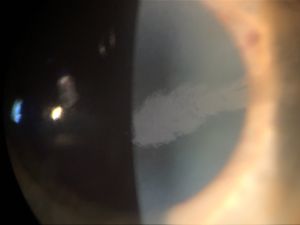
Lisch Corneal Dystrophy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Lisch Corneal Dystrophy (ICD-10 #H18.59 –other hereditary corneal dystrophies)
Disease
Lisch corneal dystrophy (LCD) is a rare form of superficial corneal dystrophy characterized by gray whorled or feathery microcysts in the shape of a band or club. The International Committee for Classification of Corneal Dystrophies places it in IC3D category 2: “A well-defined corneal dystrophy that has been mapped to one or more specific chromosomal loci, but the gene(s) remains to be identified.”[1]
Etiology
Sporadic and familial cases have been reported.[2] Familial cases are X-linked dominant with evidence for linkage to the short arm of the X-chromosome at the Xp22.3 locus. The specific gene is unknown. [1][3]
Risk Factors
Positive family history
General Pathology
The hallmark histopathological feature of LCD is diffusely vacuolated cytoplasm of epithelial cells. On light microscopy, these vacuoles are most evident in the wing cell layer but are found in cells throughout the epithelial layers of the cornea, with sharp demarcation between affected and unaffected areas.[2] Reports vary on whether the cells are periodic acid-Schiff (PAS) positive or negative.[4] They are also diastase labile, Luxol fast blue negative and Sudan black negative. [1] On electron microscopy, the intracytoplasmic vacuoles look empty, with a tendency of the vacuoles to combine and create the appearance of transparent and structureless cytoplasm.[2] Conofocal microscopy demonstrates hyperreflective cytoplasm and hyporeflective nuclei with involvement of the limbal area. [1]
Pathophysiology
The association of LCD with mutations on the short arm of the X-chromosome at Xp22.3 gene was demonstrated in a study of a single-family with high rates of LCD. Lack of male-to-male transmission in this study point towards an X-linked dominant inheritance pattern, but sporadic cases have also been reported. [2][3] There have been no consistently observed concomitant pathologies, and there are no associated physical factors to suggest a mechanical mechanism.[4] There is some evidence that the abnormal cells responsible for the epithelial defects originate from the limbus. [5]
Primary prevention
No preventive strategies have been studied to date. Soft or hard contact lens use may be protective given that their use has been shown to result in regression of lesions. However, cases of LCD have been reported in daily contact-lens wearers, so the protection offered is incomplete.
Diagnosis
Diagnosis should be made with a combination of characteristic slit-lamp and histopathological findings. Although a patient complaint of painless visual blurring is common, it is not required for diagnosis as the lesions may be found incidentally in cases where they are not encroaching on the visual axis. Family history of similar lesions may or may not be present. Genetic and metabolic testing to investigate more common causes of corneal dystrophy may be useful in diagnosis.
History
Onset of LCD is believed to be in childhood, but most reported cases are in adults.[1] It is possible that the lesions begin in childhood and are slowly progressive, not becoming clinically significant until adulthood in many patients. Patient age on presentation in available case reports ranges from the 3rd to the 8th decade. Available case reports also suggest that a unilateral presentation is more common, but bilateral involvement can also occur.
Physical examination
A thorough examination of both eyes should be conducted for any patient suspected of having LCD. Patients may have bilateral lesions but unilateral visual impairment if only some of the lesions involve the visual axis. A physical exam should include the following:
- Measurement of corrected visual acuity
- Slit-lamp examination with retro-illumination
Signs
- Painless, gradually worsening visual acuity that is not correctable with lenses.
- Gray, feathery, band-shaped corneal lesions, sometimes in whorled patterns on slit lamp examination.
- Dense collections of clear intraepithelial micro-cysts on retro-illumination.
- Unilateral or bilateral lesions.
- Lesions do not stain with fluorescein or rose bengal.
- No epithelial erosions are observed.
- High-resolution optical coherence tomography of the cornea may have abnormal findings of epithelial hyper-reflectivity with normal thickness and no stromal involvement,[6] but corneal topography may also be normal.[7]
- Diffusely vacuolated cytoplasm on histopathology.
Symptoms
LCD typically presents as gradually worsening blurry vision that cannot be corrected with eyeglasses. Monocular diplopia has also been reported. In some cases, patients are asymptomatic and the corneal lesions are found incidentally. [4]
Clinical diagnosis
Diagnosis should be made upon seeing the histopathological features of diffusely vacuolated cytoplasm in a patient with characteristic slit-lamp findings of feathery corneal micro-cysts, with or without a complaint painless visual blurring in one or both eyes.
Diagnostic procedures
Diagnostic procedures include:
- Slit lamp examination
- Light microscopy
- Electron microscopy
- Confocal microscopy
- High resolution optical coherence tomography
- Genetic testing
- Metabolic testing
Differential diagnosis
- CD has clinical similarities to Meesmann corneal dystrophy, but they have been mapped to different genes. In contrast to LCD, Meesmann corneal dystrophy is related to the KRT3 and KRT12 loci, with an autosomal dominant mode of inheritance.[8]
- Lesions in Meesmann dystrophy are generally symmetric and diffuse, in contrast to the asymmetric and dense lesions in LCD.
- Ocular pain is a frequent complaint in Meesmann dystrophy that is typically absent in LCD.
Bron and Brown blebs
- Resemble Lisch microcysts, but Bron and Brown blebs are smaller and typically painful. [4]
- May appear similarly to LCD lesions, but are always bilateral and symmetric. [4]
- Metabolic or genetic testing may be helpful to investigate Fabry disease in unclear cases
Contact lens-induced corneal de-epithelialization
- Can present as microcysts with a similar appearance to LCD. Lack of improvement with cessation of contact lens use and/or antibiotics should prompt consideration of LCD.
