All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Indications for Strabismus Surgery
Strabismus surgery is typically recommended when a patient’s eye alignment can no longer be treated with conservative measures such as eyeglasses, eye patching, prisms, and orthoptic exercises. Like many other ophthalmic procedures, strabismus surgery is very safe and effective, but complications can occur and need to be diagnosed and treated early to optimize post-operative outcome. Generally, complications of strabismus surgery have an excellent prognosis for recovery with proper treatment. Many of the complications lessen or disappear with time and conservative treatment, while others respond well to additional surgery.
Intraoperative Surgical Site Complications
Scleral Perforation
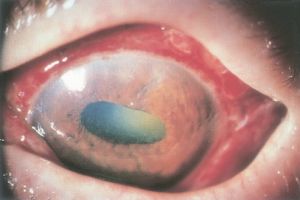
- Scleral perforation is the passage of the suture needle through the sclera from an inadvertent deep pass[1]. Perforations may occur when the suture is placed in the normally thinned sclera directly behind the muscle insertion, as well as during the dissection, isolation, and disinsertion of the muscle tendon. The reported incidence of scleral perforation varies widely with current estimates ranging from 0.3% to 7.8%[2].
- Risk factors: The risk of scleral perforation is increased if the sclera is thinned, such as in high myopia with a staphyloma, and if there is significant scarring or hemorrhage (more common during a re-operation) that may impede exposure and visualization of the sclera for suture placement[2]. Scarring may also make exposure and isolation of the muscle tendon more challenging. The risk is also greater with very posterior suture placement, such as for posterior fixation sutures, because it is more difficult to visualize and place the suture at the proper depth in the sclera in the retroequatorial globe[3].
- Primary prevention: Scleral perforation may be prevented by using a magnified view of the surgical field and placing flexible spatulated needles through the sclera with good exposure and clear visibility of the needle tip at all times[3]. Also, the depth of the scleral suture may easier to judge when placing the scleral sutures through the original insertion and using a “hang-back” technique for recessions[1].
- Diagnosis: A dilated fundus examination should be performed when there is a potential scleral perforation to identify potential retinal or vitreal problems that may require further treatment[3].
- Management:
- Laser retinopexy may be required at the time of perforation if a large retinal tear or a small retinal tear in a patient at high risk for retinal detachment is noted. Laser retinopexy may not be necessary for a child with well-formed vitreous at low risk for detachment[1].
- A scleral perforation may or may not require additional surgery. Most cases resolve without treatment, but the presence of infection, significant hemorrhage, or retinal detachment may require a vitrectomy or other surgeries as indicated[3].
- Though there is no validated protocol for prophylactic antibiotic administration, some surgeons will use subconjunctival, topical, oral, or intravenous antibiotics postoperatively[1][4].
- Prognosis: A scleral perforation usually does not create a problem other than a chorioretinal scar, but in some cases can trigger endophthalmitis, vitreous hemorrhage, or retinal detachment[3].
Lost Muscle
- A lost muscle occurs when the extraocular muscle slips free of the sutures or surgical instruments during surgery[5]. There is no direct attachment between the muscle tendon and the globe, allowing the muscle and its capsule to both retract posteriorly into the orbit. This constitutes a surgical emergency and immediate surgical attempts to recover the muscle should be made. One way in which a muscle can be lost is by rupturing, known as Pulled In Two Syndrome (PITS)[6]. PITS typically occurs at the junction between the muscle belly and tendon, resulting in loss of the posterior muscle belly. The incidence of PITS in pediatric strabismus surgery has been estimated to be approximately 0.02% (1 in 5000)[6].
- Risk factors: A lost muscle is more common when operating on the medial rectus and inferior rectus because of the shorter arc of contact with the globe[7]. A lost muscle is also more common with tight or contracted muscles because the increased passive tension increases the possibility of the muscle tendon pulling free of sutures or clamps during surgery. The risk of a lost muscle increases with poor overall systemic health, with extensive scarring from prior eye surgery, and with a tight muscle, such as with dysthyroid orbitopathy or the contracted antagonist of a paretic muscle.
- Primary prevention: The risk of a lost muscle may be minimized by using gentle surgical techniques when isolating and securing the muscle belly and rotating the globe towards a resected muscle rather than pulling the tight muscle anteriorly. The risk can also be reduced by direct placement of scleral sutures at the site of the new insertion, instead of utilizing a hang-back technique from the original insertion.
- Diagnosis: During the motility exam, weakness of the lost muscle will be noted on extraocular motility testing and the patient may present with a large angle, incomitant strabismus. Specialized testing such as saccadic velocities, force generations, and forced ductions can distinguish between lost or slipped muscles and excessive scarring or fat adherence. Imaging can also help localize lost muscles.
- Management: For a lost muscle, an attempt should be made to retrieve the muscle promptly, during the same surgery if possible[5]. If the muscle cannot be retrieved, a transposition surgery can be considered, although there is a risk of anterior segment ischemia by performing surgery on three eye muscles (the lost muscle plus two transposed muscles) at the same time. Surgery for a lost muscle can benefit from pre-operative imaging to help locate the muscle. Depending on the location, a posterior orbital approach with the aid of an orbital surgeon can sometimes successfully isolate and retrieve the lost muscle.
