Ocular Ischemic Syndrome
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Ocular ischemic syndrome (OIS) is a rare, but vision-threatening condition associated with severe carotid artery occlusive disease (stenosis or occlusion) leading to ocular hypoperfusion. Principal symptoms include visual loss, transient visual loss, and ischemic ocular pain. [1][2][3]OIS commonly occurs in the elderly with men more affected than women, owing to the higher incidence of atherosclerosis and carotid artery disease in these patients. OIS has important systemic implications, as disease of the common (CCA) or internal (ICA) carotid arteries may cause ipsilateral ocular signs and symptoms that in turn could herald a cerebral infarction. [4]
Epidemiology and Pathogenesis
Ocular ischemic syndrome most commonly occurs in the sixth decade of life; it is uncommon before 50, has no racial predilection. Men are affected twice as often as women [5][6][7] likely due to the higher incidence of atherosclerotic disease in males. Bilateral involvement may occur in up to 22% of cases. [8][9][10] The degree of stenosis, the presence or absence of collateral vessels, chronicity of carotid artery disease, its bilaterality, and associated systemic vascular diseases help contextualize the severity of OIS. [11]
The incidence of OIS has been estimated at 7.5 cases per million persons per year. [12] However, this is probably an underestimation as OIS may be misdiagnosed as a retinal vein occlusions and diabetic retinopathy. Approximately 30% of patients with symptomatic carotid occlusion manifest retinal vascular changes that are usually asymptomatic, and 1.5% of them per year progress to symptomatic OIS. [13] Of patients with ICA occlusion undergoing surgical anastomosis between the superficial temporal artery and the middle cerebral artery, 18% presented with OIS. [6]
OIS develops especially in patients with poor collateral circulation between the ICA and external carotid artery (ECA) systems or between the right and left ICA. Patients with a healthy collateral circulation may not develop OIS even with total occlusion of the ICA, whereas in those with poor collaterals an ICA stenosis <50% may be sufficient to develop OIS. Patterns of occlusion vary from less than 50% stenosis to complete occlusion of at least one CCA or one ICA, often accompanied by occlusion or stenosis of the opposite carotid arterial system. [14] (Figure 1)
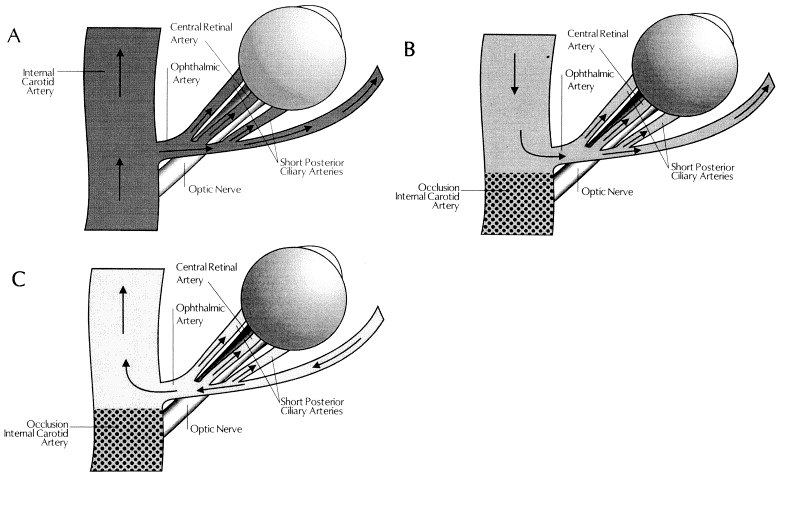
Patients who develop OIS show decreased blood flow in the retrobulbar vessels and reversal of blood flow in the ophthalmic artery (OA). [15] The OA may behave as a steal artery shunting blood flow away.[16] (Figure 2)
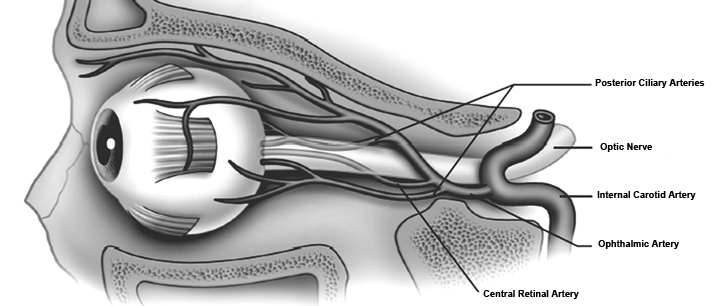
Figure 1. Arterial supply of the orbit.
Figure 2. Blood flow in the ophthalmic artery (OA) and its branches. (A) in a normal individual; (B) in a patient with internal carotid artery occlusion and collateral circulation via the circle of Willis (forward ophthalmic artery flow) (Figure 3); and (C) in a patient with internal carotid artery occlusion and collateral circulation via the ophthalmic artery (reversed flow). Reproduced with permission from Costa et al.[16]
Arterial Blood Supply
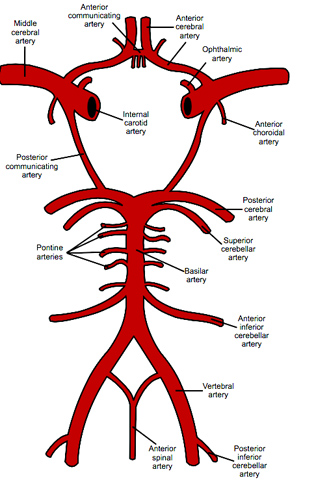
Figure 3. The Circle of Willis. Note the locations of the internal carotid artery (ICA) and ophthalmic artery (OA).