General treatment
Treatment options are entirely based on case reports, as the rarity of the disease prevents large-scale study. Epithelial debridement resulted in resolution of the lesions for at least 6 months in one case, although recurrence with this strategy appears to be a more common outcome. [2][4] Hard and soft contact lenses have reportedly resulted in improvement of lesions in several cases, but lesions worsen when contact lens use is stopped. [2][9][10] One case was successfully treated with epitheliectomy and cauterization of the limbal origin of the lesion, with no evidence of recurrence at 2 year follow up.[11] Minimal recurrence was noted in a case treated with photorefractive keratectomy and mitomycin C.[12] In a case refractive to epithelial debridement, contact lenses, and mitomycin C, removal of the limbal origin with autologous limbal transplant prevented recurrence.[13]
Medical therapy
Both hard and soft contact lens use have been reported to reduce lesion amount and improve visual acuity. No systemic or topical pharmaceutical agents have been investigated for the treatment of LCD, with the exception of intraoperative mitomycin C.
Medical follow up
The use of contact lenses to treat LCD has sporadic effectiveness, so patients should be followed closely to evaluate the need for surgical intervention.
Surgery
Epithelial debridement, epitheliectomy, photorefractive keratectomy with mitomycin C, and limbal removal with autologous limbal transplant have been used with varying success. Epithelial debridement may be an appropriate first step when a less invasive approach is preferred, although lesions are likely to recur. Epitheliectomy was successful at preventing lesion recurrence for at least two years. Photorefractive keratectomy with mitocmycin C did not prevent recurrence, but recurrence was minimal. It may be appropriate for patients also desiring surgical correction of refractive errors. Autologous limbal transplant prevented recurrence, but was advised to be performed only when other treatments had failed due to its risks and invasiveness.
Surgical follow up
The short and medium-term success of all surgical modalities in treating LCD varies by patient. Because of this, patients should be followed very closely for the first year after surgery to evaluate for lesion recurrence. Long-term success of surgical treatments in preventing recurrence is not known, and it is not clear if any methods are truly curative. Lifelong annual or bi-annual follow up may be advisable to increase the likelihood of capturing and treating recurrence early.
Complications
Adverse events related to surgery were not reported in the small number of cases available for review. However, possible risks include those present with any corneal surgery, including infection, worsened visual acuity, corneal ulceration and mechanical or toxic injury to the cornea or surrounding structures.
Additional Resources
Ocular Pathology Atlas. American Academy of Ophthalmology Website: https://www.aao.org/resident-course/pathology-atlas.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Weiss JS, Møller HU, Aldave AJ, Seitz B, Bredrup C, Kivelä T, et al. IC3D classification of corneal dystrophies--edition 2. Cornea. 2015;34(2):117-59.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Lisch W, Steuhl KP, Lisch C, Weidle EG, Emmig CT, Cohen KL, et al. A new, band-shaped and whorled microcystic dystrophy of the corneal epithelium. Am J Ophthalmol. 1992;114(1):35-44.
- ↑ 3.0 3.1 Lisch W, Büttner A, Oeffner F, Böddeker I, Engel H, Lisch C, et al. Lisch corneal dystrophy is genetically distinct from Meesmann corneal dystrophy and maps to xp22.3. Am J Ophthalmol. 2000;130(4):461-8.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Alvarez-Fischer M, de Toledo JA, Barraquer RI. Lisch corneal dystrophy. Cornea. 2005;24(4):494-5.
- ↑ Chou CY, Lockington D. A corneal tulip? Assessing corneal opacities in Lisch corneal epithelial dystrophy. Clin Exp Optom. 2018;101(4):596-8.
- ↑ Pole C, Sise A, Joag M, Galor A, Bermudez-Magner JA, Dubovy S, et al. High-Resolution Optical Coherence Tomography Findings of Lisch Epithelial Corneal Dystrophy. Cornea. 2016;35(3):392-4.
- ↑ Charles NC, Young JA, Kumar A, Grossniklaus HE, Palay DA, Bowers J, et al. Band-shaped and whorled microcystic dystrophy of the corneal epithelium. Ophthalmology. 2000;107(9):1761-4.
- ↑ Klintworth GK. The molecular genetics of the corneal dystrophies--current status. Front Biosci. 2003;8:d687-713.
- ↑ Robin SB, Epstein RJ, Kornmehl EW. Band-shaped, whorled microcystic corneal dystrophy. Am J Ophthalmol. 1994;117(4):543-4.
- ↑ Lisch W, Wasielica-Poslednik J, Lisch C, Saikia P, Pitz S. Contact lens-induced regression of Lisch epithelial corneal dystrophy. Cornea. 2010;29(3):342-5.
- ↑ Salvador-Culla B, Alonso-Agesta M, Álvarez de Toledo J, Barraquer RI. Combined Keratectomy and Localized Limbal Cauterization for Treating Lisch Epithelial Corneal Dystrophy. Cornea. 2019;38(2):243-5.
- ↑ Wessel MM, Sarkar JS, Jakobiec FA, Dang N, Bhat P, Michaud N, et al. Treatment of lisch corneal dystrophy with photorefractive keratectomy and mitomycin C. Cornea. 2011;30(4):481-5.
- ↑ Celis Sánchez J, Mesa Varona DV, Avendaño Cantos E, López-Romero Moraleda S, Cebrian Rosado E, González Del Valle F. Keratolimbal autograft transplantation as a possible new treatment of Lisch epithelial corneal dystrophy. Arch Soc Esp Oftalmol. 2016;91(7):333-6.