Slipped Muscle
- A slipped muscle occurs when less than full-thickness sutures are used to capture the muscle tendon, effectively only capturing the superficial muscular capsule instead of securing the muscle belly[5]. Post-operatively, the muscle belly retracts within the muscle capsule when force is exerted during contraction, leading to clinical weakness of the operated muscle. One report found that 10.6% of patients seen for corrective surgery after an unsatisfactory outcome from a previous surgery had a slipped muscle[8].
- Risk factors: The risk factors for a slipped muscle are similar to those for a lost muscle. The risk of a slipped muscle increases when the muscle is tight, such as with dysthyroid orbitopathy or the contracted antagonist of a paretic muscle. It can be difficult to create space at the muscle insertion to allow a full-thickness suture pass to encompass both the muscle capsule and muscle belly.
- Primary prevention: The risk of a slipped muscle may be minimized by using full-thickness bites when passing the muscle suture[1]. Special groove hooks or muscle clamps can be used with tight muscles to provide more space to securely place the muscle sutures to encompass the muscle belly in addition to the muscle capsule. Some physicians prefer to use two locking bites instead of the traditional one locking bite to ensure adequate capture of the muscle fibers.
- Diagnosis: See diagnosis of a lost muscle.
- Management: A slipped muscle can be repaired during surgery by locating the muscle capsule attached to the globe from the prior surgery. By following the muscle capsule posteriorly, the muscle itself can be found, isolated, and attached to the globe[1].
Oculocardiac Reflex
- The oculocardiac reflex (OCR) is a vagal bradycardic response to compression of the eyeball and/or tension on an extraocular muscle tendon, which stimulates the trigeminal nerve[9]. The incidence of oculocardiac reflex has been reported to be anywhere from 14% to 90% and decreases with age[10].
- Risk factors: Some studies suggest an association between operation on the medial rectus muscle and increased OCR incidence, though these results are not consistent throughout the literature[10].
- Primary prevention: Intravenous anticholinergics, such as atropine or glycopyrrolate, decrease the incidence of oculocardiac reflex. A ketamine infusion as the primary anesthetic agent decreases the incidence of OCR, as well as postoperative nausea, vomiting, and agitation. Retro- or peri-bulbar anesthetic blocks can blunt the afferent limb of the reflex arc to protect against OCR. [10]
- Diagnosis: The oculocardiac reflex most commonly presents as sinus bradycardia but it may also be associated with reduced arterial pressure, arrhythmia, asystole, and even cardiac arrest.[10]
- Management: Stopping the triggering stimulus will terminate the reflex. Pharmacologic management includes intravenous administration of anticholinergics, such as atropine. [10]
Post-operative Surgical Site Complications
Post-operative infection
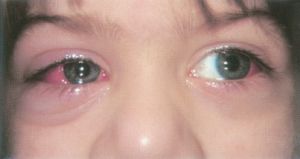
- Post-operative infection can occur if sterile technique is violated or if the patient has a pre-existing condition such as blepharitis or nasolacrimal stenosis that increases the bacteria count at the surgical site[1]. Most infections occur around the initial surgical incision into the conjunctiva and present within the first week after. Rarely, infections can penetrate deeper into the orbit with proptosis, eyelid swelling, chemosis, and erythema in the classical presentation of orbital cellulitis. Sometimes endophthalmitis can develop, either with or without a scleral perforation (see Scleral Perforation). The most commonly cultured organisms include Staphylococcus aureus (MRSA and MSSA), group A Streptococcus, and coagulase-negative Staphylococcus[11]. One study reported an overall incidence of post-operative infection of 0.14%[11], while estimates of the incidence of endophthalmitis range from 1 in 350,000 cases to 1 in 18,500 cases[12].
- Risk factors: The risk of post-operative infection is increased in very young patients, particularly those with developmental delay, that may have difficulty cooperating with hygiene and antibiotic eye drops after surgery[11]. Infection is also associated with previous skin or ear infection and acute or chronic rhinitis.
- Primary prevention: The risk of post-operative infection may be minimized by aggressively treating any superficial infection or bacterial overgrowth pre-operatively, by using meticulous sterile technique during surgery, and by using post-operative antibiotics.
- Diagnosis:
- Signs of infection include conjunctival injection, eyelid erythema and swelling, a bump or bulge over the muscle insertion, discharge, eye pain and/or increased tenderness over the surgical site, systemic signs, fever, and photophobia. Patients with severe infections tend to present with systemic infections[11].
- Orbital imaging with magnetic resonance imaging (MRI) or computed tomography (CT) can help identify pre-septal and orbital cellulitis.
