Kimura's Disease
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Kimura’s disease is a chronic, benign inflammatory disease of the subcutaneous tissue, which most often presents with painless, subcutaneous nodules of the head and neck in association with eosinophilia and elevated serum IgE.[1][2] The orbit and associated adnexal tissues may be affected, as well as the salivary glands. The disease was first reported as “eosinophilic hyperplastic lymphogranuloma” by Kim and Szeto in the Chinese literature in 1937, although it derives its eponym from a later report by Kimura et al. in the Japanese literature in 1948.[3][4] The English literature has often confused Kimura’s disease with angiolymphoid hyperplasia with eosinophilia (ALHE), although there is a growing consensus that these represent two distinct entities (see below for a detailed discussion).
Etiology
The etiology of Kimura’s disease is poorly understood, but is hypothesized to represent an autoimmune or allergic process.[1][5] Elevated levels of TNF-alpha, IL-4, IL-5 and IL-13 have been documented in patients with Kimura’s disease, and may account for alterations in Th cell profiles and in eosinophil and mast cell activity in the disease process.[5] Other studies have demonstrated an association between Kimura’s disease and the presence of anti-Candida IgE, however, no definitive, causal relationship has ever been demonstrated between Kimura’s disease and any known infectious or toxic insult.[1]
Epidemiology
Kimura’s disease is more prevalent in young males of Asian ancestry.[1][2][6] However, cases have been documented in patients of all ethnicities and in patients aged 1 through 66.[6] A study of patients in the United States demonstrated a male-to-female ratio of 6:1, and identified cases in patients of East Asian, Caucasian, African American, Hispanic, and Middle-Eastern descent.[7] Other studies have reported male-to-female ratios ranging from 3.5:1 to 12:1.[5][6] It must be stressed, therefore, that while young, Asian males are most commonly affected, Kimura’s disease may affect both men and women from a variety of different ethnicities and age groups. While cases in the United States tend to reflect a more ethnically diverse range of patients than in the originally described Chinese and Japanese literature, the clinical presentation and underlying histopathology is identical.[7]
Diagnosis
Symptoms and History
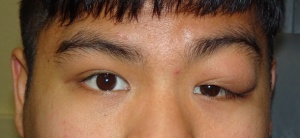
Kimura’s disease most typically presents with subcutaneous nodules of the head or neck, which are firm, painless, and may be single or multiple.[1] Pruritus is a common feature of these lesions and there may rarely be overlying hyperpigmentation.[1][6] Lesions may remain stable over time and may undergo either slow enlargement or spontaneous regression.[1] The most common sites of involvement are the preauricular and submandibular regions, although orbital involvement does occur.[8] Patients who present with orbital involvement usually report painless swelling, eye redness, tearing, and occasionally headache or diplopia.[1]
Physical Examination
Patients with orbital involvement may display palpable adnexal lesions, exophthalmos due to intraorbital lesions, eyelid edema, and conjunctival injection.[1] Swelling of the extraocular muscles may occur and can be mistaken for thyroid eye disease.[9][10] Within the orbit, the most commonly involved locations are, in descending order of frequency, the superior orbit, the eyelids and lacrimal gland.[1] Nodules are typically unilateral, although bilateral involvement has been reported.[1][8][9] There is at least one documented case of a white, elevated chorioretinal lesion with associated serous retinal detachment in a patient with Kimura’s disease.[11]
Systemic Associations
Kimura’s disease is associated with various systemic conditions, including asthma, nephrotic syndrome, sinusitis, tuberculosis and Loeffler’s syndrome.[1][8][12] Nephrotic syndrome in Kimura’s disease may be due to heterogeneous histopathologic entities, such as mesangial proliferative glomerulonephritis, membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, IgA nephropathy and acute tubular injury.[5] Early literature hypothesized a possible association between Kimura’s disease and Mikulicz’s disease, which has subsequently been refuted.[1]