Diagnosis and Ancillary Tests
Fluorescein Angiogram
Fluorescein angiography can help to establish the diagnosis of OIS. [5][17] Particularly characteristic findings are delayed choroidal filling time (most specific angiographic sign) and prolonged arteriovenous (AV) transit time (most sensitive angiographic sign). Overall, a prolonged arteriovenous transit time is seen in 95% of cases, retinal vascular staining in 85%, and delayed choroidal filling in 60% (Figure 4). [5]
The choroid normally fills completely within 5 seconds after the first appearance of the dye in the choroidal arteries. Patchy or delayed choroidal filling is seen in a majority of eyes with OIS with severe filling delay of >1 minute in some cases. Prolonged retinal arteriovenous time is present in up to 95% of eyes with OIS. Arterial and early and late venous circulation times are also prolonged in eyes with OIS. However, delayed retinal circulation time is also observed in eyes with central retinal artery occlusion (CRAO) or central retinal vein occlusion (CRVO). Staining of the retinal vessels is also commonly seen and has been suggested to be due to endothelial cell damage with rupture of the inner blood-retina barrier due to chronic ischemia. This staining often may imitate the angiographic appearance of frosted branch angiitis seen in inflammatory conditions.
About 15% of eyes with OIS show macular edema at the late phase of the fluorescein angiography. [18] Leakage from microaneurysms or from telangiectasia may occur resulting in increased retinal thickness. Intraretinal fluid accumulation is usually mild to moderate, does not have a cystoid pattern and is often associated with hyperfluorescence of the optic disk attributed to leakage from blood vessels. Retinal capillary nonperfusion can also be seen in some eyes. The staining of arteries is more prominent with OIS. Also, optic disc hyperfluorescence, peripheral microaneurysms, peripheral perivascular staining are other angiographic features.
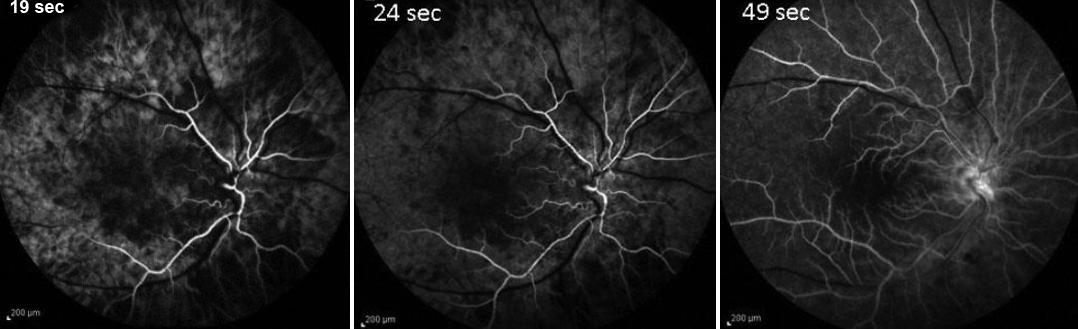
Figure 4. Fluorescein Angiogram (FA) in ocular ischemic syndrome (OIS). Prolonged Retinal and Choroidal Circulation in OIS in internal carotid artery (ICA) Occlusion at 19, 24 and 49 seconds (left, middle and right). Reproduced with permission from Open Journal of Ophthalmology.
Indocyanine Green Angiography
Indocyanine green (ICG) angiography allows for better evaluation of the choroidal vascular abnormalities. [19] The arm-to-choroid circulation time (time from the injection of the dye to the first appearance of the dye in the choroidal arteries, usually ~10 seconds), and the intra-choroidal circulation time (time from the first appearance of the dye in the choroidal arteries to complete dye filling in the choroidal veins, usually ~5–6 seconds) are both prolonged in OIS. Choroidal hypoperfusion results in occlusions of the choriocapillaries with areas of vascular filling defects in the posterior pole or the mid-periphery. Delayed filling or occlusion of the choriocapillaris in the peripheral watershed zones can also be observed. (Figure 5)
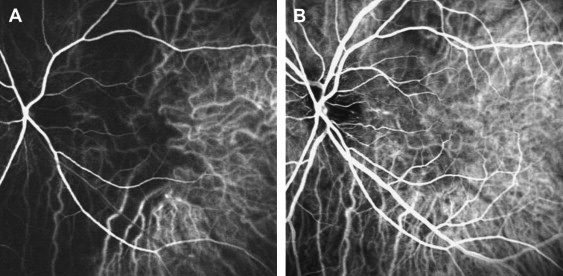
Figure 5. Indocyanine green angiography in ocular ischemic syndrome (OIS). A: Choroidal hypofluorescence corresponding to the main posterior watershed zone between the medial and lateral posterior ciliary arteries. B: Filling of the choroidal watreshed zone is delayed, with some part of it remaining hypofluorescent 10 seconds later. Reproduced with permission from Mendrinos et al. 2010 [20]
Electroretinography
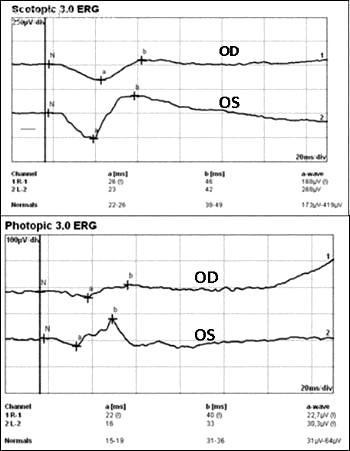
The b-wave of the electroretinograph (ERG) corresponds to activity of Müller and/or bipolar cells and reflects the functional status of the inner retinal layer, whereas the a-wave corresponds to activity of photoreceptors. In CRAO, where there is ischemia of the inner retina, the amplitude of the b-wave is decreased. In contrast, in eyes with OIS where both the retinal and choroidal circulation are compromised, there is ischemia of the inner and outer retina that results in decreased amplitude of both a and b waves. [5] Reduction in the amplitude of the oscillatory potential of the b-wave has been demonstrated in eyes with carotid artery stenosis even if the fluorescein angiography is normal. Carotid artery surgery increases ocular blood flow leading to improved retinal function and has been suggested to increase a and b wave amplitudes (Figure 6). [21]
Figure 6. Reduced scotopic and photopic ERG in ocular ischemic syndrome (OIS). Obtained with permission from Online Journal of Ophthalmology.