- Increasing redness, swelling, and a change in vision may be secondary to a post-operative infection, but may also be a sign of an allergic reaction or anterior segment ischemia, particularly in the early post-operative period. The slit lamp examination can help to distinguish signs of anterior uveitis, an early indicator of anterior segment ischemia, from conjunctival inflammation secondary to an infection or allergic reaction. For the most common area of confusion, allergic reaction versus early post-operative infection, an exact diagnosis at presentation may be difficult and an empiric change in antibiotic medication may be warranted with careful follow up to monitor the clinical course.
- For infections, gram stains and cultures (either of the conjunctiva or, in the case of scleral perforation and possible endophthalmitis, of the vitreous) can aid with diagnosis and treatment.
- Management: For infections, topical antibiotics are used to treat conjunctivitis, systemic antibiotics are used to treat preseptal and orbital cellulitis, and intravitreal antibiotics are used to treat endophthalmitis. The medical follow up is determined by the severity of the clinical problem. For serious infections, the patient may require inpatient hospitalization for intravenous antibiotics and close monitoring. For less serious problems, the follow up period can be longer, with specific instructions to call for any apparent deterioration in clinical status. If imaging demonstrates a subconjunctival or orbital abscess, then abscess drainage be necessary[1][11]. Also see management of scleral perforation.
- Prognosis: Although most cases of post-operative infection have a good prognosis, severe orbital cellulitis can cause optic neuropathy and blindness, and endophthalmitis carries a significant risk of permanent vision loss even with prompt treatment[11][12].
Allergic Reaction
- Allergic reactions can occur sporadically in patients that are sensitive to the materials or medications used in the peri-operative period.[13][14]
- Risk factors: The risk of an allergic reaction is increased in patients with a history of hypersensitivity reactions or systemic allergies or asthma.
- Primary prevention: The risk of an allergic reaction may be minimized by carefully reviewing the patient’s medical history and avoiding medications that might cross-react with known medication allergies.
- Diagnosis: See diagnosis of post-operative infection for differentiation.
- Management: For allergies, the antibiotic eye drops should be changed, with the possible addition of a topical corticosteroid or antihistamine if the symptoms persist.
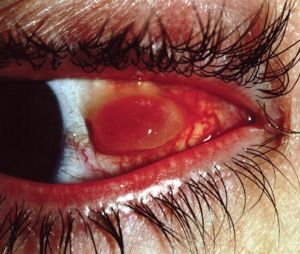
Foreign Body Granuloma / Pyogenic Granuloma
- A foreign body granuloma can develop sporadically in susceptible patients, usually a few weeks after surgery[15]. The occurrence rate appears equal in primary and secondary surgeries and the existence of a prior granuloma does not appear predictive of subsequent granulomas. Pyogenic granuloma, also known as lobular capillary hemangioma, occurs with proliferation of capillareies with edema typically at the conjunctival incision.
- Risk factors: The risk of a foreign body granuloma occurring appears to be related to the suture material. With the elimination of gut sutures in most strabismus surgeries, granulomas have become uncommon. Pyogenic granuloma can occur after any incision or trauma to the conjunctiva.
- Primary prevention: The risk of a foreign body granuloma may be reduced by avoiding gut sutures and by proper draping to keep lashes out of the surgical field[16].
- Diagnosis: Persistent post-operative foreign body sensation can be caused by a foreign body granuloma. Examination will demonstrate a localized, elevated, hyperemic mass at the suture site typically less than 1 cm in diameter.
- Management: Topical corticosteroids are used for several weeks, with possible surgical excision if no clinical response is observed[17].
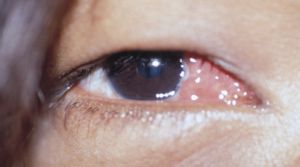
Conjunctival Inclusion Cyst
- A conjunctival inclusion cyst can occur when conjunctival epithelial cells are buried beneath the conjunctival surface during surgery[16]. These cells can multiply over time to create a subconjunctival cyst days to years after the original surgery. The incidence of conjunctival inclusion cysts range from 0.25% to 0.4%[18][19].
- Risk factors: The risk of a conjunctival inclusion cyst is increased when the conjunctival wound is not closed meticulously. In particular, for fornix incisions, relying on simple apposition of the conjunctival wound without sutures appears to increase the risk of subsequent cyst formation[1].
- Primary prevention: The risk of a conjunctival inclusion cyst may be minimized with careful and complete wound closure[16]. Preplaced marking sutures can allow easy identification of the edges of the conjunctival wound at the end of surgery, allowing easy distinction from the underlying Tenon’s capsule.
- Diagnosis: May not be noticed for many months or years. See diagnosis of foreign body granuloma.
- Management: See management of foreign body granuloma[20].
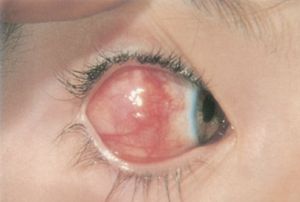
Conjunctival Scarring
- Conjunctival scarring occurs when, instead of returning to the typical translucent white appearance, the conjunctiva remains chronically hyperemic and pink, particularly after a second or third operation[21]. This complication can be exacerbated by advancement of Tenon’s capsule too close to the limbus, particularly during a resection, or by advancement of the plica semilunaris onto the bulbar conjunctiva. If severe, the conjunctival scarring itself can cause a restrictive strabismus.
