Refractive Surgery Pearls in Topography
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Topography is derived from the Greek: “topos” (place) and “graphein” (to draw). Corneal topography relates to a representation of the geometrical properties of the corneal surface it is a technique for measuring and evaluating its shape and curvature. [1][2] The advances in refractive surgery techniques have created the necessity for precise analysis of the corneal surface and shape. The conventional keratometer only measures close estimations of the anterior corneal curvature but is insufficient for the assessment of patients for refractive surgery. In recent years, rapid advances in corneal topography and tomography have paralleled those of excimer laser refractive surgery, and computerized corneal topography has become the standard of care in clinical practice.[1]
History
In the seventeenth century, Christopher Scheiner compared pictures formed by the impression of marbles on the cornea. [3] In 1880, Placido created a disk, involving the reflection of a series of concentric highly contrasting rings.[4] In the late nineteenth century, Javal and Schoitz, created the keratometer. This device focuses on the 3 - 4mm central area of the cornea, and assumes that the rest of the cornea has a sphero-cylindrical surface. More recently, the photokeratoscopes have been developed to assess the rest of the corneal surface. [1]
Preoperative Assessment
A fundamental component of the refractive surgery evaluation is the preoperative assessment of the cornea with topography. Its main goal is to detect epithelial irregularities and stromal abnormalities, evaluate corneal astigmatism, refractive stability or undetected corneal diseases.[5] It is essential that the patient maintains an adequate position and fixation in order to ensure a high-quality image.[1]. This is does not imply non-utility of a manifest refraction, however, as a 2023 prospective cohort study of 64 eyes by Liu et al showed slightly inferior clinical results in primary topography-guided FS-LASIK using Pentacam-measured anterior corneal astigmatism axis compared to using the manifest refractive astigmatism axis[6]. Khamar et al also published in 2023 attempting to standardize a nomogram for use in cases in which the subjective (manifest refraction) and objective (topographic) cylinder differ by different amounts. They report success with distance vision and felt further refinements were needed for near vision outcomes[7].
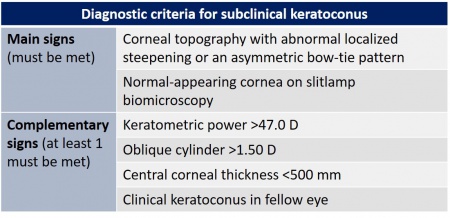
Laser refractive surgery is now widely used. In order to avoid ectasia secondary to the procedure, it is mandatory the detect subclinical keratoconus before surgery. There are several tools we can use to assess this disorder: corneal topography, pachymetry, and corneal aberrometry. Recent studies have reported tomographic measurements using optical coherence tomography and corneal biomechanical indices. Several factors can increase the risk of corneal ectasia after the procedure, such as age, keratometric indices, corneal elevation data, and corneal thickness.[8]
Incipient or subclinical keratoconus is usually asymptomatic and can be undetected in routine clinical practice. It is the most significant risk factor for the development of ectasia after laser refractive surgery.[9] To assess incipient keratoconus, it is necessary to use multiple parameters, algorithms and predictive models.[8]
An additional measurement that may be assessed is corneal densitometry. Some data has been published suggesting this may be a useful measure to quantify the development of corneal haze after PRK, though further research is needed to validate an approach to this[10].
As technology continues to advance, more and more data elude to the possibility of artificial intelligence playing a role in future imaging interpretation. Specifically, in late 2022, Ambrosio Jr et al published data suggesting that AI may be able to augment existing tomography and biomechanical assessments to improve ectasia detection[11]. Cohen et al also reported a machine learning algorithm that approached the accuracy of a human corneal specialist in the discrimination between normal, suspect irregular and keratoconic corneas in their tested population[12].
