Elevated Episcleral Venous Pressure (EVP)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Elevated episcleral venous pressure (EVP) is a clinical finding which may be associated with elevated intraocular pressure (IOP) and glaucoma if left chronically untreated. In most cases this is secondary to an underlying etiology. However it can also be idiopathic if all other causes are excluded. The idiopathic form can be familial or sporadic. In German literature idiopathic elevated EVP leading to secondary open angle glaucoma has been termed Radius-Maumenee Syndrome[1].
Pathophysiology
Based on the Goldmann equation, intraocular pressure (IOP) is the rate of aqueous humor production divided by the facility of outflow plus EVP[2]. The average EVP ranges from 8-10 mmHg[3], although can transiently change based on head positioning[4]. There is a linear relationship and 0.83±0.21 mmHg rise in EVP correlates to a 1 mmHg rise in IOP[5][6].
In the conventional drainage pathway, aqueous humor flows through the efferent channels of the canal of Schlemm and then through the anterior ciliary venous circulation before converging with the episcleral venous plexus at Tenon’s capsule and the conjunctiva. Given the paucity of capillary networks in episcleral vessels, arteriovenous vasculature anastomosis predominate[2]. The aqueous in the episcleral venous plexus will then drain into the superior ophthalmic vein and enters the cavernous sinus above the annulus of Zinn, before flowing into the internal jugular vein and into the right atrium via the superior vena cava[7][8]. Any obstruction along this pathway may cause elevated EVP.
If there is increased venous pressure downstream to the episcleral anastomoses, then there is subsequent decrease in outflow of blood from the orbit and aqueous. Thus, in chronic cases, elevated EVP can cause blood reflux into Schlemm’s canal. This can subsequently raise IOP chronically, which can lead to glaucomatous damage due to secondary open angle glaucoma[9].
Etiology
Etiologies can range from venous obstruction to arteriovenous anomalies and can be acute or chronic in nature.
Any history of head trauma is a risk factor for developing a carotid cavernous sinus, dural fistula or other arteriovenous anomaly which can lead to the development of elevated EVP. Therefore, it is important to elicit the timing of any trauma and do a full review of systems, including any previous infectious exposures, to rule out life or vision threatening causes of elevated EVP.
|
Venous Obstruction |
Arteriovenous Anomalies |
Idiopathic |
|---|---|---|
|
Retrobulbar Tumor Orbital Amyloidosis Jugular Vein Obstruction Congestive Heart Failure Thrombosis of cavernous sinus or orbital vein Vasculitis involving episcleral or orbital vein Superior Vena Cava Syndrome (Mediastinal Tumor) |
Carotid-Cavernous Sinus Fistula (acute vs. chronic) Dural Sturge-Weber Syndrome Orbital-meningeal shunts Carotid-jugular shunts |
Sporadic Familial |
Diagnosis
History
Typically, when patients present for evaluation of elevated EVP it is due to chronic eye redness that has not responded to previous treatment and often is misdiagnosed as chronic conjunctivitis[6]. They are usually unaware of their condition or underlying cause and typically do no describe pain or irritation. It is important to ask about recent trauma, specifically craniofacial, that can suggest a carotid cavernous sinus (high flow) or dural (low flow) fistula[6][10]. A complete past medical history should be obtained to rule out etiologies that may cause venous obstruction, including but not limited to hyperthyroidism, amyloidosis, congestive heart failure, hypercoagulable states, vasculitis, superior vena cava syndrome and Sturge-Weber Syndrome.
Physical examination
Clinical exam is the gold standard for diagnosing elevated EVP. Typically, on physical examination, the episclera is injected and demonstrates the pathognomonic corkscrew episcleral vessels without inflammation (Figure 1). It is important to perform gonioscopy, which can show an open angle with blood reflux in Schlemm’s canal or hyalinization of the wall of Schlemm’s canal due to chronic accumulation of blood[11]. Other signs include chemosis, proptosis and the presence of an orbital bruit, however, these are not specific signs for elevated EVP[11]. If the elevated EVP is secondary to a carotid-cavernous fistula, then pulsatile exophthalmos can sometimes be seen[12]. The IOP can often times be elevated in the affected eye. Ancillary testing would include a optic coherence tomography (OCT) scan and Humphrey Visual Field testing to assess for glaucomatous changes.
Differential Diagnosis:
- Episcleritis
- Scleritis
- Conjunctivitis
- Ataxia Telangiectasia
- Acute Closed Angle Glaucoma
- Rosacea
- Episcleral Nodule
- Corneal lesion near limbus
- Foreign body
- Herpetic Keratitis
- Uveal Neoplasm
- Polycythemia vera
- Leukemia[13]
Diagnostic procedures
A complete workout to rule out underlying etiology should be done when elevated EVP is suspected. In the setting of detecting an arteriovenous fistula, cerebral angiography is the gold standard, however, orbital Doppler ultrasound is a non-invasive method which can confirm dilation of the superior ophthalmic vein[8]. Other modalities for diagnosis include orbital ultrasound, ultrasound biomicroscopy (UBM), computerized axial tomography, and magnetic resonance imaging.