- Risk factors: The risk of conjunctival scarring is increased for a re-operation and after a surgical resection. Care must be taken to avoid creation of conjunctival foreshortening or symblepharon.
- Primary prevention: The risk of conjunctival scarring may be minimized by careful wound closure. For resections, debridement of Tenon’s capsule from the undersurface of the conjunctiva and recession of the conjunctival wound from the corneal limbus may help prevent the thickened anterior conjunctival scar that can occur after a large resection.
- Diagnosis: Slit lamp examination reveals conjunctival scarring. Also see diagnosis of a lost muscle, as conjunctival scarring may result in restrictive strabismus.
- Management: Persistent conjunctival scarring may require surgery to remove scar tissue and smooth the conjunctival surface. The risk of excessive conjunctival scarring increases with each surgery performed.
- Prognosis: A visible conjunctival scar is present in over 90% of cases postoperatively and is usually subtle and clinically insignificant, although it can rarely be severe[21].
Fat Adherence
- Fat adherence is caused by violation of Tenon’s capsule with prolapse of orbital fat. The orbital fat can cause a fibro-fatty scar that is adherent to the muscle and globe, potentially leading to a restrictive strabismus[1].
- Risk factors: The risk of fat adherence is increased with more posterior strabismus surgery, such as operating on the inferior oblique muscle, exploring posteriorly to retrieve a lost muscle, or dissecting more posteriorly to place a posterior fixation suture[1].
- Primary prevention : The risk of fat adherence may be minimized by recognition of the violation of the retro-orbital fat intra-operatively[1]. The posterior opening in Tenon’s capsule can be repaired in layers with absorbable sutures to prevent contact between the globe and muscles and orbital fat.
- Diagnosis: See diagnosis of a lost muscle.
- Management: Treatment is surgical, but success is limited, and recurrence is high. In the case of fat adherence following surgery on the inferior oblique muscle, exploration can release adhesions, and recession of the inferior rectus recession can improve ocular motility, though full resolution is unlikely. Inferior rectus recession in addition to extensive conjunctival peritomy, recessing the conjunctiva, and allowing the sclera to re-epithelialize has also been used[22].
Dellen
- Dellen are shallow, clearly defined excavations at the margin of the cornea or sclera. The occur when thickened bulbar conjunctiva (either from scarring, hemorrhage, or swelling) prevents adequate and even lubrication of the ocular surface during a blink. Any disruption of the tear layer on the sclera or cornea causing local dehydration can create a dellen.[1]
- Risk factors: The risk of dellen formation is higher for a limbal incision (6.5%) than a non-limbal (2.2%) incision, because the subsequent irregularity of the perilimbal conjunctiva can cause a disruption of the tear layer in the anterior sclera and cornea[16]. Dellen are more common after resection surgery than recession surgery.[1]
- Primary prevention: The risk of dellen formation may be minimized by utilizing a fornix incision instead of a limbal incision or by recessing the conjunctiva away from the limbus after a limbal incision.
- Diagnosis: The patient may complain of persistent post-operative foreign body sensation, though dellen may be underdiagnosed because clinical findings are subtle and symptoms may be absent[1]. On slit lamp examination, fluorescein will pool within the indentation of the cornea or sclera without creating true staining.
- Management: For dellen formation, aggressive topical lubrication with artificial tears, sometimes in conjunction with eye patching, can help until the chemosis and swelling subside and the ocular surface becomes smooth again[23]. A dellen may in rarer cases require surgery to remove scar tissue and smooth the conjunctival surface.
- Prognosis: Although the thinning can appear very dramatic, the rate of perforation appears very low, and dellen are typically asymptomatic and self-limiting[23][24].
Anterior Segment Ischemia
- Anterior segment ischemia occurs when blood supply to the anterior segment through the ciliary arteries within the four rectus muscles is interrupted[24]. Simultaneous surgery on three rectus muscles in the same eye, or two rectus muscles in a patient with compromised blood flow from vascular disease, can cause ischemia. The incidence of significant anterior segment ischemia has previously been estimated to be approximately 1 in 13,000 cases, although this is likely an underestimate because mild cases probably occur without clinical detection[25].
- Risk factors: The risk of anterior segment ischemia is higher when operating on multiple muscles in the same eye, in older patients with microvascular disease, and in patients with prior extensive eye surgery that might also disrupt the ciliary vessels, such as scleral buckling procedures[25].
- Primary prevention: The risk of anterior segment ischemia may be minimized by limiting the number of muscles operated on in each eye, by utilizing botulinum toxin, and by using special surgical techniques to spare the ciliary vessels during muscle surgery. A fornix conjunctival incision may have a slightly reduced effect on anterior segment perfusion compared with a limbal incision[24].
