note that these smaller lesions alter the visual perception significantly.
Optical Coherence Tomography
Spectral-domain OCT (SD-OCT) has been the preferred method of following myopic CNV over time. Although FA or ICG angiography is more sensitive for detection, SD-OCT is a noninvasive, quantifiable, and widely available method for monitoring CNV. The CNV will be visible as a subretinal hyperreflective lesion with or without intraretinal fluid, subretinal fluid, or pigment epithelial detachment. The physical topography of staphyloma and thinned retinal layers pose challenges to interpreting OCT in the myopic patient. However, the resolution is appropriate for most patients. Spectral-domain OCT also allows for detection of myopic foveoschisis or macular hole formation. For this reason, evaluating patients with SD-OCT permits better demonstration of the macular anatomy compared to a biomicroscopic examination.[8] OCT angiography (OCTA) has more recently emerged as a noninvasive technique for detecting and confirming the presence of myopic CNV.[14][15] One study identified higher vessel length density in the CNV on OCTA was correlated with more resistance to anti-VEGF therapy and may portend a worse prognosis without more frequent injections.[16] Another study has identified vascular branching and anastomoses/loops on OCTA as indicators of CNV activity.[17]
Recently, swept-source and ultra-wide-field (UWF) OCT have been implemented to evaluate various tissues affected by pathologic myopia. Swept-source OCT uses a wavelength-sweeping laser as the light source and has less sensitivity roll-off with tissue depth than conventional spectral-domain OCT. Using a longer central wavelength, penetration into deeper tissues and enhanced evaluation of the choroid and sclera are potentially possible. UWF-OCT is similar to swept-source OCT but uses multiple scan lines to generate scan maps, which has been utilized to visualize posterior staphylomas, myopic macular retinoschisis, and dome-shaped macula. The data provided by these newer imaging techniques may help in understanding the pathophysiology of pathologic myopia as well as new therapeutic approaches.[18]
Management
Patients with stable high myopia may be followed annually for visual acuity, refraction, and general ophthalmic health. If CNV or other complications develop, patients are followed more closely as determined by their treatment regimen.
Medical Therapy
There is no topical, local, or systemic pharmacotherapy or surgery that is known to alter effectively the increase in axial length and thinning that occurs in the sclera, choroid, and retina of eyes with pathologic myopia. Animal and in vitro studies have shown some promise in scleral collagen crosslinking to arrest the progression of pathologic myopia, but further research is needed to elucidate these effects.[19] There are, however, treatments available for CNV, a major complication of pathologic myopia.
Laser Photocoagulation
The first widely adopted therapy for CNV in pathologic myopia was photothermal laser ablation of the new vessels. This treatment was complicated by a high rate of recurrence and the tendency of the photocoagulation scars to expand over time, increasing the risk of central vision loss as the border of the laser scar encroached or expanded into the fovea.[8]
Photodynamic Therapy
Photodynamic therapy (PDT) replaced thermal laser in the later 1990s, supported by evidence from the Verteporfin in Photodynamic Therapy (VIP) study. The advantage of PDT was the potential to selectively target neovascular vessels with lesser collateral damage to the retina, RPE, and choroid and to limit the development of large scars seen in photothermal laser treatment. The VIP study showed that PDT was better than placebo in reducing moderate vision loss at 12 months. However, by 24 months, there was no statistically significant difference between treatment arms.[2] PDT has been limited by the observation that up to 13% still have moderate vision loss despite treatment and up to 57% have persistent leakage at 1 year.