Visual-evoked potentials
Visual-evoked potentials (VEP) after exposure to intense light stimulation (photostress) show an increase in latency and a decrease in amplitude. Photostress induces transient VEP changes consisting of an increase in response latency and a decrease in amplitude. The time it takes the VEP to recover to the baseline status (ie. recovery time after photostress) ranges in normal subjects between 68 and 78 seconds. This recovery time after photostress is prolonged in patients with severe carotid artery stenosis and improves following endarterectomy surgery. The VEP may elicit visual dysfunction prior to the appearance of ophthalmological and may be a useful investigation.
Ophthalmodynamometry
Ophthalmodynamometry is used to estimate the pressure in the ophthalmic artery (OA) at the site of origin of the central retinal artery (CRA). Measurements are performed by increasing IOP by gradually applying pressure to the globe and observing the arteries on the optic disc until they begin to pulsate. [22][23] The pressure required to produce artery pulsations on the optic disc reflects the OA diastolic pressure, whereas the force required to cause cessation of arterial pulsations reflects the OA systolic pressure. In OIS, the CRA perfusion pressure is low and for this reason the pressure necessary for the pulsations to appear is reduced. Diastolic readings are decreased in OIS, and may improve or return to normal after carotid artery surgery. They are considered to be more reliable than systolic readings.
Imaging Methods for the Evaluation of Carotid Occlusive Disease
Carotid Duplex Ultrasound
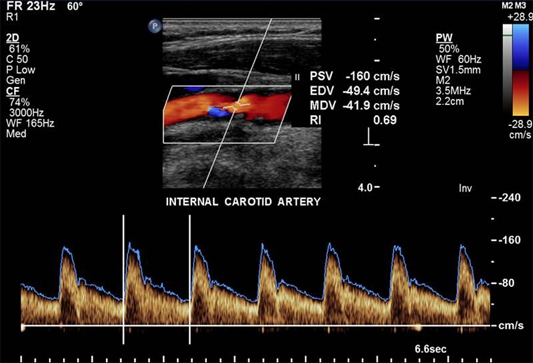
Duplex carotid ultrasonography is the most commonly used non-invasive test and combines B-mode ultrasound and Doppler ultrasound, providing both anatomical imaging of the vessel and flow velocity information. The parameters used to classify severity of stenosis include peak systolic velocity (PSV), end-diastolic velocity, and the ICA/CCA PSV ratio. Compared to conventional intra-arterial digital substraction angiography (DSA) for detection of high-grade symptomatic carotid artery stenosis, duplex ultrasound has a sensitivity of 89% and a specificity of 84%. For detecting occlusion, duplex ultrasound has a sensitivity of 96% and a specificity of 100%. (Figure 7)
Figure 7. Carotid Duplex Ultrasonography.
Peak Systolic Velocity (cm/sec) ICA Stenosis (% diameter)
125 – 225 50 – 70
225 – 350 70 – 90
>350 >90
Color Doppler Imaging of Retrobulbar Vessels
Color Doppler imaging of retrobulbar vessels is a useful adjunct to conventional duplex ultrasound for carotid artery examination. Distal to a hemodynamic significant stenosis, the blood pressure is decreased and the Doppler effect is dampened, causing diminished blood flow velocity. Therefore, studying the flow profile in the OA, the CRA and the short posterior ciliary arteries (SPCA) may provide hemodynamic information about the carotid and retrobulbar circulations such as velocities and pulsatility indices (an indicator of vascular resistance) of the orbital arteries. These parameters are significantly reduced in patients with significant carotid artery stenoses as compared to a normal, age-matched population with no carotid disease. Doppler imaging of retrobulbar vessels also allows studying the flow direction in the OA. Reversed OA flow pattern is a highly specific indicator of ipsilateral high-grade ICA stenosis or occlusion. (Figure 8)
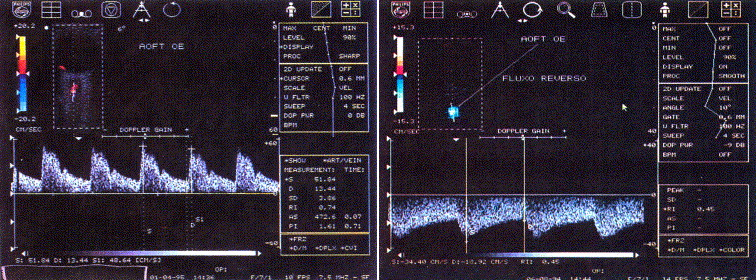 Figure 8. Color Doppler imaging of a normal ophthalmic artery. Note pulsatile, positive waveform with a dicrotic notch during systole (left) and a negative, pulsatile waveform in reversed ophthalmic artery (OA) flow with a higher end diastolic velocity suggesting the existence of a shunt. Reproduced with permission from Costa et al. [16]
Figure 8. Color Doppler imaging of a normal ophthalmic artery. Note pulsatile, positive waveform with a dicrotic notch during systole (left) and a negative, pulsatile waveform in reversed ophthalmic artery (OA) flow with a higher end diastolic velocity suggesting the existence of a shunt. Reproduced with permission from Costa et al. [16]
Magnetic Resonance Angiography and Computed Tomographic Angiography
New non-invasive or minimally invasive methods, and combinations of them, are increasingly used as the second-line investigation tool after duplex ultrasound for carotid stenosis. Magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) have gradually replaced DSA.
A review of carotid MRA performance data found that for the diagnosis of 70–99% carotid stenosis, MRA had a pooled sensitivity of 95% and a pooled specificity of 90% which are superior to a conventional DSA. [24] For detection of complete occlusions, MRA yielded a sensitivity of 98% and a specificity of 100% which is similar to duplex ultrasound. Limitations of MRA include claustrophobia, pacemakers and metallic stents or implantable defibrillators, and obesity.