Topographic maps
Dioptric values in the topographic map are represented with colors: warmer colors illustrate steeper curvatures (higher D), and cooler colors exhibit flatter curvatures (lower D). Near the periphery, normal corneas tend to flatten, and it is represented with cooler colors. Both eyes tend to have mirror topographies (enantiomorphism). [13]
Topographic patterns
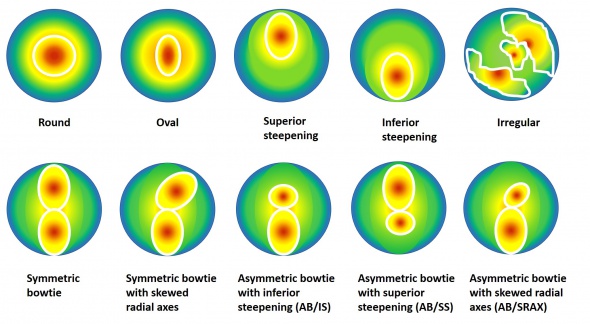
In 1996, Rabinowitz et al., created a database of videokeratography patterns and quantitative indices based on the normal corneas of 390 eyes. They proposed 10 different topographical patterns.[14] They are:
Studies have shown a good accuracy of the mean keratometry (K) in detecting keratoconus. The cutoff points between 45.2 D and 45.7 D have a sensitivity >80% and specificity >70%. This parameter is useful for differentiating subclinical keratoconus and normal eyes, [15][16][17] Astigmatism has been shown to be higher in keratoconus. The cutoff point is 2.5 D, it has an acceptable ability for the screening of keratoconus (sensitivity and specificity >75%). The specificity decreases in incipient keratoconus (<65%).[16]
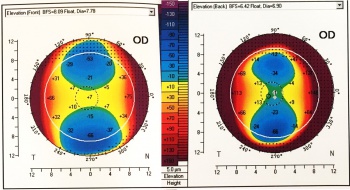
Elevation map
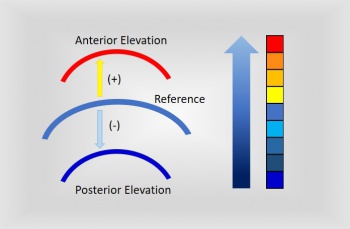
Elevation maps illustrate the variation in height within the measured corneal surface and some reference surface. The most common reference is a sphere, referred to as “best-fit sphere” (BFS). Other shapes include the "best-fit asphere (BFA) or best-fit toric asphere (BFTA)". The values lying above the reference surface are represented with warmer colors, while cooler colors indicate areas below it.[13] There are several patterns described for topography maps: “island, regular ridge, irregular ridge, incomplete ridge, and unclassified”.[18] Jafarinasab et al. studied the sensitivity and specificity of the anterior and posterior corneal elevation by Orbscan II topography (Bausch and Lomb, Rochester, NY, USA). They determined an optimal cutoff point for the posterior elevation of 51 µm for keratoconus and 35 µm for subclinical keratoconus. While for the anterior elevation the optimal cutoff points were 19 µm for keratoconus and 16 µm for subclinical keratoconus.[19] In Pentacam Scheimpflug corneal tomography, the posterior elevation: normal ≤+17 µm, suspicious +18 µm - + 20 µm and risky >+20 µm. In the anterior map: normal ≤+12 µm, suspicious +13 µm + 15 µm and risky >+15 µm.[20] Recently, the posterior elevation has been determined as an excellent predictor of keratoconus. Most of the studies have reported a sensitivity and specificity of >90%.[15][17] When detecting incipient keratoconus, there is significant variability. De Sanctis et al. detected a sensitivity of 73.3% and specificity of 86.5% for posterior elevation in subclinical keratoconus.[21] Jafarinasab et al. obtained a lower sensitivity of 50.00% and specificity 88.65%.[19] While posterior corneal elevation may be a useful metric in the evaluation of keratoconus, Wallerstein et al published data in Dec 2022 suggesting that patients with both low and high levels of posterior corneal astigmatism had statistically similar outcomes when topography-guided LASIK patients were treated targeting the manifest refraction[22].