Recent reports have also documented concomitant elevations of serum IgG4 as well as intralesional IgG4 in a small number of patients with Kimura’s disease.[13][14][15] It is hypothesized that the presence of elevated IgG4 in these patients represents an epiphenomenon of Kimura’s disease.[13]
Diagnostic procedures
Lesions of the orbit and soft tissues of the head and neck can be evaluated with MRI. Diagnosis is confirmed by excisional biopsy. A-scan ultrasonography of subcutaneous lesions has demonstrated low-to-medium, irregular internal reflectivity.[1]
Laboratory test
Systemic workup includes CBC to evaluate for eosinophilia and serum immunoglobulin levels to evaluate for elevations in IgE and IgG4. Urinalysis or 24-hour urine collection may demonstrate proteinuria or nephrotic syndrome. Nephrotic syndrome is usually marked when present, with a mean daily protein excretion of 8 g per 24 hours.[5]
Histopathology
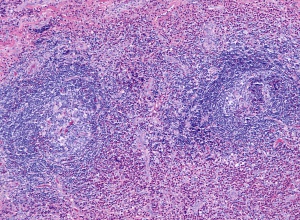
The subcutaneous lesions of Kimura’s disease are characterized by follicular hyperplasia with active germinal centers, as well as a proliferation of capillary and arteriolar vasculature, surrounded by concentric rings of reactive fibrosis composed of collagen and hyaline.[1][2][6] The follicles present in Kimura’s disease are usually well-formed, but tend to undergo degradation once infiltrated by eosinophils, at which point follicular capillary vascularization and eosinophilic microabscesses are commonly observed.[6] The vasculature present in Kimura’s disease is primarily composed of capillaries and arterioles with delicate walls and flat or cuboidal endothelium with typical nuclei.[6] The interfollicular zones are populated by numerous lymphocytes with prominent eosinophilia, although plasma cells and mast cells are also present.[6] In advanced Kimura’s disease affecting glandular tissues, normal glandular tissue is destroyed and replaced with fibrotic tissue and lymphocytic infiltrate.[6]
Differential diagnosis
The differential diagnosis of Kimura’s disease primarily includes soft tissue masses of the orbit, including but not limited to:[1][2][9]
- Angiolymphoid hyperplasia with eosinophilia (ALHE)
- N.B.: There is significant confusion between these two entities in the English literature, which is discussed in detail below.
- Orbital hemangioma
- Lymphangioma
- Orbital pseudotumor
- Hodgkin’s lymphoma
- Castleman’s disease
- Langerhans cell histiocytosis
- Angioimmunoblastic T cell lymphoma
- Eosinophilic granulomatosis with polyangiitis
- Drug-induced and parasitic lymphadenitis
- Thyroid Eye Disease (Graves' disease)
Differentiation from ALHE
There is long-standing confusion in the English literature between Kimura’s disease and angiolymphoid hyperplasia with eosinophilia (ALHE). There is superficial resemblance between Kimura’s disease and ALHE based on the presence of painless, subcutaneous nodules of the head and neck, the presence of eosinophilia, and the common histopathologic presentation of a lymphatic, vascularized lesion populated with leukocytes and eosinophils.[1][6] Conflation of these two entities stems in part from a report by Wells and Whimster in 1969.[16] In their report of a case of ALHE, they noted histologic similarities between these two entities and posited that they represented a single entity observed at different times in its natural history, with Kimura’s disease representing a later stage in the development of ALHE.[1][16] Other authors have noted, however, that the vascular endothelial cells in ALHE typically manifest atypical, histiocytic features, whereas those of Kimura’s disease do not.[17] A comprehensive review of the literature of Kimura’s disease has not demonstrated a case of ALHE progressing to Kimura’s disease, and there is a growing body of evidence that has thoroughly characterized the histopathologic and clinical distinctions between these two entities.[1] Fundamentally, the two entities differ in that Kimura’s disease stems from an autoimmune or allergic etiology, while ALHE is thought to be caused by a benign proliferation of vascular endothelium.[1][18]