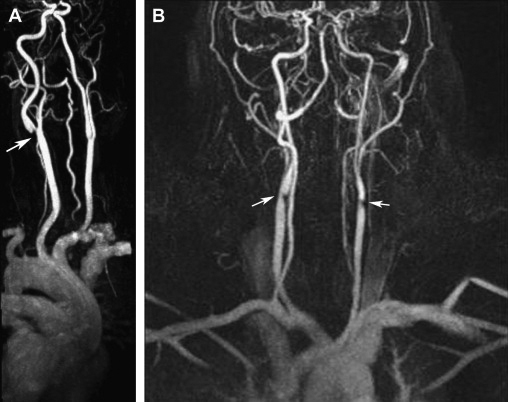
Potential advantage of MRA and CTA over conventional angiography is their ability to allow better carotid plaque characterization and identification of a plaque as well as to evaluate for cerebrovascular accident simultaneously. [25] The combined use of MRA, CTA, and doppler ultrasound improves diagnostic accuracy for high-grade symptomatic carotid stenoses and minimizes the need for invasive carotid arteriography. Disadvantages of CTA are the necessity of administering a nephrotoxic iodinated contrast agent and ionizing radiation and/or artifacts related to heavily or circumferentially calcified arterial walls. (Figure 9) (Figure 10)
Figure 9. 3D- reconstructed magnetic resonance angiography (MRA) in carotid artery disease. A: High-grade stenosis of the left internal carotid artery origin (arrow) in a 58-year-old diabetic woman. B: Severe bilateral stenosis at the origin of the internal carotid arteries (arrows) in a 48-year-old woman. Reproduced with permission from Mendrinos et al. [20]
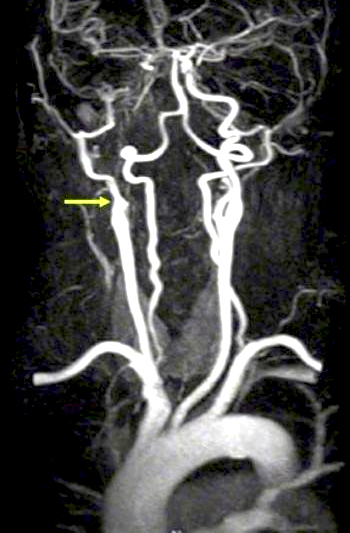
Figure 10. Internal carotid artery (ICA) occlusion in ocular ischemic syndrome (OIS).
Carotid Arteriography
Conventional intra-arterial digital substraction angiography has been considered as the gold standard for imaging the cerebrovascular system. Nevertheless, it is not ideal for screening and follow-up both because of the risks of disabling cerebral infarction, systemic complications and high cost. In patients with arteriosclerosis, a 4% risk of transient ischemic attack or minor cerebral infarction, a 1% risk of major cerebral infarction, and a <1% risk of mortality have been reported. [26] In patients with OIS, who have poor collateral circulation and severe carotid artery disease, arteriography-related risks may be even higher.
Duplex ultrasound should be chosen as the first-line investigation of patients suspected of having carotid stenosis. If surgically significant stenosis is identified or in equivocal cases, further imaging with either MRA or CTA should be performed. Only if results are contradictory or inconclusive, should DSA be performed. [20] (Figure 11)
Figure 11. Internal carotid artery (ICA) occlusion in ocular ischemic syndrome (OIS). Reproduced with permission from Open Journal of Ophthalmology.
Symptoms
Overview
As OIS is an ocular manifestation of systemic disease, patients with OIS may initially present with constitutional rather than ocular symptoms. In a review of 42 patients with OIS, only 6 reported developing ocular symptoms as their initial complaint. Additionally, patients may overlook their ocular symptoms due to the prominence of other complaints. The same study found that 37 of 42 patients with OIS initially visited non-ophthalmology clinics for evaluation, yet upon further questioning, all 37 reported experiencing ocular symptoms to some degree. Symptoms associated with OIS warrant a thorough history combined with careful evaluation to avoid misdiagnosis. [27]
Patients with OIS may be asymptomatic or report multiple nonspecific ocular symptoms, making correct diagnosis a challenge, especially in patients with comorbid ocular disease. Luo et al. found that among 42 patients with OIS, 35 presented with more than two ocular symptoms, including visual loss, ocular/periorbital pain, amaurosis fugax, photophobia, floaters, metamorphopsia, phosphenes, and diplopia. [27]
Visual Loss
Decrease in visual acuity in OIS may be severe, with acute or subacute presentation. In a series of 43 patients, at initial presentation, 35% of eyes had a VA of 20/20 to 20/40 and in 35% had vision of counting fingers or worse. [5] Similarly, in 52 patients studied by Sivalingam et al, 43% of the affected eyes had an initial VA of 20/20 to 20/50, and 37% had vision of counting fingers or worse. [10] Mizener et al reported initial VA of 20/40 or better in 15% of their cases, whereas 65% had VA of 20/400 or less. [11]
Visual fields on presentation can vary greatly from normal, to a central scotoma, nasal defects, centrocecal defects, and the presence only of a central or temporal island.[11]
A history of transient visual loss is present in approximately 10–15% of patients with OIS. This is most frequently caused by transient embolization of the CRA or its branches, but vasospasm may also play a role. In these cases, a dark or black shade spreads across the visual field that lasts for a few seconds to minutes. Less commonly, severe carotid artery disease causes transient visual loss as a result of choroidal hypoperfusion. Conditions that either increase retinal metabolic demands or decrease perfusion pressure can precipitate transient visual loss and reflect the inability of a borderline ocular circulation to maintain stable the ocular blood flow. This has been reported following exposure to bright light, postural change, or after eating a meal. Visual loss resulting from hypoperfusion of the choroid often lasts several minutes to hours and may be associated with positive visual phenomena.