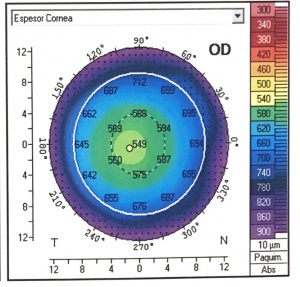
Pachymetric map
The distribution of corneal thicknesses is represented in pachymetric maps. It provides a global map of corneal thickness which eliminates errors due to probe decentration with the manual ultrasound. An accurate measurement is essential before refractive surgery, as it is an important tool in the diagnosis and progression of corneal ectasias.[13] It can be determined by the rotating Scheimpflug camera (Pentacam) and the Scheimpflug camera combined with Placido corneal topography (Sirius). These systems measure the corneal thickness at different points of the cornea.[8]
The pachymetric map can useful in detecting incipient keratoconus. The diagnostic criteria are a central corneal thickness (CCT) less than 500 mm, in addition to topographic asymmetry. Also, a study has shown a high probability (97.5%) of corneal ectasia when there is a difference between the central and minimum thickness of more than 27 mm.[23]
Although the pachymetric parameters can accurately detect keratoconus, there is variability in the cutoff points between different studies according to the sample or the device used. Therefore, we should not use this parameter alone to detect keratoconus. When detecting subclinical keratoconus, sensitivities, and specificities of less than 70% have been reported in all studies.[8]
Pachymetric measurements assessed with OCT are similar to those obtained with Scheimpflug systems.[24]Optical coherence tomography can determine the difference between the thickness of the upper and lower area of the cornea. Also, it shows the vertical location of the thinnest point of the cornea. Studies have shown a high precision in detecting keratoconus.[8]
Reinstein et al. determined the total corneal thickness, as well as the epithelial and stromal thickness in patients with keratoconus. They found that there was a localized central thinning surrounded by an annulus of thick epithelium (epithelial doughnut pattern). The position of the thinnest point in the epithelium was displaced 0.48 ± 66 mm temporally and 0.32 ± 0.67 mm, and by 0.31 ± 0.45 mm and 0.45 ± 0.37 mm inferiorly in the stroma.[25]
Schallhorn et al., distinguished between corneal ectasia and contact lens-related warpage by OCT epithelial thickness and corneal topography maps. The keratoconus and forme fruste keratoconus eyes had coincident topographic steepening with epithelial thinning. Also, the locations of maximum axial power agreed with the thinnest epithelial value in 90% of the cases. On the other hand, the warpage eyes had topographic steepening with epithelial thickening and normal pachymetry. The maximum mean power agreed with the maximum epithelial thickness (93%). Topographic maps and epithelial thickness assess with optical coherence tomography are synergistic tools to differentiate both entities.[26]
Indices
The next indices are used to distinguish normal corneas from those with a pathology (keratoconus or forme fruste keratoconus) and are based on the pachymetry, anterior corneal curvature, and elevation patterns.[13]
Keratoconus percentage index (KISA%) value
The keratoconus percentage index (KISA%) value is obtained from 3 videokeratographic parameters:
- Central keratometric power
- I–S index
- Corneal simulated astigmatism
- SRAX index
The SRAX index has an accuracy of 99.6% in detecting keratoconus. The KISA % index varies from 60% to 100% in subclinical keratoconus.[27]
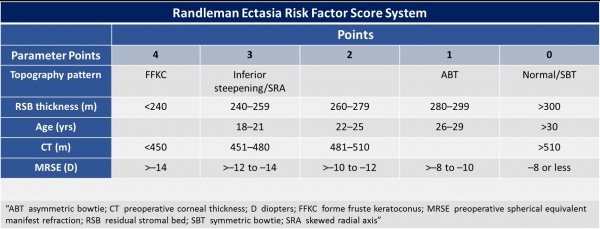
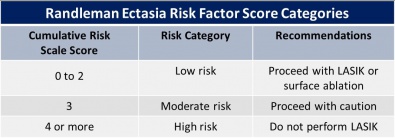
Randleman Ectasia Risk Score System
In 2008, Randleman et al. studied the epidemiologic features of ectasia after refractive surgery. There were included 171 ectasia cases. Of these cases, 164 were LASIK and the remaining PRK. They evaluated the preoperative characteristics, including: “age, gender, spherical equivalent refraction, pachymetry, and topographic patterns, type of surgery performed, flap thickness, ablation depth, and residual stromal bed (RSB) thickness”; and postoperative features: time elapsed since the appearance of ectasia. They created a “risk factor stratification scale”, considering each characteristic. This is an important method to assess risk of ectasia after LASIK.[28]
PTA Index
Santhiago et al., investigated the association between the percent tissue altered (PTA) and the development of ectasia in patients with normal topography, who underwent LASIK surgery. They included 30 eyes with normal topography that developed ectasia and another group of 174 eyes without this complication.