- Diagnosis: Typical findings in anterior segment ischemia include iritis, corneal edema, folds in Descemet’s membrane and, if severe, anterior segment necrosis and phthisis bulbi of the operated eye[20]. Pupillary reactions should be assessed because an abnormally slow or asymmetric pupillary reaction to light can be an early sign of anterior segment ischemia. Other forms of specialized testing, such as an iris angiogram for anterior segment ischemia, are typically performed in a research setting rather than in standard clinical practice. Also see diagnosis of post-operative infection.
- Management: For anterior segment ischemia, topical and systemic corticosteroids can relieve inflammation until collateral vascularization can occur[20].
- Prognosis: When severe, anterior segment ischemia can progress to necrosis and phthisis bulbi, but most cases resolve with corticosteroids over time[20].
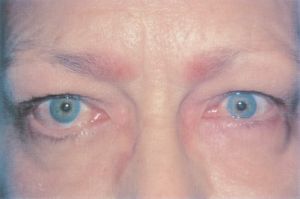
Eyelid Retraction or Ptosis
- Eyelid retraction or ptosis may occur after strabismus surgery. This can occur because the eyelid retractors, particularly in the lower eyelid, are adherent to the intermuscular septum and fascial tissue around the vertical rectus muscles. This connection creates a shift in eyelid position during standard recession or resection surgery of the vertical rectus muscles[26]. One study found that 91% of patients who had a superior rectus recession developed upper-lid retraction, 94% of patients who had an inferior rectus recession developed lower-lid retraction, and 100% of patients who had an inferior rectus resection developed lower-lid advancement with accompanying flattening[27].
- Risk factors: The risk of eyelid retraction or ptosis is increased in subjects undergoing vertical rectus recessions or resection with high surgical dosages, particularly surgeries involving the inferior rectus. Eyelid retraction can also be seen in subjects undergoing anterior transposition of the inferior oblique muscles following previous large superior rectus muscle recessions[28].
- Primary prevention: The risk of eyelid retraction or ptosis may be minimized by limiting the surgical dosages applied to the vertical rectus muscles and by carefully dissecting the lid retractors away from the extraocular muscles during surgery at least 12-15 mm posterior to the insertion[26].
- Diagnosis: change in eyelid position is assessed with clinical ophthalmic examination including measurements of MRD1, MRD2, palpebral fissure, and inferior and superior scleral show.
- Management: Eyelid retraction or ptosis may require oculoplastic surgery to restore the normal lid configuration, particularly if the defect prevents proper eyelid closure or affects peripheral vision.
Change in Refraction
- Change in the refraction post-operatively occurs from a change in the force the extraocular muscle places on the cornea through its attachment to the sclera. Over time, this change in force usually reaches a new equilibrium, typically with restoration of the original corneal refractive shape[29].
- Risk factors: Operating on more than one muscle per eye, particularly utilizing larger amounts of recession or resection, increases the risk for change in refraction. For example, operating on two horizontal muscles can induce a small with-the-rule astigmatism[30].
- Primary prevention: Limiting surgical dosages could reduce the risk of a change in the post-operative refraction, but as a practical matter, simply monitoring post-operative vision and adjusting the refractive correction to maximize vision can treat this self-limited complication.
- Diagnosis: For decreased vision post-operatively, checking a refraction can help distinguish a refractive change from other post-operative complications. Retinoscopy or corneal topography can be used to assess for irregular astigmatism.
- Management: Optical therapy is directed at correcting any change in refractive error following surgery.
- Prognosis: Change in refraction is typically temporary and resolves after a few months.
Post-operative Strabismus Complications
Unsatisfactory Eye Alignment
- Unsatisfactory eye alignment can occur sporadically after otherwise uncomplicated strabismus surgery in cooperative patients. It can also occur from pre-operative measurement errors of the eye misalignment, intra-operative measurement errors in extraocular muscle position, and excessive scarring or inflammation. Late causes of poor alignment can come from poor sensory adaptation to the new eye position. This is the most common complication despite careful pre-operative measurements and utilization of common surgical dosage tables. Around 20% of adults with strabismus do not achieve satisfactory alignment with one procedure[31].
- Risk factors: Unsatisfactory eye unalignment is more likely after surgery in patients with poor fusion potential and in patients with more complicated types of strabismus. Patients with dense amblyopia or structural problems in one or both eyes have limited potential for binocular vision and will not employ fusional mechanisms to improve or maintain eye alignment. Similarly, patients with neuro-developmental anomalies have been shown to have higher rates of undercorrection and overcorrection after strabismus surgery. Also, patients with more unusual and severe forms of strabismus, such as 3rd nerve palsies, are more difficult to align satisfactorily with surgery[29].