Pain
Pain may be present in up to 40% of the eyes with OIS.[11][6] Pain may be the result of increased IOP or may be ischemic in origin. Ischemic pain begins gradually over hours to days and is described as a dull, constant ache in the affected eye, over the orbit, upper face, and temple, and may worsen when the patient is upright. Lying down relieves or lessens pain. The description "ocular angina" may be used to describe this discomfort. Ischemic pain may be confused with that from secondary glaucoma, but pain in eyes with OIS would not be explained by a slight IOP elevation. Pain from both increased IOP and ischemia may be present in the same patient. In older patients, giant cell arteritis should be excluded. The signs are summarized in Table 1. [20]
Signs
| Anterior Segment | Posterior Segment | Orbital |
|---|---|---|
| Conjunctival and Episcleral Injection | Narrowed retinal arteries | Orbital pain |
| Corneal edema and Descemet's folds | Dilated retinal veins | Anterior and posterior segment ischemia with intraocular inflammation and hypotony |
| Corneo-scleral melting | Retinal hemorrhages (mid peripheral) | Ophthalmoplegia |
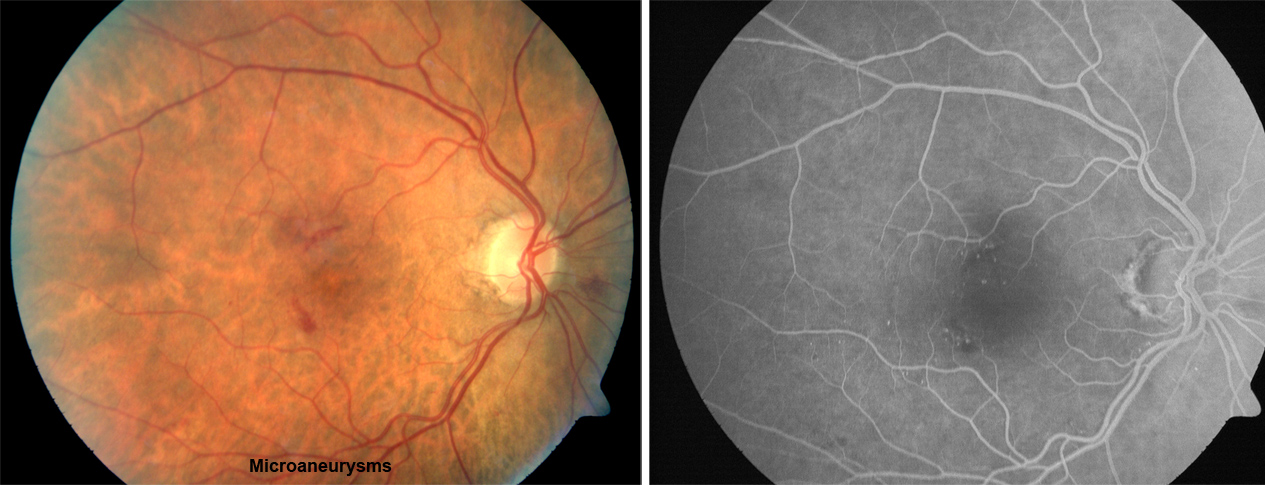
| Corneal hypoesthesia | Microaneusyms (mid peripheral) (Figure 13) | Ptosis |
| Spontaneous hyphema | Macular capillary telangiectasia | |
| Iris atrophy | Retinal arteriovenous communications | |
| Fixed semi-dilated pupil or sluggish reaction to light with relative afferent pupil defect | Cotton-wool spots | |
| Anterior and posterior synechiae | Cherry-red spot | |
| Uveal ectropion | Neovascularization of disc | |
| Rubeosis iridis (Figure 12) | Neovascularization elsewhere | |
| Neovascular glaucoma | Choroidal neovascular membrane | |
| Iridocyclitis (cell, flare, keratic precipitates) | Vitreous hemorrhage | |
| Asymmetric Cataract/arcus/ | Cholesterol Emboli, Spontaneous retinal arterial pulsations, anterior ischemic optic retinopathy, wedge shaped areas of chorio-retinal atrophy | |
| Asymmetric diabetic retinopathy | ||
| Hard exudates are usually not seen in OIS alone |
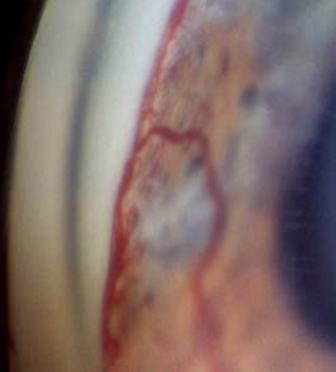
Figure 12. Gonioscopic image showing rubeosis iridis.
Figure 13. Microaneurysms from ocular ischemic syndrome. Left: Fundus photograph; Right: Fluorescein angiogram (FA).
Systemic Associations
Atherosclerosis of the carotid vascular system is the major cause of OIS and may be its initial manifestation. [5][28][29] Atherosclerotic plaques are usually located at the site of carotid bifurcation or at the proximal segment of the ICA. However, an intracranial carotid artery stenosis may also lead to OIS. Generally, ≥ 90% stenosis of the ipsilateral carotid arterial system is present in eyes with OIS and rarely the site of occlusion is the ophthalmic artery or bilateral ECAs. OIS has been reported to be the initial manifestation of carotid occlusive disease in approximately 70% of the patients. Patients with OIS often have systemic vascular diseases that are related to atherosclerosis. These include ischemic heart disease, previous cerebrovascular accident or transient ischemic attack, peripheral vascular disease, systemic arterial hypertension, and diabetes. [30] The prevalence of both systemic arterial hypertension and diabetes is significantly higher among patients with OIS compared to controls. The average stroke rate has been reported to be significantly higher in patients with OIS (4% per year) compared to 0.49% per year in controls. OIS may also appear in patients with arteritis, including giant cell arteritis (GCA), aortic arch syndrome, Takayasu arteritis, carotid artery dissection, hyperhomocysteinemia, radiotherapy of the neck with carotid occlusion, thyroid orbitopathy, and Moyamoya disease as well as neurofibromatosis and scleroderma.