In terms of epidemiology, Kimura’s disease tends to affect young males of Asian ancestry. Most patients present in their teenage years. In contrast, ALHE does not have a preponderance in any particular ethnicity, and most commonly affects women in the 3rd to 4th decade of life.[2]
Clinically, the subcutaneous lesions of Kimura’s disease are firm, large, with a size ranging from 2 to 5 cm in diameter, and are deeply seated within the subcutaneous tissue with indistinct margins.[6] The overlying skin in Kimura’s disease is usually normal but may demonstrate hyperpigmentation.[1] In contrast, the lesions of ALHE are smaller, with a diameter of about 1 cm, more nodular, and more superficial within the subcutaneous tissue, and are more likely to bleed when irritated.[1][6] The overlying skin is more likely to be erythematous in ALHE.[1] Involvement of the lymph nodes, lacrimal and salivary glands is more common in Kimura’s disease.[1][2][6] Kimura’s disease is, by definition, accompanied by peripheral eosinophilia, whereas this is an infrequent finding in ALHE.[1] Notably, Kimura’s disease is associated with a variety of systemic conditions, discussed above, while ALHE is not associated with systemic disease.[1]
The histology of subcutaneous lesions of Kimura’s disease is described above. The histology of the subcutaneous lesions of ALHE is similar, but differs in important ways. The lesions of ALHE tend to manifest larger and more prominent vasculature with thick walls and atypical endothelial cells with histiocytoid features and vacuolization, and which tend to protrude into the vascular lumen.[2][6] The vascular endothelium of ALHE can also be distinguished from that of Kimura’s disease by immunohistochemistry and electron microscopy.[1] Fibrosis is much less prominent in ALHE compared to Kimura’s disease, and may be entirely absent at the periphery of the lesion.[6]
Management
There is no clearly defined optimal treatment for Kimura’s disease. Orbital or adnexal lesions are often amenable to surgical excision, although there is a risk of vigorous bleeding during the excision of these often quite vascular lesions.[1][8] Complete excision may also be difficult given the unclear margins of these lesions, while recurrence is common if excision is incomplete.[1] Careful observation may therefore be warranted unless the orbital congestion or cosmetic appearance are unacceptable.[8] Radiotherapy has been used successfully in a small number of cases, although the side effects of radiotherapy may be considerable, particularly for young patients and for orbital lesions.[1][19] Lesions typically respond to oral or intravenous steroids, although they often recur once steroids are withdrawn.[1] Patients who are refractory to steroid treatment have been treated successfully with systemic immunosuppression, including cyclophosphamide, cyclosporine and leflunomide.[1][5]
Prognosis
Kimura’s disease has a chronic, albeit benign course. Lesions tend to remain stable or may slowly enlarge over time. Spontaneous regression may occur. Malignant transformation has not thus far been documented in a case of Kimura’s disease.[1] The prognosis of nephrotic syndrome associated with Kimura’s disease is generally favorable. In one study of 26 patients, systemic treatment led to resolution of nephrotic syndrome in 24 patients, while 2 progressed to end stage renal disease necessitating hemodialysis.[5]
References
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 1.28 1.29 1.30 1.31 1.32 Buggage RR, Spraul CW, Wojno TH, Grossniklaus HE. Kimura disease of the orbit and ocular adnexa. Surv Ophthalmol., 1999 Jul-Aug;44(1):79-91.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Clarós P, Fokouo V, Nyada F, Clarós A. Kimura’s disease of the orbit: A modern diagnostic challenge. Eur Ann Otorhinolaryngol Head Neck Dis., 2017 Sep;134(4):287-289.
- ↑ Kim H, Szeto C. Eosinophilic hyperplastic lymphogranuloma, comparison with Mikulicz’s disease. Chin Med J., 1937;23:699-700.