- Primary prevention: The risk of unsatisfactory eye alignment may be minimized by a careful and complete pre-operative evaluation and by meticulous surgical technique with emphasis on good exposure, lighting, and precise intraoperative measurements to verify that proper surgical dosages were obtained. The pre-operative evaluation can include multiple consistent measurements of the eye deviation and prism adaptation to assess both surgical angle and post-operative fusion potential. However, one study has demonstrated that the surgical effect of strabismus correlates better with the preoperative deviation rather than with the surgical dose chosen.[32] The intra-operative procedure can include steps to minimize the measurement error induced by the curvature of the globe, particularly for large recessions, and to ensure proper placement of the muscle sutures to capture of the muscle belly, not just the muscle capsule, and minimize sag of the new muscle insertion.
- Diagnosis: Unsatisfactory eye alignment is diagnosed with sensorimotor examination at post-operative visits following strabismus surgery.
- Management: An initially unsatisfactory post-operative alignment may or may not require surgical correction[29]. Sometimes prisms can be incorporated temporarily (Fresnel prisms) or permanently into eyeglasses and further surgery deferred. In other situations, the prisms can be weaned slowly over time as fusional mechanisms build, allowing single binocular vision without prisms. Many cases of unsatisfactory eye alignment will need another strabismus surgery, particularly in the case of a slipped muscle or restrictive scarring where the eye alignment will not improve over time.
Diplopia
- Diplopia can occur in patients capable of vision in each eye from an imperfect eye alignment. Small amounts of residual vertical or torsional misalignments can be difficult to fuse into a single image, particularly if the misalignment is opposite to the pre-operative misalignment (overcorrection). Rarely, patients may have horror fusionis, the inability to fuse despite well-aligned eyes.[33]
- Risk factors: Diplopia is more likely in adult patients who possess limited ability to suppress the second image. The risk is also higher in more complicated types of strabismus, particularly vertical, torsional, or paretic forms of strabismus. In those patients, it may be more difficult to create a useful area of single binocular vision[34][35]. One study found that 9% of adult patients had temporary diplopia after surgery, which typically resolved within 6 weeks. Only 0.8% developed persistent intractable diplopia[34]. Postoperative diplopia, both temporary and intractable, is more likely in adults who experienced diplopia with preoperative prism testing[34]. Visual field loss, whether from neurologic or ocular etiology, can also cause diplopia.[36][37][38]
- Primary prevention: Diplopia can be prevented with careful assessment of the binocular potential prior to surgery. Amblyoscope testing can be used to assess a patient's fusional potential. Additionally, choosing conservative surgical dosages and employing adjustable suture techniques may help to minimize the chance of an overcorrection. In many cases, diplopia is a necessary but short-lived complication of eye muscle surgery.
- Diagnosis: Post-operative diplopia can be diagnosed based on patient history and sensorimotor examination at post-operative visits.
- Management. Prisms may also be used in select cases to alleviate diplopia. Monocular occlusion with a patch or occlusion foil may be used when prisms are not effective. In some cases, additional strabismus surgery may be necessary to correct the diplopia.
Iatrogenic Brown Syndrome
- Iatrogenic Brown syndrome can occur following superior oblique tightening procedures. Brown syndrome is characterized by limitation of elevation of the eye in adduction.
- Risk factors: Brown syndrome was noted more commonly following superior oblique tuck when concomitant weakening of the antagonist inferior oblique tendon was performed.[39]
- Primary prevention: The risk can be minimized by avoiding overtightening of the superior oblique tendon during surgery. When placing sutures for the superior oblique tuck, the sutures can be tied in a reversible shoe-string technique, and then forced duction testing should be performed to assess for limitation of elevation in adduction. If there is limitation, then the the amount of tuck should be reduced.
- Diagnosis: Diagnosis is made with sensorimotor examination in the post-operative period and can be confirmed with forced duction testing.
- Management: Many cases of iatrogenic Brown syndrome can be observed, since superior oblique tucks often relax with time.[40]
Anti-Elevation Syndrome
- Anti-elevation syndrome is characterized by restriction of elevation of the eye in abduction following inferior oblique anteriorization. [41]
- Risk factors: Anti-elevation syndrome was noted to more commonly occur in patients who had placement of the inferior oblique muscle more than 1mm anterior to the insertion of the inferior rectus muscle.[28]
- Primary prevention: Risk of anti-elevation syndrome can be decreased by avoiding inserting the inferior oblique muscle anterior to the inferior rectus insertion, and by keeping the two corners of the inferior oblique insertion close to each other to avoid lateral spreading of the inferior oblique fibers.[28][40]
- Diagnosis: Diagnosis is made with sensorimotor examination in the post-operative period and can be confirmed with forced duction testing.
- Management: Surgery may be required to disinsert or recess the inferior oblique muscle insertion.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 S.E. Olitsky, D.K. Coats, Complications of Strabismus Surgery, Middle East Afr J Ophthalmol. 22 (2015) 271–278. https://doi.org/10.4103/0974-9233.159692.
- ↑ 2.0 2.1 I. Hashim, C. Al-Haddad, Scleral Perforation as a Complication of Strabismus Surgery: A Literature Review, J Pediatr Ophthalmol Strabismus. (2021) 1–10. https://doi.org/10.3928/01913913-20211019-02.