Differential diagnosis
Diabetic retinopathy and Central Retinal Vein Occlusion (CRVO) are the two most likely conditions to be confused with OIS. A basic differentiating feature is the low retinal artery pressure in eyes with OIS. Table 2 lists the clinical and angiographic signs that help to differentiate these three. [20]
The differential diagnosis of OIS should also include the hyperviscosity syndromes. Fundus manifestations caused by serum or blood hyperviscosity include optic disk swelling, retinal capillary microaneurysms, cotton-wool spots, retinal hemorrhages, dilated retinal veins, and retinal venous occlusion. A basic workup should therefore include a complete blood cell count with differential, serum protein electrophoresis, and immunoelectrophoresis.
| Clinical Sign | Ocular Ischemic Syndrome | Central Retinal Vein Occlusion | Diabetic Retinopathy |
|---|---|---|---|
| Retinal Veins | Dilated but not tortuous | Dilated and tortuous | Dilated and beaded |
| Age | 50's to 80's | Variable | 50's to 80's |
| Hemorrhages (location) | dot and blot (mid-periphery) and in deeper retinal layers | Flame shaped (all quadrants) in nerve fiber layer | Dot and blot (posterior pole and mid-periphery) |
| Microaneurysms (Location) |
Common (mid-periphery) |
Uncommon | Common (Posterior pole) |
| Other Microvascular abnormalities | Macular telangiectasias, retina AV communications, capillary dropout | opto-ciliary shunts, capillary dropouts | intraretinal microvascular abnormalities, capillary dropout |
| Hard exudates | No | Rare | Common |
| Optic Disc | Normal | Edema (common) | Rarely diabetic papillopathy |
| Central retinal artery perfusion pressure | Decreased | Normal | Normal |
| Fluorescein Angiography | |||
| A-V transit time | Prolonged | Prolonged | Usually normal |
| Retinal Vessel Staining | Arteries>Veins | Veins>Arteries | Usually absent |
| Macular edema | Rare | Common | Common |
| Choroidal filling | Delayed, patchy | Normal | Usually normal |
| Microaneurysm | mid peripheral | usually at posterior pole | usually at posterior pole |
| Optic disc hyperfluorescence | may be present | Usually present | absent except papillopathy or new vessels at the disc or optic neuropathy |
Management of Ocular Ischemic Syndrome
The management of OIS involves a multidisciplinary approach. The aim is threefold, firstly to treat the ocular complications and prevent further damage, secondly to investigate and treat the associated vascular risk factors, and thirdly to perform vascular surgery whenever indicated.
Ocular treatment
The ocular treatment is directed toward control of anterior segment inflammation, retinal ischemia, increased IOP, and neovascular glaucoma. Topical therapy includes steroids to suppress anterior segment inflammation and cycloplegics to stabilize the blood–aqueous barrier and limit iris movement in order to decrease the likelihood of a spontaneous hyphema. Medical treatment of increased IOP consists of ocular hypotensive agents that reduce aqueous outflow (topical β-adrenergic blockers, or α-agonists that also increase uveoscleral flow), along with topical and/or oral carbonic anhydrase inhibitors. Prostaglandins, pilocarpine and other anticholinergic agents, should be avoided whenever possible because they may increase ocular inflammation.
When neovascular glaucoma develops, IOP control is usually refractory to medical therapy and surgery (trabeculectomy with antimetabolites or aqueous shunt implants) or diode laser cyclophotocoagulation is often required. In patients with no useful vision and in pain who are thought to have limited potential for visual recovery, cycloablation is a viable option. If the eye remains painful, retrobulbar injection of alcohol or chlorpromazine may provide relief. If these fail to alleviate pain in a blind eye, enucleation or evisceration should be considered. Panretinal photocoagulation may be effective in same patients with ocular neovascularization caused by carotid occlusive disease and may prevent development of neovascular glaucoma. In eyes with poor fundus visualization, 360° transconjunctival cryotherapy or transscleral diode laser retinopexy may be considered.[31][32]
Intravitreal anti vascular endothelial growth factor (bevacizumab and ranibizumab) and triamcinolone have been used in the treatment of iris neovascularization and cystoid macular edema complicating OIS.
Systemic Medical treatment
OIS patients should be referred to a primary care physician as well as a neurologist for full medical and neurological assessment. It is essential to treat associated systemic diseases. In addition lifestyle modifications, cessation of smoking and weight reduction need to be recommended.
Surgical Treatment
Carotid Artery Endarterectomy
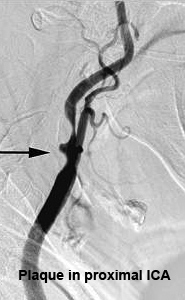
The landmark North American Symptomatic Carotid Endarterectomy (NASCET) trial demonstrated the superiority of carotid endarterectomy (CEA) and aspirin therapy in preventing stroke compared with aspirin therapy alone for both symptomatic and asymptomatic carotid artery stenosis. [33][34] Based on these trials, the American Academy of Neurology and the American Heart Association/American Stroke Association recommend CEA for symptomatic stenosis of 50–99% if the perioperative risk of stroke or death is <6%. [35] In asymptomatic patients, CEA is recommended for a stenosis of 60–99% if the perioperative risk of stroke or death is <3%. (Figure 14)
CEA has been shown to be effective in reversing or preventing the progression of chronic ocular ischemia or in increasing ocular blood flow. [36][37] [38] Peak systolic velocity of flow in the ophthalmic artery rises after surgery [39] and any reversal of ophthalmic artery flow is corrected.[40] Carotid artery surgery therefore can reduce ocular ischemia and improve hypotensive retinopathy as well as reduce the risk of stroke. However, it is important to note that the presence of iris neovascularization implies a greater degree of ocular ischemia and damage, making reversal of ischemia and visual recovery unlikely and limiting any beneficial effect of CEA on visual acuity.
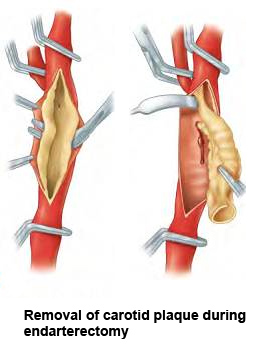
Figure 14. Removal of carotid artery plaque during endarterectomy.