There are diagnostic modalities that measure EVP however are not routinely used in clinical practice. Direct cannulation is a direct method and can be done with the complete occlusion or partial vessel occlusion method; this is the most accurate test. Indirect methods include the venomanometer pressure chamber, torsion balance, and air jet.
The episcleral venomanometer is a device that has a dome-shaped silicone ring on its tip. It is mounted on the slit-lamp and the silicone ring is used to gradually compress the episcleral veins until they blanch. EVP is considered the pressure needed to blanch the episcleral veins.
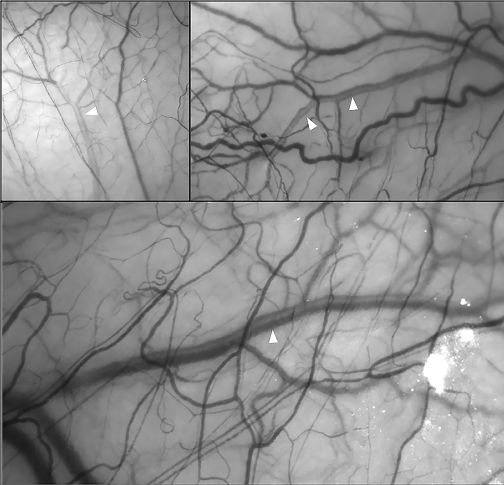
Recently, several non-invasive in-vivo methods have been introduced to measure the episcleral venous outflow. One method is hemoglobin video imaging which assesses the laminar flow in the episcleral veins (i.e., the separation between blood and aqueous within the veins). An interference filter is used and the eye is exposed to a light with a wave length corresponding to the hemoglobin absorption spectrum. The aqueous appears clear since it is devoid of hemoglobin, while the blood appear darker (Figure 2).[14]
Another non-invasive method is erythrocyte-mediated angiography. This technique is based on injecting autologous, indocyanine green–labelled red blood cells into the individual and then direct visualization of these erythrocytes using scanning laser ophthalmoscopy. This allows for accurate quantification of the episcleral blood flow.[15]
Management
The aim of management should revolve around first treating the primary underlying etiology. If no etiology is determined for the elevated EVP then treatment is similar to that of primary open angle glaucoma (POAG)[16].
Medical therapy
Traditionally, medical therapy is aimed at decreasing aqueous humor production and increasing uveoscleral outflow. Medications that enhance outflow through the conventional pathway are not as effective[8]. Beta blockers and carbonic anhydrase inhibitors are classically favored in treatment of elevated EVP. Given its action on the arterial vasculature, Apraclonidine may be considered as it decreases blood flow to the eye[17].
Recently, several glaucoma medications have been proposed to improve the episcleral venous outflow and reduce the EVP[18]:
A- Topical Rho-Kinase inhibitors
Several mechanisms have been proposed to explain the IOP-lowering effect of topical netarsudil (Rhopressa; Aerie Pharmaceuticals), including reducing aqueous humor production and facilitating aqueous outflow. However, recently, several studies have shown its effect on episcleral venous outflow.
Sit and colleagues[19] used automated venomanometry to measure the EVP after the instillation of topical netarsudil 0.02%. They reported a statistically significant reduction in the mean diurnal EVP by -0.79 mm. Another prospective study by Kim and colleagues[20] used a new technique called erythrocyte-mediated angiography to measure the episcleral venous outflow. They showed a significant increase in the episcleral venous outflow after the instillation of topical netarsudil.
Kaufmann and colleagues[21] retrospectively evaluated the effectiveness of topical netarsudil in 6 eyes with Sturge-Weber-syndrome-associated glaucoma with elevated EVP. The mean follow-up period was 18.7±11.8 months. They reported a statistically significant IOP reduction from a mean of 26.2±4.5 mmHg on 3.3±1.2 glaucoma medications into 17.6±1.4 mmHg at the last follow-up after adding topical netarsudil (P=0.003).
Topical ripasudil is another rho-kinase inhibitor that is approved in Japan and Korea. A prospective study by Suzuki and colleagues[22] investigated its effect on aqueous outflow in episcleral venous vasculature compared to latanoprost. The study included 16 healthy, non-glaucomatous eyes. They used hemoglobin video imaging to evaluate the changes in the aqueous column before and after topical ripasudil. They reported a significant increase in the aqueous column width eight hours after the use of topical ripasudil, suggesting enhancement of episcleral venous outflow.