- ↑ 3.0 3.1 3.2 3.3 3.4 D.K. Coats, S.E. Olitsky, eds., Scleral Perforation and Penetration, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 211–221. https://doi.org/10.1007/978-3-540-32704-2_21.
- ↑ R.J. Morris, P.H. Rosen, P. Fells, Incidence of inadvertent globe perforation during strabismus surgery., Br J Ophthalmol. 74 (1990) 490–493.
- ↑ 5.0 5.1 5.2 D.K. Coats, S.E. Olitsky, eds., Slipped and Lost Muscles, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 233–246. https://doi.org/10.1007/978-3-540-32704-2_23.
- ↑ 6.0 6.1 E.M. Ellis, M. Kinori, S.L. Robbins, D.B. Granet, Pulled-in-two syndrome: a multicenter survey of risk factors, management and outcomes, J AAPOS. 20 (2016) 387–391. https://doi.org/10.1016/j.jaapos.2016.06.004.
- ↑ K.I. Chatzistefanou, B.J. Kushner, L.R. Gentry, Magnetic resonance imaging of the arc of contact of extraocular muscles: implications regarding the incidence of slipped muscles, J AAPOS. 4 (2000) 84–93. https://doi.org/10.1067/mpa.2000.103434.
- ↑ S.I. Chen, P.C. Knox, P. Hiscott, I.B. Marsh, Detection of the Slipped Extraocular Muscle After Strabismus Surgery, Ophthalmology. 112 (2005) 686–693. https://doi.org/10.1016/j.ophtha.2004.11.048.
- ↑ R.W. Arnold, The Oculocardiac Reflex: A Review, Clin Ophthalmol. 15 (2021) 2693–2725. https://doi.org/10.2147/OPTH.S317447.
- ↑ 10.0 10.1 10.2 10.3 10.4 L.M. Dunville, G. Sood, J. Kramer, Oculocardiac Reflex, in: StatPearls, StatPearls Publishing, Treasure Island (FL), 2021. http://www.ncbi.nlm.nih.gov/books/NBK499832/ (accessed January 1, 2022).
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 R.J. House, J.C. Rotruck, L.B. Enyedi, D.K. Wallace, E. Saleh, S.F. Freedman, Postoperative infection following strabismus surgery: case series and increased incidence in a single referral center, J AAPOS. 23 (2019) 26.e1-26.e7. https://doi.org/10.1016/j.jaapos.2018.09.007.
- ↑ 12.0 12.1 D.K. Coats, S.E. Olitsky, eds., Postoperative Infection, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 223–232. https://doi.org/10.1007/978-3-540-32704-2_22.
- ↑ D.K. Coats, S.E. Olitsky, eds., Equipment, Operating Room Supplies, and Patient Preparation, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 57–65. https://doi.org/10.1007/978-3-540-32704-2_7.
- ↑ E.M. Helveston, M.A. Callahan, Synthetic absorbable suture for strabismus surgery, Am J Ophthalmol. 82 (1976) 300–302. https://doi.org/10.1016/0002-9394(76)90437-2.
- ↑ E.R. Gaskin, M.D. Childers Jr., Increased Granuloma Formation from Absorbable Sutures, JAMA. 185 (1963) 212–214. https://doi.org/10.1001/jama.1963.03060030070035.
- ↑ 16.0 16.1 16.2 16.3 D.K. Coats, S.E. Olitsky, eds., Anterior Segment and Ocular Surface Complications of Strabismus Surgery, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 185–201. https://doi.org/10.1007/978-3-540-32704-2_19.
- ↑ Z. Chen, T. Wang, Q. Pan, Z. Zhang, Z. Zhang, Clinicopathologic characteristics and surgical outcome of synthetic fiber conjunctival granuloma, BMC Ophthalmology. 20 (2020) 448. https://doi.org/10.1186/s12886-020-01717-1.
- ↑ A.M. Guadilla, P.G. de Liaño, P. Merino, G. Franco, Conjunctival cysts as a complication after strabismus surgery, J Pediatr Ophthalmol Strabismus. 48 (2011) 298–300. https://doi.org/10.3928/01913913-20100818-02.
- ↑ X. Min, H. Jiang, L. Shi, Descriptive Study of Conjunctival Cysts: A Rare Complication after Strabismus Surgery, Journal of Ophthalmology. 2018 (2018) e1076818. https://doi.org/10.1155/2018/1076818.
- ↑ 20.0 20.1 20.2 20.3 J.W. Simon, Complications of strabismus surgery, Current Opinion in Ophthalmology. 21 (2010) 361–366. https://doi.org/10.1097/ICU.0b013e32833b7a3f.
- ↑ 21.0 21.1 M.J. Wan, D.G. Hunter, Complications of Strabismus Surgery: Incidence and Risk Factors, Seminars in Ophthalmology. 29 (2014) 421–428. https://doi.org/10.3109/08820538.2014.959190.
- ↑ P. Merino, I. Blanco, P.G. de Liaño, Fat adherence syndrome following inferior oblique surgery: Treatment and outcomes, J Optom. 9 (2016) 240–245. https://doi.org/10.1016/j.optom.2015.07.002.