Carotid Artery Stenting
Endovascular carotid artery stenting (CAS) is a treatment alternative in patients who need CEA. CAS has been used for patients who are considered to be at high-risk for complications after CEA including those with anatomic conditions rendering surgery technically difficult, such as previous neck irradiation or radical neck surgery, recurrent stenosis after CEA, contralateral recurrent laryngeal-nerve palsy, tracheostomy, and carotid stenosis above the C2 vertebral body. [41] Medical conditions that increase the risk of surgery, such as unstable angina, recent myocardial infarction, multivessel coronary disease, congestive heart failure, are also indications for CAS. Carotid artery stenting has been shown to improve the ocular blood flow in patients with acute and chronic forms of OIS. [42] (Figure 15)
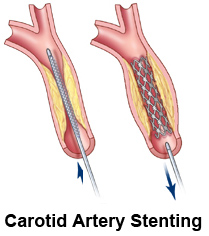
Figure 15. Carotid artery stenting.
Extracranial–Intracranial (EC-IC) Arterial Bypass Surgery
EC-IC bypass surgery involves the surgical anastomosis of the superficial temporal artery (STA) with a branch of the middle cerebral artery (MCA). It is indicated when there is complete occlusion of the ICA or the CCA or when ICA stenosis is inaccessible (at or above the C2 vertebral body) to CEA. [43] The aim is to increase cerebral blood flow and prevent the development of cerebral ischemia. CEA usually improves ocular hemodynamic parameters, but stabilization or improvement in vision seems to occur only when performed early before development of iris neovascularization or neovascular glaucoma. Carotid endarterectomy is also beneficial in preventing cerebral infarctions. A multidisciplinary approach is essential when considering surgery in patients with OIS factoring in the patients anatomic considerations and associated co-morbidities.
Prognosis
Although many patients present with relatively good vision, it has been shown that up to 50% of patients who present with OIS will have vision at count fingers (CF) or less within one year. Rubeosis iridis, also known as neovascularization of the iris (NVI), has been shown to be a poor prognostic indicator, with progression to CF or worse in 80% of those who presented with NVI or had NVI within three months of presentation. Treatment with panretinal photocoagulation (PRP) may have a positive effect with regards to reduction of neovascularization glaucoma. [10]
In a review of 25 patients with OIS, 17 patients (68%) subsequently developed neovascular glaucoma (NVG). In this study, development of NVG was significantly associated with IOP at time of diagnosis, length of time between symptom onset and diagnosis, and extent of ipsilateral carotid artery stenosis. Development of NVG was also shown to be associated with multiple pre-existing conditions, including hypertension, diabetes, and dyslipidemia. However, there was no significant difference in follow-up best-corrected visual acuity (BCVA) between NVG and non-NVG groups, with both groups yielding a poor prognosis. [44]
Interestingly, the duration of macular ischemia may be a better prognostic indicator than the initial visual acuity. [45]
Ultimately, the overall mortality rate for patients with OIS is 40% at 5 years, with the leading cause of death being cardiovascular disease, usually myocardial infarction (67%), followed by cerebral infarction (19%). [10] It is therefore essential that physicians adopt appropriate therapeutic options aiming at primary prevention of myocardial and cerebral infarction.
References
- ↑ Gordon N. Ocular manifestations of internal carotid artery occlusion. The British journal of ophthalmology. May 1959;43(5):257-267.
- ↑ Hedges TR, Jr. Ophthalmoscopic findings in internal carotid artery occlusion. American journal of ophthalmology. May 1963;55:1007-1012.
- ↑ Bullock JD, Falter RT, Downing JE, Snyder HE. Ischemic ophthalmia secondary to an ophthalmic artery occlusion. American journal of ophthalmology. Sep 1972;74(3):486-493.
- ↑ Biousse V. Carotid disease and the eye. Current opinion in ophthalmology. Dec 1997;8(6):16-26.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. International ophthalmology. Feb 1988;11(4):239-251.
- ↑ 6.0 6.1 6.2 Kearns TP, Siekert RG, Sundt TM, Jr. The ocular aspects of bypass surgery of the carotid artery. Mayo Clinic proceedings. Mayo Clinic. Jan 1979;54(1):3-11.
- ↑ Kearns TP, Siekert RG, Sundt TM. The ocular aspects of carotid artery bypass surgery. Transactions of the American Ophthalmological Society. 1978;76:247-265.
- ↑ Brown GC. Macular edema in association with severe carotid artery obstruction. American journal of ophthalmology. Oct 15 1986;102(4):442-448.
- ↑ Imrie FR, Hammer HM, Jay JL. Bilateral ocular ischaemic syndrome in association with hyperhomocysteinaemia. Eye. Jul 2002;16(4):497-500.
- ↑ 10.0 10.1 10.2 10.3 Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. International ophthalmology. Jan 1991;15(1):15-20.
- ↑ 11.0 11.1 11.2 11.3 Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. May 1997;104(5):859-864.
- ↑ Sturrock GD, Mueller HR. Chronic ocular ischaemia. The British journal of ophthalmology. Oct 1984;68(10):716-723.
- ↑ Klijn CJ, Kappelle LJ, van Schooneveld MJ, et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke; a journal of cerebral circulation. Mar 2002;33(3):695-701.
- ↑ Ros MA, Magargal LE, Hedges TR, Jr., Simeone FA. Ocular ischemic syndrome: long-term ocular complications. Annals of ophthalmology. Jul 1987;19(7):270-272.
- ↑ Kerty E, Eide N, Horven I. Ocular hemodynamic changes in patients with high-grade carotid occlusive disease and development of chronic ocular ischaemia. II. Clinical findings. Acta ophthalmologica Scandinavica. Feb 1995;73(1):72-76.
- ↑ 16.0 16.1 16.2 Costa VP, Kuzniec S, Molnar LJ, Cerri GG, Puech-Leao P, Carvalho CA. Collateral blood supply through the ophthalmic artery: a steal phenomenon analyzed by color Doppler imaging. Ophthalmology. Apr 1998;105(4):689-693.