B- Intracameral Bimatoprost Implant (Durysta)
The effect of the intracameral prostaglandin analogues on the EVP differs from that of topical prostaglandin analogues.[23] Intracameral bimatoprost induces a selective dilation of the episcleral venous system without affecting the episcleral arteries or connecting arteriovenous anastomoses. This results in enhancing the episcleral venous outflow. On the other hand, topical prostaglandin analogues cause dilation of the episcleral vasculatures (arteries, veins, and connecting anastomoses).[23]
C- QLS-111
QLS-111 (Qlaris Bio) is a novel, promising topical drug that selectively targets the episcleral vasculature, reducing EVP. It acts as a vasodilator through an ATP-sensitive potassium channel modulation mechanism. The drug is currently being developed, with preliminary data showing significant IOP reduction and no significant adverse events.[24] [25]
Surgery
Surgical therapy should be considered if patients are refractory to medical therapy. The aim should be to bypass the trabecular outflow, therefore selective laser trabeculoplasty (SLT) and micro-pulse laser trabeculoplasty (MLT) has not been classically recommended[8]. However, a recent study by Khatib and colleagues evaluated episcleral venous outflow in eight eyes who had undergone SLT. Using hemoglobin video imaging, they demonstrated a significant increase in the aqueous column in the episcleral vasculature following SLT.[14] Several studies are currently conducted to evaluate the episcleral venous outflow before and after various glaucoma procedures using erythrocyte-mediated angiography.[18]
Trabeculectomy and sclerotomy are appropriate surgical options. Eyes with elevated EVP have been reported to be at higher risk for uveal effusion syndrome[1][26]. Therefore, special consideration is needed to prevent hypotony during surgery. Prophylactic sclerotomies or scleral windows may be necessary[10]. One case recommends a surgical technique where a tight trabeculectomy with multiple adjustable sutures, the sutures can then be used to titrate the IOP gradually and therefore prevent acute shallowing of the anterior chamber intraoperatively[27]. Other reports suggest maintaining the anterior chamber with injection of balance salt solution and viscoelastic, in addition to adjustable suture placement on the scleral flap[8]. Medical management post operatively can include use of cycloplegic agents and glucocorticoids to reduce inflammation[8].
Complications
The major complication for untreated elevated EVP is development of secondary open angle glaucoma. Elevated EVP can also lead to acute angle closure glaucoma as suprachoroidal hemorrhage with subsequent forward displacement of the lens-iris diaphragm[28][29]. Rarely, neovascular glaucoma can occur as a result of ocular ischemia[30].
Follow Up
Follow up for these cases varies depending on the underlying etiology and IOP. If following for secondary open angle glaucoma, frequent follow up is needed with routine IOP checks, gonioscopy, OCT, and visual fields to monitor and prevent glaucomatous progression.
References
- ↑ 1.0 1.1 Rhee DJ, Gupta M, Moncavage MB, Moster ML, Moster MR. Idiopathic elevated episcleral venous pressure and open angel glaucoma. Br J Ophthalmol. 2009;93(2):231-234.
- ↑ 2.0 2.1 Moster M, Ichpujani P. Episcleral venous pressure, and glaucoma. . Journal of Current Glaucoma Practice 1996;3:1143-1155
- ↑ Allingham RR, Damji KF, Freedman SF, Moroi SE, Rhee DJ, Shields MB. Shields textbook of Glaucoma 2012.
- ↑ Arora N, McLaren JW, Hodge DO, Sit AJ. Effect of Body Position on Episcleral Venous Pressure in Healthy Patients. Invest Ophthalmol Vis Sci. 2017;58(12):5151-5156.
- ↑ Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987;103(4):523-526.
- ↑ 6.0 6.1 6.2 Cioffi GA, Durcan FJ, Girkin CA. Basic and Clinical Science Course: Glaucoma. San Francisco: American Academy of Ophthalmology. 2013;26.
- ↑ Higginbotham EJ. Glaucoma associated with increased episcleral venous pressure. Philadelphia WB Saunders 2000.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Rong X, Li M. Advanced glaucoma secondary to bilateral idiopathic dilated episcleral veins - a case report BMC Ophthalmology 2018;18:207.
- ↑ Minas TF, Podos SM. Familial glaucoma associated with elevated episcleral venous pressure. Archives of Ophthalmology 1980;80(1):202-208.
- ↑ 10.0 10.1 Girkin CA, Bhorade AM, Crowton JG, et al. Glaucoma United States of America 2018.
- ↑ 11.0 11.1 Roll P, Benedikt O. Dilatation and tortuosity of episcleral vessels in open angle glaucoma II. Electron microscopy study of the trabecular meshwork Klin Monatsbl Augenheikd. 1980;176:297-301.