The equation for PTA is: PTA = (FT + AD) / CCT
(PTA) Percent Of Tissue Altered
(FT) Flap Thickness
(AD) Ablation Depth
(CCT) Preoperative Central Corneal Thickness
They concluded that PTA values below 40.0% correlate with the low incidence of ectasia. This formula is a precise method for assessing ectasia risk, and it is more sensitive than the individual components that comprise it.[29]
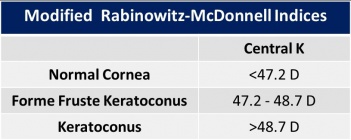
Index Rabinowitz-McDonnell
They described a new index for the assessment of keratoconus. Three criteria were considered:
- “Central K (central steepening)”: is used to distinguish central cones.
- “I-S values (reflecting inferior-superior dioptric asymmetry)”: Is the refractive power difference between the five inferior points and the superior points. It is used to detect inferior corneal steepening.
- “SRAX (relative skewing of the steepest radial axes above and below the horizontal meridian)”.
They found, that a central K greater than 47.20 D, I-S higher than 1.2 and SRAX index above 21°, identified 98% of the patients with keratoconus.[30]
Belin Ambrosio Enhanced Ectasia Display
This is a comprehensive refractive surgical screening tool developed by Belin and Ambrosio. It combines elevation and pachymetry based corneal evaluation in an all inclusive display and allows for quick and effective screening of ectatic disease.
The criteria used for identification of ectasia are standard deviation of: 1. Mean pachymetric progression (dp); 2. mean changes in back elevation (db); 3. Mean changes in front elevation (df); 4. Mean thinnest point thickness (dt); and 5. Mean thinnest point displacement (dy). The final "D" score is based on the previous mentioned 5 parameters . For each criteria, <1.6 is considered normal and colored white; between 1.6 and 2.6 is considered suspicious for keratoconus and colored yellow and if >2.6, indicates development of keratoconus and is colored red.[31]
Epithelial Thickness Maps
Epithelial thickness mapping with optical coherence tomography (OCT) is becoming more widely used for evaluation and planning for refractive surgery. When there is a suspicious steepening pattern on topography or tomography alone, patients may be deemed poor candidates for refractive surgery due to risk of ectasia. Corneal steepening in cases of ectatic disease are often accompanied by epithelial thinning. When evaluating a patient with suspicion for ectatic disease, if the epithelium is thin, then this may confirm steepening is likely secondary to ectasia. If the epithelium is thick, then this would suggest nonpathologic causes.[32][33] [34]Reports have used this method to confirm thicker epithelium over an area of localized thinning to exclude keratoconus and perform refractive surgery.[35][36] This additional screening may provide more insight into surgical planning, but more studies are needed to further establish its utility in refractive surgery.