- ↑ Choromokos EA, Raymond LA, Sacks JG. Recognition of carotid stenosis with bilateral simultaneous retinal fluorescein angiography. Ophthalmology. Oct 1982;89(10):1146-1148.
- ↑ Brown GC. Macular edema in association with severe carotid artery obstruction. American journal of ophthalmology. Oct 15 1986;102(4):442-448.
- ↑ Utsugi N, Takahashi K, Kishi S. Choroidal vascular occlusion in internal carotid artery obstruction. Retina. Dec 2004;24(6):915-919.
- ↑ 20.0 20.1 20.2 20.3 20.4 Mendrinos E, Machinis TG, Pournaras CJ. Ocular ischemic syndrome. Survey of ophthalmology. Jan-Feb 2010;55(1):2-34.
- ↑ Story JL, Held KS, Harrison JM, Cleland TP, Eubanks KD, Brown WE, Jr. The ocular ischemic syndrome in carotid artery occlusive disease: ophthalmic color Doppler flow velocity and electroretinographic changes following carotid artery reconstruction. Surgical neurology. Dec 1995;44(6):534-535.
- ↑ Decarlo LJ. Ophthalmodynamometry--a review. McGill medical journal. Oct 1961;30:134-140.
- ↑ Sisler HA. A review of ophthalmodynamometry. American journal of ophthalmology. Sep 1960;50:419-424.
- ↑ Nederkoorn PJ, van der Graaf Y, Hunink MG. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review. Stroke; a journal of cerebral circulation. May 2003;34(5):1324-1332.
- ↑ Yuan C, Zhao XQ, Hatsukami TS. Quantitative evaluation of carotid atherosclerotic plaques by magnetic resonance imaging. Current atherosclerosis reports. Sep 2002;4(5):351-357.
- ↑ Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke; a journal of cerebral circulation. Feb 1990;21(2):209-222.
- ↑ 27.0 27.1 Luo J, Yan Z, Jia Y, Luo R. Clinical analysis of 42 cases of ocular ischemic syndrome. J Ophthalmol. 2018;2018:2606147. Published 2018 Mar 11. doi:10.1155/2018/2606147
- ↑ Marx JL, Hreib K, Choi IS, Tivnan T, Wertz FD. Percutaneous carotid artery angioplasty and stenting for ocular ischemic syndrome. Ophthalmology. Dec 2004;111(12):[[1]].
- ↑ Foncea Beti N, Mateo I, Diaz La Calle V, Ruiz J, Gomez Beldarrain M, Garcia-Monco JC. The ocular ischemic syndrome. Clinical neurology and neurosurgery. Dec 2003;106(1):60-62.
- ↑ Sivalingam A, Brown GC, Magargal LE, Menduke H. The ocular ischemic syndrome. II. Mortality and systemic morbidity. International ophthalmology. May 1989;13(3):187-191.
- ↑ Flaxel CJ, Larkin GB, Broadway DB, et al.(1997) Peripheral transscleral retinal diode laser for rubeosis iridis. Retina 17:421–429.
- ↑ Bloom PA, Tsai JC, Sharma K, et al.(1997) Cyclodiode: trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 104:1508–1519.
- ↑ Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. The New England journal of medicine. Nov 12 1998;339(20):1415-1425.
- ↑ North American Symptomatic Carotid Endarterectomy Trial C. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. The New England journal of medicine. Aug 15 1991;325(7):445-453.
- ↑ Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. Jun 20 2006;113(24):e873-923.
- ↑ Cohn EJ, Jr., Sandager GP, Benjamin ME, Lilly MP, Hanna DJ, Flinn WR. Assessment of ocular perfusion after carotid endarterectomy with color-flow duplex scanning. Journal of vascular surgery. Apr 1999;29(4):665-671.
- ↑ Ishikawa K, Kimura I, Shinoda K, et al. In situ confirmation of retinal blood flow improvement after carotid endarterectomy in a patient with ocular ischemic syndrome. American journal of ophthalmology. Aug 2002;134(2):295-297.
- ↑ Neupert JR, Brubaker RF, Kearns TP, et al.(1976) Rapid resolution of venous stasis retinopathy after carotid endarterectomy. Am J Ophthalmol 81:600–602.
- ↑ Wong YM, Clark JB, Faris IB, et al.(1998) The effects of carotid endarterectomy on ocular haemodynamics. Eye 12:367–373.
- ↑ Costa VP, Kuzniec S, Molnar LJ, et al.(1999) The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. Ophthalmology 106:306–310
- ↑ Narins CR, Illig KA. Patient selection for carotid stenting versus endarterectomy: a systematic review. Journal of vascular surgery. Sep 2006;44(3):661-672.
- ↑ Neroev VV, Kiseleva TN, Vlasov SK, Pak NV, Gavrilenko AV, Kuklin AV. Visual outcomes after carotid reconstructive surgery for ocular ischemia. Eye. Oct 2012;26(10):1281-1287.
- ↑ Sundt TM, Jr., Siekert RG, Piepgras DG, Sharbrough FW, Houser OW. Bypass surgery for vascular disease of the carotid system. Mayo Clinic proceedings. Mayo Clinic. Nov 1976;51(11):677-692.
- ↑ Kim YH, Sung MS, Park SW. Clinical features of ocular ischemic syndrome and risk factors for neovascular glaucoma. Korean J Ophthalmol. 2017;31(4):343-350. doi:10.3341/kjo.2016.0067
- ↑ Haouas M, Campolmi N, Chikh M, Guillemot C, Grivet D, Michel C, Rizzi P, Duprey A, Gain P, Thuret G. Évolution favorable d’un cas de syndrome d’ischémie oculaire chronique bilatéral traité en urgence par chirurgie de revascularisation carotidienne [Favorable outcome in a case of bilateral ocular ischemic syndrome treated by emergency carotid revascularization surgery]. Journal Français d'Ophtalmologie. 2015 Mar;38(3):e43-5. doi:10.1016/j.jfo.2014.04.029. Epub 2015 Jan 15.