Waldenstrom Macroglobulinemia and Hyperviscosity-related Retinopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Waldenstrom macroglobulinemia, a type of non-Hodgkin lymphoma, is also known as lymphoplasmacytic lymphoma. ICD 10: C88.0.
Disease
Waldenstrom macroglobulinemia (WM) is a malignant lymphoplasmo-proliferative disorder which overproduces IgM, a monoclonal pentameric immunoglobulin. In patients with WM, the bone marrow or the lymph nodes are occupied by pleomorphic B lymphocytes in various maturation stages ranging from small lymphocytes, lymphoplasmacytoid cells with basophilic cytoplasm to plasma cells. Mast cells also increase significantly in the bone marrow of these patients while overexpressing CD40 Ligand, an inducer of B-cell proliferation. Patients present with cytopenias due to bone marrow infiltration, lytic bone disease, hepatomegaly, splenomegaly, lymphoplasmacytic infiltration of the pulmonary parenchyma, or Bing-Neel syndrome.
Bing-Neel syndrome is a rare complication due to lymphoplasmacytoid infiltration and IgM deposits in the CNS. Perivascular malignant infiltration occurs as a result of long-standing hyperviscosity and secondary CNS vascular hyperpermeability. Symptoms include vertigo, headache, ataxia, diplopia, nystagmus, and coma.
Ocular manifestations of the disease include infiltration of the conjunctivae, malignant vitritis, and hyperviscosity-related retinopathy (Figure 1). [1] Most signs and symptoms occur due to the hyperviscosity syndrome and include retinal hemorrhages, microaneurysms, retinal vein tortuosity, retinal vein occlusion, macular edema, serous macular detachments, retinal neovascularization.
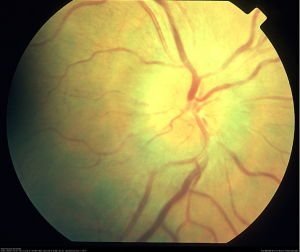
Etiology
WM is primarily considered a sporadic disease, but some studies have suggested a possibility of a single genetic defect. Deletions in 6q21-22.1 were found in majority of patients with WM, and B-cell disorders were also found in first degree relatives of these patients. Serum hyperviscosity leading to vascular disturbances plays a major role in the microvascular changes of the retina causing vascular dilation. [3]
Risk Factors
Waldenstrom macroglobulinemia has an incidence of 3 per million people per year. This accounts for 1-2% of hematological cancers. WM is found to be more prevalent amongst Caucasians and males. About 5% of the patients are Black and 55% to 70% are men. WM is rare in patients younger than 50 years old. [1][4] About 40% of patients with WM will have retinal issues due to serum hyperviscosity. Additionally, having a pre-existing IgM–Monoclonal Gammopathy of Undetermined Significance (MGUS) significantly increases the risk of developing WM later in life. There are some studies showing an association between Hepatitis C and WM. Heredity (certain mutated genes) and certain autoimmune diseases like Sjogren syndrome also increase the risk of developing WM.
General Pathology
Waldenstrom macroglobulinemia is characterized by cytopenia due to bone marrow infiltration, anemia, lytic bone disease, organomegalies, diffuse pulmonary infiltrates or pleural effusion due to lymphoplasmacytic infiltration of the parenchyma, and malignant infiltration of the bowel, stomach, skull base, orbit, and CNS.
Pathophysiology
WM is a lymphoproliferative B-cell disorder that is an indolent form of B-cell non-Hodgkin’s lymphoma, also known as lymphoplasmacytic lymphoma. The two main landmarks of this disease are bone marrow infiltration with clonal lymphoplasmacytic cells and IgM proliferation. In the majority of patients, WM follows Monoclonal Gammopathy of Undetermined Significance (MGUS). Pleomorphic lymphoid cells and Dutcher bodies (cytoplasmic inclusion bodies which stain positive for periodic acid Schiff) may be seen in a bone marrow biopsy. Mast cell overgrowth is also common and plays a role in stimulating bone marrow and IgM proliferation. WM is characterized by hyperviscosity/paraproteinemia due to overproduction and deposition of pentamer IgM. Due to the large size of IgM antibodies, the majority remain intravascular and form aggregates by binding to water. Additionally, because of their cationic nature, they attract the negatively charged red blood cells and lower their repulsive forces. All this leads to hyperviscosity syndrome and its associated symptoms as well as rouleaux formation.
Normal serum viscosity is 1.4 to 1.8 times that of water at body temperature, and multiple studies state that symptoms due to hyperviscosity are unlikely if serum viscosity is less than 4 cP (centipoise, unit of dynamic viscosity) and more likely if values are higher than 5.0 cP. [3][5] Viscosity is highest in small venules; therefore, the force exerted by viscous fluids will tear the venule wall, resulting in microvascular bleeding. [6] This is most readily observable in the retina as central retinal hemorrhages and vascular dilation, and is commonly seen in WM. According to Menke et al., both the central and peripheral retina are involved in the most severe hyperviscosity-related retinopathy. On the other hand, only peripheral hemorrhages and dilated veins were found in milder hyperviscosity-related retinopathy (intermediate serum viscosity levels). [3]
Primary Prevention
Since the major risk factors like MGUS and older age are non-modifiable, there are no existing recommendations for prevention of Waldenstrom macroglobulinemia.