- ↑ Kimura T, Yoshimura S, Ishikawa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissues. Trans Soc Pathol Jpn. 1948;37:179-80.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Ren S, Li XY, Wang F, Zhang P, Li GS, Wang L, Zhong X. Nephrotic syndrome associated with Kimura’s disease: a case report and literature review. BMC Nephrol., 2018 Nov 8;19(1):316.
- ↑ Jump up to: 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 Li TJ, Chen XM, Wang SZ, Fan MW, Semba I, Kitano M. Kimura’s disease: a clinicopathologic study of 54 Chinese patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod., 1996 Nov;82(5):549-55.
- ↑ Jump up to: 7.0 7.1 Chen H, Thompson LD, Aguilera NS, Abbondanzo SL. Kimura disease: a clinicopathologic study of 21 cases. Am J Surg Pathol., 2004 Apr;28(4):505-13.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 Kennedy SM, Pitts JF, Lee WR, Gibbons DC. Bilateral Kimura’s disease of the eyelids. Br J Ophthalmol., 1992;76(12):755-757.
- ↑ Jump up to: 9.0 9.1 9.2 Goncalves AC, Moritz RB, Aldred VL, Monteiro ML. Bilateral extraocular muscles enlargement from Kimura’s disease of the orbit. Indian J Ophthalmol., 2016 Jul;64(7):538-40.
- ↑ Chakraborti C, Saha AK, Bhattacharjee A, Lakra R. Kimura’s disease involving bilateral lacrimal glands and extraocular muscles along with ipsilateral face: a unique case report. Indian J Ophthalmol., 2019 Dec;67(12):2107-2109.
- ↑ Aonuma H, Kawamura K, Ohara T, Takahashi K, Ueno M. A chorioretinal lesion in a patient with Kimura’s disease. Jpn J Ophthalmol. 1995;39(2):193-7.
- ↑ Carrera W, Silkiss RZ. Kimura’s disease of the lacrimal gland with concomitant chronic sinusitis. Orbit. 2020 Apr 13. DOI: 10.1080/01676830.2020.1753784
- ↑ Jump up to: 13.0 13.1 Li J, Ge X, Ma J, Li M, Li J. Kimura’s disease of the lacrimal gland mimicking IgG4-related orbital disease. BMC Ophthalmol., 2014;14:158.
- ↑ Tsubouchi K, Imanaga T, Yamamoto M, Hirata K, Nakano T. A case of IgG4-positive multiorgan lymphoproliferative syndrome associated with Kimura disease. Nihon Kokyuki Gakkai Zasshi., 2010 Jul;48(7):524-8.
- ↑ Kottler D, Barete S, Quereux G, Ingen-Housz-Oro S, Fraitag S, Ortonne N, Deschamps L, Rybojad M, Flageul B, Crickx B, Janin A, Bagot M, Battistella M. Retrospective Multicentric Study of 25 Kimura Disease Patients: Emphasis on Therapeutics and Shared Features with IgG4-Related Disease. Dermatology., 2015;231(4):367-77.
- ↑ Jump up to: 16.0 16.1 Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol., 1969(81):1-14.
- ↑ Rosai J. Angiolymphoid hyperplasia with eosinophilia of the skin. Its nosological position in the spectrum of the histiocytoid hemangioma. Am J Dermatopathol., 1982 Apr;4(2):175-84.
- ↑ Googe PB, Harris NL, Mihm MC Jr. Kimura’s disease and angiolymphoid hyperplasia with eosinophilia: two distinct histopathological entities. J Cutan Pathol., 1987 Oct;14(5):263-71.
- ↑ Monzen Y, Kiya K, Nishisaka T. Kimura’s disease of the orbit successfully treated with radiotherapy alone: a case report. Case Rep Ophthalmol., 2014 Mar 13;5(1):87-91.