- ↑ 23.0 23.1 D.H. Lee, M.A. Herion, D.R. Unwin, O.A. Cruz, Scleral dellen after bilateral adjustable suture medial rectus muscle resection, J AAPOS. 7 (2003) 221–222. https://doi.org/10.1016/s1091-8531(03)00004-1.
- ↑ 24.0 24.1 24.2 D.K. Coats, Strabismus Surgery Complications, International Ophthalmology Clinics. 50 (2010) 125–135. https://doi.org/10.1097/IIO.0b013e3181f0fa21.
- ↑ 25.0 25.1 R.A. Saunders, E.C. Bluestein, M.E. Wilson, J.E. Berland, Anterior segment ischemia after strabismus surgery, Surv Ophthalmol. 38 (1994) 456–466. https://doi.org/10.1016/0039-6257(94)90175-9.
- ↑ 26.0 26.1 D.K. Coats, S.E. Olitsky, eds., Complications Involving the Ocular Adnexa, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 259–266. https://doi.org/10.1007/978-3-540-32704-2_26.
- ↑ E.M. Pacheco, D.L. Guyton, M.X. Repka, Changes in eyelid position accompanying vertical rectus muscle surgery and prevention of lower lid retraction with adjustable surgery, J Pediatr Ophthalmol Strabismus. 29 (1992) 265–272. https://doi.org/10.3928/0191-3913-19920901-03.
- ↑ 28.0 28.1 28.2 B.J. Kushner, The effect of anterior transposition of the inferior oblique muscle on the palpebral fissure, Arch Ophthalmol. 118 (2000) 1542–1546. https://doi.org/10.1001/archopht.118.11.1542.
- ↑ 29.0 29.1 29.2 D.K. Coats, S.E. Olitsky, eds., Unexpected Postoperative Alignment, in: Strabismus Surgery and Its Complications, Springer, Berlin, Heidelberg, 2007: pp. 291–294. https://doi.org/10.1007/978-3-540-32704-2_29.
- ↑ D. Mezad-Koursh, A. Leshno, T. Ziv-Baran, C. Stolovitch, Refractive Changes Induced by Strabismus Corrective Surgery in Adults, J Ophthalmol. 2017 (2017) 2680204. https://doi.org/10.1155/2017/2680204.
- ↑ B.J. Kushner, The efficacy of strabismus surgery in adults: a review for primary care physicians, Postgraduate Medical Journal. 87 (2011) 269–273. https://doi.org/10.1136/pgmj.2010.108670.
- ↑ Archer SM. Why strabismus surgery works: the legend of the dose-response curve. J AAPOS. 2018 Feb;22(1):1.e1-1.e6. doi: 10.1016/j.jaapos.2017.12.001. Epub 2017 Dec 27. PMID: 29288836.
- ↑ R.P. Rutstein, B. Bessant, Horror fusionis: a report of five patients, J Am Optom Assoc. 67 (1996) 733–739.
- ↑ 34.0 34.1 34.2 B.J. Kushner, Intractable diplopia after strabismus surgery in adults, Arch Ophthalmol. 120 (2002) 1498–1504. https://doi.org/10.1001/archopht.120.11.1498.
- ↑ J.Y. Wang, D.A. Leske, S.R. Hatt, J.M. Holmes, Diplopia after strabismus surgery for adults with nondiplopic childhood-onset strabismus, J AAPOS. 23 (2019) 313.e1-313.e5. https://doi.org/10.1016/j.jaapos.2019.07.005.
- ↑ Borchert MS, Lessell S, Hoyt WF. Hemifield slide diplopia from altitudinal visual field defects. Journal of Neuro-ophthalmology : the Official Journal of the North American Neuro-ophthalmology Society. 1996 Jun;16(2):107-109. PMID: 8797166.
- ↑ Khanna CL, Holmes JM. Strabismus and binocular diplopia due to advanced glaucomatous visual field loss. J AAPOS. 2017 Aug;21(4):263-267. doi: 10.1016/j.jaapos.2017.06.009. Epub 2017 Jun 20. PMID: 28642003.
- ↑ Pula JH, Fischer M, Kattah JC. Hemifield slide from traumatic optic chiasmopathy. J Clin Neurosci. 2014 Aug;21(8):1446-7. doi: 10.1016/j.jocn.2014.01.001. Epub 2014 Jan 24. PMID: 24613428.
- ↑ Helveston EM, Ellis FD. Superior oblique tuck for superior oblique palsy. Aust J Ophthalmol. 1983 Aug;11(3):215-20. PMID: 6639513.
- ↑ 40.0 40.1 American Academy of Ophthalmology. Pediatric Ophthalmology and Strabismus. Basic and Clinical Science Course, 2021-2022.
- ↑ Kushner BJ. Restriction of elevation in abduction after inferior oblique anteriorization. J AAPOS. 1997 Mar;1(1):55-62. doi: 10.1016/s1091-8531(97)90024-0. PMID: 10530986.