Conclusions
Corneal topography is an essential tool to perform the preoperative assessment in refractive surgery.[5] It is important to diagnose a variety of corneal abnormalities that can lead to poor outcomes with LASIK, such as keratoconus, forme fruste keratoconus, and pellucid marginal degeneration.[37] Also, we need to determine the presence of astigmatism and refractive stability. It is especially relevant to screen patients carefully and to diagnose those who have an elevated risk for ectasia. Some artifacts can be confused with keratoconus: "Displaced apex syndrome, CL-induced warpage, a prominent tear meniscus, misalignment when obtaining the topography, dry eye disease with poor precorneal tear film and accidental external pressure on the globe".[5]
In LASIK, PRK, SMILE and LASEK, it is relevant to assess the refractive error and corneal topography and determine whether it will lead to an abnormally steep or flat corneal curvature. The risk for buttonholes is bigger for steep corneas, whereas the risk for free caps is higher for flatter corneas. These complications are reported mainly with the use of mechanical microkeratomes; on the other hand, femtosecond LASIK flaps do not seem to have these complications.[37] Postoperative corneal topography is helpful in determining the ablation bed, the quality of the surgery and the uniformity of laser. The study should be performed at least one week after surgery. It is also useful for detecting ectasia and evaluating progression. "Corneal topography-guided custom ablation patterns" is a helpful technique to prevent undesirable postoperative results.[5]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Gatinel D. Corneal Topography and Wave Front Analysis. In: Albert & Jakobiec's Principles & Practice of Ophthalmology, 3rd ed. Philadelphia, PA: Saunders/Elsevier; 2008:921-963. Retrieved from https://www.clinicalkey.com/#!/content/book/3-s2.0-B9781416000167500734 , accessed on 08.01.2017
- ↑ Taravella M, Davidson RS. Corneal Topography and Wavefront Imaging. In: Ophthalmology, 4th ed. Philadelphia, PA: Saunders/Elsevier; 2014:168-173. Retrieved from https://www.clinicalkey.com/service/content/pdf/watermarked/3-s2.0-B9781455739844001111.pdf?locale=en_US, accessed on 08.01.2017
- ↑ Naroo SA, Cervino A. Corneal topography and its role in refractive surgery. In: Refractive surgery: a guide to assessment and management. London, UK: Elsevier; 2004:9–16.
- ↑ Placido A. Novo instrumento per analyse immediate das irregularitades de curvatura da cornea. Periodico Oftalmol Practica. 1880; 6:44–49.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 Yeu E, Belin MW, Khachikian SS. Topographic Analysis in Keratorefractive Surgery. In: Cornea, 4th ed. Holland: Elsevier; 2017:1728-1735.
- ↑ Liu C, Luo T, Fang X, Hu M, Su Y, Li J, Wang Y. Clinical results of topography-guided laser-assisted in situ keratomileusis using the anterior corneal astigmatism axis and manifest refractive astigmatism axis. Graefes Arch Clin Exp Ophthalmol. 2023 Jan;261(1):247-256. doi: 10.1007/s00417-022-05775-7. Epub 2022 Jul 27. PMID: 35895108.
- ↑ Khamar P, Shetty R, Annavajjhala S, Narasimhan R, Kumari S, Sathe P, Roy AS. Impact of crossplay between ocular aberrations and depth of focus in topo-guided laser-assisted in situ keratomileusis outcomes. Indian J Ophthalmol. 2023 Feb;71(2):467-475. doi: 10.4103/ijo.IJO_191_22. PMID: 36727342.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 Martínez-Abad A. and Piñero DP. New perspectives on the detection and progression of keratoconus. Journal of Cataract & Refractive Surgery. 2017: 43(9):1213-1227.
- ↑ Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003; 110:267–275.