Diagnosis
To make the diagnosis of Waldenstrom macroglobulinemia, it is important to follow the diagnostic criteria strictly and rule out all other lymphoproliferative diseases. The key to diagnosis includes two main criteria (Mayo Clinic) of detecting IgM monoclonal protein (M-spike) of any size and at least 10% lymphoplasmacytic lymphoma cells in the bone marrow. [7] The lymphocytes are of B-cell profile with expression of surface IgM, CD19, CD20, CD22, and CD79a antigens. Diagnostic workup includes blood cell count with differential to look for cytopenia, immunoglobulin levels (IgM would be high and IgG is usually low), electrophoresis, viscosity measurement, and bone marrow aspiration and biopsy. Biopsy specimens undergo additional testing which includes immunohistochemistry, flow cytometry, and cytogenetics. [7] When a condition that leads to hyperviscosity syndromic state (such as any lymphoproliferative diseases) is suspected, a thorough ophthalmic examination should be performed. Characteristic findings on a dilated fundoscopic examination may prompt an initial diagnosis. [6] (See below for more details under physical examination).
History
In 1944, Jan Gosta Waldenström first described and identified two patients with macroglobulinemia with symptoms which he attributed to elevated serum viscosity. This included a triad of mucosal bleeding, visual alterations, and neurologic abnormalities and was consistent with reports of increased serum viscosity in multiple myeloma previously documented between 1932 and 1937. J. L. Fahey coined the term “hyperviscosity syndrome” in 1965. [6]
Physical examination
The physical exam is consistent with hyperviscosity syndrome, presenting with symptoms of neurologic dysfunction such as headache and fatigue, bleeding diathesis such as epistaxis, and visual abnormalities associated with retinopathy. [7] Ocular signs of hyperviscosity manifest as a hyperviscosity-related retinopathy. In the early stages of Waldenstrom macroglobulinemia, it is postulated that small retinal hemorrhages are seen in the far periphery that can be observed on indirect ophthalmoscopy with scleral depression. [3] Later, the dilated fundoscopic examination also reveals vascular tortuosity and sausage-like dilation of retinal veins, as well as more centrally located retinal hemorrhages, macular edema, and optic disc edema. [8] The hemorrhages observed are microaneurysms, dot-blot, and flame hemorrhages. It is important to note that retinal hemorrhages can occur without affecting visual acuity if the macula is spared. Optical coherence tomography (OCT) is often used to monitor for macular edema, which can progress to neurosensory serous macular detachments. Bilateral central retinal vein occlusions, as a result of hyperviscosity syndrome, have also been reported. [6] Fluorescein angiography demonstrates increased retinal circulation time, and areas of capillary non-perfusion and microaneurysms. [8]
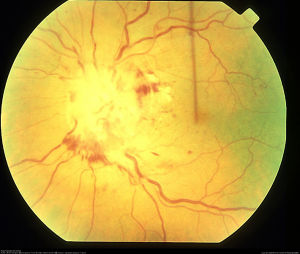
Signs and Symptoms
Most patients with Waldenstrom macroglobulinemia have symptoms due to tumor infiltration and/or monoclonal immunoglobulin M leading to hyperviscosity syndrome.
Signs/Symptoms due to tumor infiltration
- B symptoms
- Pancytopenia/Anemia
- Lytic bone disease
- Lymphadenopathy
- Splenomegaly
- Hepatomegaly
- Diffuse pulmonary infiltrates or pleural effusion symptoms
- Malignant infiltration of stomach and bowel
- Malignant vitritis
- Conjunctival infiltration
Signs/Symptoms due to Hyperviscosity
- Bleeding/nose bleeding
- Headache
- Vertigo
- Impaired hearing and vision
- Ataxia
- Nystagmus
- Diplopia
- Neurological deficits
- Eventually coma[1]
Ocular symptoms due to hyperviscosity: Ocular symptoms of vision changes in Waldenstrom Macroglobulinemia are seen in 30% to 40% of patients and are due to hyperviscosity syndrome. Retinal findings include retinal vein engorgement with vessel tortuosity, retinal hemorrhages and microaneurysms, and optic disc edema. Decreased blood flow occurs with increased concentration of macroglobulins and blood viscosity; rise in intravascular pressure, causes retinal outflow venous obstruction. [10]
- Retinal hemorrhages
- Retinal vascular tortuosity and venous dilation
- Macular edema
Diagnostic procedures
- Blood cell counts: cytopenia or anemia
- Serum protein electrophoresis: amount of Monoclonal Immunoglobulin M
- Bone marrow biopsy: pleomorphic lymphocytic proliferation
- CT scan: signs of lymphoma in the chest, abdomen, and pelvis
- Indirect ophthalmoscopy with scleral depression/ dilated funduscopic exam: retinal hemorrhages, dilated and tortuous retinal veins, macular edema, optic disc edema, serous retinal detachment
- Optical coherence tomography: macular edema
- Fluorescein angiography: increased retinal circulation time - delayed arm to eye and prolonged AV transit time may be seen.