Anti–Vascular Endothelial Growth Factor Injections
Anti–vascular endothelial growth factor (VEGF) therapy is now considered the first-line intervention for eyes with myopic CNV.[8] The initial evidence was based primarily on retrospective studies and clinician experience. A growing number of prospective and randomized trials have been published or are currently underway. One such trial was RADIANCE (A Randomized Controlled Study of Ranibizumab in Patients With Choroidal Neovascularization Secondary to Pathologic Myopia), a multicenter, randomized, controlled trial comparing intravitreal ranibizumab to PDT in the treatment of myopic CNV. This study reported improved visual acuity at 12 months in the ranibizumab treatment arm.[20] The REPAIR study (Prospective, Multicenter Trial of Ranibizumab in Choroidal Neovascularization Due to Pathological Myopia) also demonstrated the efficacy and safety of ranibizumab in myopic CNV.[21] Meanwhile, the MYRROR study (Intravitreal Aflibercept Injection in Patients With Myopic Choroidal Neovascularization) found aflibercept to be efficacious and safe in myopic CNV in an Asian population.[22] Current data indicate that patients are more likely to have clinical response and resolution of CNV within 1-3 injections as compared to long-term continuing injections in macular degeneration complicated by CNV.[20] Currently, ranibizumab 0.5 mg is approved by the US Food and Drug Administration (FDA) for the treatment of myopic CNV.
Surgery
Patients with decreased vision in the setting of maculoschisis or foveoschisis may benefit from vitrectomy to relieve traction on the fovea and prevent formation of macular holes or macular retinal detachment. This is further discussed in the article myopic traction maculopathy. Patients with maculoschisis complicated by macular holes or significant chorioretinal atrophy have a worse visual prognosis. However, 80% of those with foveal detachment and 50% of those with retinoschisis may have improved vision following surgery.[2] Gas or silicone oil tamponade is essential in cases of macular hole with or without detachment as this encourages re-apposition of retinal layers. Internal limiting membrane peeling, likewise, is seen as an important asset for relief of traction and improved macular hole closure rates.
Retinal detachments may also develop. If confined to the area of staphyloma these may sometimes be monitored without intervention. Prompt surgery is indicated if any progression is identified. The use of a macular buckle to treat the staphyloma as well as ongoing vitreous traction or detachment is reported to have higher foveal reattachment rates than vitrectomy alone in cases of recurrent detachment. Direct macular buckling even without vitrectomy has had good rates of retinal reattachment, likely because of alteration of distribution of vector forces allowing for improved contact of the RPE with the neurosensory retina. However, this approach is generally considered second line due to postoperative complications such as metamorphopsia and alteration of choroidal circulation. It has also been suggested that concomitant resolution of foveoschisis, retinal detachment, and macular hole has been achieved more frequently with macular buckling than with vitrectomy.[19] However, the role of macular buckling is still controversial.
Complications
Complications associated with visual morbidity in pathologic myopia include progressive thinning and atrophy resulting in photoreceptor loss, development of CNV, macular hole, pigment epithelial detachments, and macular or foveal detachments. Ninety percent of patients with CNV are expected to have atrophy surrounding any previously regressed CNV.[2] Peripheral retinal detachment is another complication.
Prognosis
Progressive visual decline in the form of progressive chorioretinal thinning, atrophy, and stretching of existing scars is expected in about 40% of patients with pathologic myopia.[2] In one study over a 6-year period, 1.2% of myopic eyes developed pathologic myopia and 17% with existing pathologic myopia experienced progression. Baseline myopia severity and axial length were strong predictors of worsening prognosis, and these factors were associated with poorer visual acuity and vision-related quality of life.[23]
Prevention
Recent studies are emerging suggesting interventions that may help lower the risk of myopia progression (see https://eyewiki.aao.org/Myopia#Primary_Prevention).
References
- ↑ American Academy of Ophthalmology. Pathologic myopia with tilted disc and peripapillary atrophy of RPE and choroid. https://www.aao.org/image/pathologic-myopia-with-tilted-disc-peripapillary-a-2 Accessed May 19, 2020.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Ryan et al. Retina. 2013.
- ↑ Ohno-Matsui K. Pathologic myopia. Asia Pac J Ophthalmol (Phila). 2016;5:415-423.