- ↑ Balparda, Kepa; Mesa-Mesa, Sara1; Maya-Naranjo, Maria Isabel1; Mora-Sánchez, Carolina2; Escobar-Giraldo, Mariana1,3. Determination of the repeatability of corneal densitometry as measured with a Scheimpflug camera device in refractive surgery candidates. Indian Journal of Ophthalmology 71(1):p 63-68, January 2023. | DOI: 10.4103/ijo.IJO_1121_22
- ↑ Ambrósio R Jr, Machado AP, Leão E, Lyra JMG, Salomão MQ, Esporcatte LGP, Filho JBRDF, Ferreira-Meneses E, Sena NB Jr, Haddad JS, Neto AC, Castelo de Almeida G Jr, Roberts CJ, Elsheikh A, Vinciguerra R, Vinciguerra P, Bühren J, Kohnen T, Kezirian GM, Hafezi F, Hafezi NL, Torres-Netto EA, Lu N, Kang DSY, Kermani O, Koh S, Padmanabhan P, Taneri S, Trattler W, Gualdi L, Salgado-Borges J, Faria-Correia F, Flockerzi E, Seitz B, Jhanji V, Chan TCY, Baptista PM, Reinstein DZ, Archer TJ, Rocha KM, Waring GO 4th, Krueger RR, Dupps WJ, Khoramnia R, Hashemi H, Asgari S, Momeni-Moghaddam H, Zarei-Ghanavati S, Shetty R, Khamar P, Belin MW, Lopes B; International Corneal and Ocular and Biomechanics Study Group. Optimized artificial intelligence for enhanced ectasia detection using Scheimpflug-based corneal tomography and biomechanical data. Am J Ophthalmol. 2022 Dec 19:S0002-9394(22)00506-2. doi: 10.1016/j.ajo.2022.12.016. Epub ahead of print. PMID: 36549584.
- ↑ Cohen E, Bank D, Sorkin N, Giryes R, Varssano D. Use of machine learning to achieve keratoconus detection skills of a corneal expert. Int Ophthalmol. 2022 Dec;42(12):3837-3847. doi: 10.1007/s10792-022-02404-4. Epub 2022 Aug 11. PMID: 35953576.
- ↑ Jump up to: 13.0 13.1 13.2 13.3 Kim E, Weikert MP, Martinez CE, Klyce SD. Keratometry and Topography. In: Cornea, 4th ed. Holland: Elsevier; 2017.144-153.
- ↑ Rabinowitz, Yaron S., et al. Videokeratography database of normal human corneas. British journal of ophthalmology.1996; 80(7): 610-616.
- ↑ Jump up to: 15.0 15.1 Reddy JC, Rapuano CJ, Cater JR, Suri K, Nagra PK, Hammersmith KM. Comparative evaluation of dual Scheimpflug imaging parameters in keratoconus, early keratoconus, and normal eyes. J Cataract Refract Surg. 2014; 40:582–592.
- ↑ Jump up to: 16.0 16.1 Toprak I, Yaylalı V, Yildirim C. A combination of topographic and pachymetric parameters in keratoconus diagnosis. Cont Lens Anterior Eye. 2015; 38:357–362.
- ↑ Jump up to: 17.0 17.1 Muftuoglu O, Ayar O, Ozulken K, Ozyol E, Akıncı A. Posterior corneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. 2013; 39:1348–1357.
- ↑ Gatinel, D., Malet, J., Hoang-Xuan, T., & Azar, D. T. Corneal elevation topography: best fit sphere, elevation distance, asphericity, toricity and clinical implications. Cornea. 2011; 30(5), 508.
- ↑ Jump up to: 19.0 19.1 Jafarinasab MR, Shirzadeh E, Feizi S, Karimian F, Akaberi A, Hasanpour H. Sensitivity and specificity of posterior and anterior corneal elevation measured by Orbscan in diagnosis of clinical and subclinical keratoconus. J Ophthalmic Vis Res. 2015; 10:10–15. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4424711/pdf/JOVR-10-10.pdf. Accessed August 7, 2018
- ↑ Tanuj D, Neha M, Tarun A. New Investigations in Ophthalmology, 2nd ed. 2017;.3:30.
- ↑ de Sanctis U, Aragno V, Dalmasso P, Brusasco L, Grignolo F. Diagnosis of subclinical keratoconus using posterior elevation measured with 2 different methods. Cornea. 2013; 32:911–915.
- ↑ Wallerstein, Avi, et al. “Posterior Corneal Astigmatism Does Not Influence Manifest-Treated Topography-Guided LASIK Outcomes.” Journal of Refractive Surgery, vol. 38, no. 12, 2022, pp. 780–790., https://doi.org/10.3928/1081597x-20221108-01.