- ↑ Heichel J, Hammer T, Solymosi L, Brandt S, Winter I. Pressure-Lowering Effect of Fistula Occlusion in a Patient with Secondary Glaucoma due to an Intracranial Arteriovenous Fistula. Ophthalmology and Therapy. 2015;4(2):135-141.
- ↑ Bagheri N, Wajda BN, Calvo CM, Durrani AK. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease Philadelphia Lippincott Williams & Wilkins 2017.
- ↑ 14.0 14.1 Khatib TZ, Meyer PAR, Lusthaus J, Manyakin I, Mushtaq Y, Martin KR. Hemoglobin Video Imaging Provides Novel In Vivo High-Resolution Imaging and Quantification of Human Aqueous Outflow in Patients with Glaucoma. Ophthalmol Glaucoma. 2019 Sep-Oct;2(5):327-335.
- ↑ Asanad S, Park A, Pottenburgh J, Siddiqui A, Mayo L, Saeedi OJ. Erythrocyte-Mediated Angiography: Quantifying Absolute Episcleral Blood Flow in Humans. Ophthalmology. 2021 May;128(5):799-801.
- ↑ Stock RA, Fenandes NL, Pastro NL, de Oliveira RS, Bonamigo EL. Idiopathic dilated episcleral vessels (Radius-Maumenee syndrome): case report. Arq Bras Oftalmol. 2013;76(1):45-47.
- ↑ Mantzioros N, Weinreb RN. Apraclonidine reduces intraocular pressure in eyes with increased episcleral venous pressure. Journal of Glaucoma 1992;1(1):42-43.
- ↑ 18.0 18.1 Elhusseiny AM, Saeedi OJ. Episcleral venous pressure and flow. Glaucoma Physician. March 2023.https://www.glaucomaphysician.net/issues/2023/march-2023/episcleral-venous-pressure-and-flow
- ↑ Sit AJ, Gupta D, Kazemi A, McKee H, Challa P, Liu KC, Lopez J, Kopczynski C, Heah T. Netarsudil Improves Trabecular Outflow Facility in Patients with Primary Open Angle Glaucoma or Ocular Hypertension: A Phase 2 Study. Am J Ophthalmol. 2021 Jun;226:262-269.
- ↑ Sarah Kim, Victoria Chen, Marvin Cruz, Jessica Pottenburgh, Osamah Saeedi; Precise quantification of episcleral venous flow rates in human subjects before and after netarsudil 0.02%. Invest. Ophthalmol. Vis. Sci. 2022;63(7):3497.
- ↑ Kaufman AR, Elhusseiny AM, Edward DP, Vajaranant TS, Aref AA, Abbasian J. Topical netarsudil for treatment of glaucoma with elevated episcleral venous pressure: A pilot investigation in sturge-weber syndrome. Eur J Ophthalmol. 2023 Sep;33(5):1969-1976.
- ↑ Suzuki M, Suzuki Y, Komori R, Orii Y, Arimura S, Iwasaki K, Takamura Y, Inatani M. Aqueous column changes in the episcleral veins after the instillation of ripasudil versus latanoprost: a randomized, double-blind, crossover clinical trial. Sci Rep. 2022 Sep 10;12(1):15255.
- ↑ 23.0 23.1 Lee SS, Robinson MR, Weinreb RN. Episcleral venous pressure and the ocular hypotensive effects of topical and intracameral prostaglandin analogs. J Glaucoma. 2019;28(9):846-857.
- ↑ Charters L. Glaucoma 360: Drug targets nd reduces episcleral venous pressure in glaucoma. Ophthalmology times. Feb 3, 2023.https://www.ophthalmologytimes.com/view/glaucoma-360-drug-targets-and-reduces-episcleral-venous-pressure-in-glaucoma
- ↑ Qlaris Bio. https://qlaris.bio/our-approach/
- ↑ Bellows AR, Chylack LT, Epstein DL, Hutchinson BT. Choroidal effusion during glaucoma surgery in patients with prominent episcleral vessels Arch Ophthalmol 1979;97(3):493-497.
- ↑ Pradhan ZS, Kuruvilla A, Jacob P. Surgical management of glaucoma secondary to idiopathic elevated episcleral venous pressure. Oman Journal of Ophthalmology. 2015;8(2):120.
- ↑ Buus DR, David TT, Parish RK. Spontaneous carotid cavernous fistula presenting with acute angle closure glaucoma Arch Ophthalmol. 1989;107(4):596-597.
- ↑ Fourman S. Acute closed angle glaucoma after arteriorvenous fistulas. Am J Ophthalmol. 1989;107(2):156-159.
- ↑ Spencer WH, Thompson HS, Hoyt WF. Ischemic ocular necrosis from carotid-cavernous fistula. Pathology of stagnant anoxic inflammation in orbital and ocular tissues Br J Ophthalmol. 1973;57(3):145.