Differential diagnosis
- IgM-monoclonal gammopathy of undetermined significance (MGUS)
- B-cell chronic lymphocytic leukemia
- B-cell non-Hodgkin lymphomas: small lymphocytic lymphoma, marginal zone lymphoma, mantle-cell lymphoma, follicular lymphoma, diffuse large B-cell lymphoma
- Cryoglobulinemia
- Multiple Myeloma
- Central retinal vein occlusion
- Venous Stasis Retinopathy
- Severe non-proliferative diabetic retinopathy
- Anemic retinopathy
Management
Initial treatment of Waldenstrom macroglobulinemia involves managing the acute symptoms due to hyperviscosity syndrome since more than 80% of IgM is intravascular. Plasmapheresis is used to decrease IgM in the vasculature and is shown to be as effective as reducing serum IgM by 35%-48%. This treatment also helps with reversing the retinal findings like vascular engorgement.
Once serum IgM levels are controlled, the next step is to prevent another spike and maintain the levels with chemotherapy. Cyclophosphamide/doxorubicin/vincristine/prednisone/rituximab (CHOP-R) is a stem cell sparing regimen, and rituximab (a recent addition to the CHOP regiment) may be added without an increase in toxicity. [1][7][11][12] Other studies favor the use of DRC (dexamethasone, rituximab, and cyclophosphamide) or BR (bendamustine and rituximab) over CHOP-R as this regimen may be more tolerable and is associated with greater periods of halting disease progression.
Red blood cell transfusions are not recommended due to the risk of increasing serum viscosity from interaction of host IgM with donor RBCs.[13]
Complications
Life-threatening complications include gastrointestinal bleeding, cerebral hemorrhage, high-output cardiac failure.[5]Bilateral central retinal vein occlusions due to compression and engorgement of the retinal venous system and severe macular edema leading to serous macular detachments can lead to permanent vision loss if left untreated. [14]
Prognosis
The median survival of patients with Waldenstrom macroglobulinemia is around five years with up to 40% surviving ten years or more. In the majority of patients, the cause of death is advanced age-related comorbidities rather than WM itself. Presence of increased immunoblasts/pleomorphic cells, deletion of 6q, and absence of MYD88 L265P mutation are all associated with poor prognosis. WM can also transform to diffuse large B-cell lymphoma which has an adverse outcome.[7]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Vijay A, Gertz M. Waldenström macroglobulinemia. Blood. 2007;109.12: 5096-5103.
- ↑ Hunyor, Alex P. “hyperviscosity-retinopathy.” Retina Image Bank, 2013, imagebank.asrs.org/file/3080/hyperviscosity-retinopathy.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Menke MN, Feke GT, McMeel JW, Branagan A, Hunter Z, Treon SP. Hyperviscosity-Related Retinopathy in Waldenström Macroglobulinemia. Archives of Ophthalmology. 2006;124(11):1601–1606. doi:10.1001/archopht.124.11.1601
- ↑ Cancer.org. “Risk Factors For Waldenstrom Macroglobulinemia.” 2018, cancer.org/cancer/waldenstrom-macroglobulinemia/causes-risks-prevention/risk-factors.
- ↑ Jump up to: 5.0 5.1 Stone MJ, Pascual V. Pathophysiology of Waldenström's macroglobulinemia. Haematologica. 2010;95(3):359-364. doi:10.3324/haematol.2009.017251
- ↑ Jump up to: 6.0 6.1 6.2 6.3 Gertz M. Acute hyperviscosity: Syndromes and management. Blood. 2018 Sep 27; 132(13): 1379–1385
- ↑ Jump up to: 7.0 7.1 7.2 7.3 7.4 Kaseb H, Gonzalez-Mosquera LF, Parsi M, et al. Cancer, Lymphoplasmacytic Lymphoma (Waldenstrom Macroglobulinemia), 2020;In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- ↑ Jump up to: 8.0 8.1 Agarwal A. Gass’ Atlas of Macular Diseases, Fifth Ed, Volume 1. Elsevier Saunders; 2012; 608-609.
- ↑ Hunyor, Alex P. “hyperviscosity-retinopathy.” Retina Image Bank, 2013, imagebank.asrs.org/file/3080/hyperviscosity-retinopathy.
- ↑ Watson JA, Olson DJ, Zhang AY. Hyperviscosity Retinopathy Due to Waldenström Macroglobulinemia: A Case Report and Literature Review. J Vitreoretin Dis. 2021 Feb 11;5(6):520-524.
- ↑ Treon SP, Hunter Z, Barnagan AR. CHOP plus rituximab therapy in Waldenstrom's macroglobulinemia. Clin Lymphoma. 2005;5(4):273-277. doi:10.3816/clm.2005.n.015
- ↑ Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG). Leukemia. 2009;23(1):153-161. doi:10.1038/leu.2008.261
- ↑ Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood. 2014;124(9):1404-1411. doi:10.1182/blood-2014-03-565135
- ↑ Rogers PA, Estes M. Hyperviscosity Syndrome,2020; In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.