- ↑ Prakash G, Agarwal A, Mazhari AI, Kumar G, Desai P, Kumar DA, Jacob S, Agarwal A. A new, pachymetry-based approach for diagnostic cutoffs for normal, suspect and keratoconic cornea. Eye. 2012; 26:650–657. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3351046/pdf/eye2011365a.pdf. Accessed August 7, 2018
- ↑ Kanellopoulos AJ, Asimellis G. OCT-derived comparison of corneal thickness distribution and asymmetry differences between normal and keratoconic eyes. Cornea. 2014; 33:1274–1281.
- ↑ Reinstein DZ, Gobbe M, Archer TJ, Silverman RH, Coleman DJ. Epithelial, stromal, and total corneal thickness in keratoconus: three-dimensional display with Artemis very-high frequency digital ultrasound. J Refract Surg. 2010; 26:259–271. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles /PMC3655809/pdf/nihms211821.pdf. August 7, 2018
- ↑ Schallhorn JM., et al. Distinguishing between contact lens warpage and ectasia: Usefulness of optical coherence tomography epithelial thickness mapping. Journal of Cataract & Refractive Surgery. 2017; 43(1):60-66.
- ↑ Smadja D. Topographic and tomographic indices for detecting keratoconus and subclinical keratoconus: a systematic review. Int J Keratoconus Ectatic Corneal Dis. 2013; 2:60–64. Available at: http://www.jaypeejournals.com/eJournals/ShowText.aspx?IDZ5069&TypeZFREE&TYPZTOP&IN Z_eJournals/images/JPLOGO.gif&IIDZ391&isPDFZYES. Accessed August 7, 2018.
- ↑ Randleman JB, Woodward M, Lynn MJ, et al. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008; 115:37–50.
- ↑ Santhiago MR, Smadja D, Gomes BF, Mello GR, Monteiro ML, Wilson SE, & Randleman JB. Association Between the Percent Tissue Altered and Post–Laser In Situ Keratomileusis Ectasia in Eyes With Normal Preoperative Topography. American journal of ophthalmology. 2014; 158(1), 87-95.
- ↑ Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. Journal of Refractive Surgery. 1995; 11(5), 371-406.
- ↑ https://www.aao.org/image/belin-ambrosio-enhanced-ectasia-display
- ↑ Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg. 2013 Mar;29(3):173-9. doi: 10.3928/1081597X-20130129-08. Erratum in: J Refract Surg. 2013 Apr;29(4):234. Perez-Straziota, E [corrected to Perez-Straziota, Claudia E]. PMID: 23446013; PMCID: PMC4123636.
- ↑ Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009 Jul;25(7):604-10. doi: 10.3928/1081597X-20090610-06. PMID: 19662917.
- ↑ Reinstein DZ, Archer TJ, Vida RS. Epithelial thickness mapping for corneal refractive surgery. Curr Opin Ophthalmol. 2022 Jul 1;33(4):258-268. doi: 10.1097/ICU.0000000000000867. PMID: 35779050.
- ↑ Reinstein DZ, Archer TJ, Gobbe M. Stability of LASIK in topographically suspect keratoconus confirmed non-keratoconic by Artemis VHF digital ultrasound epithelial thickness mapping: 1-year follow-up. J Refract Surg. 2009 Jul;25(7):569-77. doi: 10.3928/1081597X-20090610-02. PMID: 19662913.
- ↑ Asroui L, Dupps WJ Jr, Randleman JB. Determining the Utility of Epithelial Thickness Mapping in Refractive Surgery Evaluations. Am J Ophthalmol. 2022 Aug;240:125-134. doi: 10.1016/j.ajo.2022.02.021. Epub 2022 Mar 3. PMID: 35247335.
- ↑ Jump up to: 37.0 37.1 Salz JJ, Trattler W. Patient Evaluation and Selection in Refractive Surgery. In: Cornea, 4th ed. Holland: Elsevier; 2017. 1719-1727.