- ↑ Jump up to: 4.0 4.1 4.2 Wong YL, Sabanayagam C, Ding Y, et al. Prevalence, risk factors, and impact of myopic macular degeneration on visual impairment and functioning among adults in Singapore. Invest Ophthalmol Vis Sci. 2018;59(11):4603-4613.
- ↑ Jump up to: 5.0 5.1 Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systemic review. Am J Ophthalmol. 2014;157(1):9-25.e12.
- ↑ Jump up to: 6.0 6.1 Ruiz-Medrano J, Montero JA, Flores-Moreno I, Arias L, Garcia-Layana A, Ruiz-Moreno JM. Myopic maculopathy: current status and proposal for a new classification and grading system (ATN). Prog Retin Eye Res. 2019;69:80-115.
- ↑ Wong YL, Hysi P, Cheung G, et al. Genetic variants linked to myopic macular degeneration in persons with high myopia: CREAM Consortium. PLoS One. 2019;14(8):e0220143.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 Adatia FA, Luong M, Munro M, Tufail A. The other CNVM: a review of myopic choroidal neovascularization treatment in the age of anti-vascular endothelial growth factor agents. Surv Ophthalmol. 2015;60(3):204-215.
- ↑ Liu HH, Xu L, Wang YX, Wang S, You QS, Jonas JB. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology. 2010;117:1763-1768.
- ↑ Jump up to: 10.0 10.1 Spaide et al. Pathologic Myopia. 2014.
- ↑ Neelam K, Cheung CM, Ohno-Matsui K, Lai TY, Wong TY. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012;31(5):495-525.
- ↑ Raecker ME, Park DW, Lauer AK. Diagnosis and treatment of CNV in myopic macular degeneration. EyeNet. April 1, 2015:35-37.
- ↑ Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159(5):877-883.e7.
- ↑ Miyata M, Ooto S, Hata M, et al. Detection of myopic choroidal neovascularization using optical coherence tomography angiography. Am J Ophthalmol. 2016;165:108-114. doi:10.1016/j.ajo.2016.03.009
- ↑ Querques L, Giuffrè C, Corvi F, et al. Optical coherence tomography angiography of myopic choroidal neovascularisation. Br J Ophthalmol. 2017;101(5):609-615. doi:10.1136/bjophthalmol-2016-309162
- ↑ Hosoda Y, Miyata M, Uji A, et al. Novel predictors of visual outcome in anti-VEGF therapy for myopic choroidal neovascularization derived using OCT angiography. Ophthalmol Retina. 2018;2(11):1118-1124. doi:10.1016/j.oret.2018.04.011
- ↑ Li S, Sun L, Zhao X, et al. Assessing the activity of myopic choroidal neovascularization: comparison between optical coherence tomography angiography and dye angiography. Retina. 2019;40(9):1757-1764. doi:10.1097/IAE.0000000000002650
- ↑ Ohno-Matsui K, Fang Y, Shinohara K, Takahashi H, Uramoto K, Yokoi T. Imaging of pathologic myopia. Asia Pac J Ophthalmol (Phila). 2019;8:172-177.
- ↑ Jump up to: 19.0 19.1 Saw SM, Matsumura S, Hoang QV. Prevention and management of myopia and myopic pathology. Invest Ophthalmol Vis Sci. 2019;60(2):488-499.
- ↑ Jump up to: 20.0 20.1 Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014;121(3):682-692.e2.
- ↑ Tufail A, Narendran N, Patel PJ, et al. Ranibizumab in myopic choroidal neovascularization: the 12-month results from the REPAIR study. Ophthalmology. 2013;120(9):1944-1945.e1.
- ↑ Ikuno Y, Ohno-Matsui K, Wong TY, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology. 2015;122(6):1220-1227.
- ↑ Wong YL, Sabanayagam C, Wong CW, et al. Six-year changes in myopic macular degeneration in adults of the Singapore Epidemiology of Eye Diseases Study. Invest Ophthalmol Vis Sci. 2020;61(4):14.