Posterior Polar Cataract
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Posterior Polar cataracts (PPC) represent a medically and surgically unique subset of cataracts. Incidence is not well characterized though they are proportionally less common than most other forms of cataract. Multiple genes have been implicated with autosomal dominance inheritance patterns[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] as well as observed spontaneous development. Loci and genes associated with these cataracts have also been implicated in global eye conditions such as anterior segment mesenchymal dysgenesis and persistent hyperplastic primary vitreous. [11] [12] PPCs often arise at the end of a hyaloid artery remnant, which can result in a range of pathology from the benign "Mittendorf dot" to a more clinically relevant cataract. Cataracts form early in life, but may become more clinically significant over time. Bilateral eye involvement is reported in 65-80% of cases. [13] [14] [15] Children at risk for possible secondary amblyopia require specific attention to multi-modal therapies that are needed. [16] [17]
Clinical Classification, Characteristics and Pre-operative assessment
Different clinical classification schemes have been proposed in the literature. Phenotypic appearance and clinical course are among the variables included. Stationary PPCs are reported have better visual acuity with anatomic features distinguished by a central opacity on the posterior capsule surrounded by rings, thus creating the appearance of a bull’s eye. Progressive PPCs are reported to manifest with enlarging radiations with increasing symptoms over time. [18] [19] Other classification schemes grade PPC s based on their clinical appearance and the presence or absence of associated posterior sub-capsular (PSC) or nuclear sclerotic (NSC) lens changes (Table 1). [15]
Table 1: Sample phenotypic classification schemes for posterior polar cataracts
| Phenotype and Clinical Course | Stationary | Progressive |
|---|---|---|
| Author (Vasavada [18][19]) | Central opacity with bull's eye ring appearance | Central opacity with enlarging radiations over time |
| Phenotype based on Clinical Appearance | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|
| Author (Lee[15]) | Opacity associated with posterior sub-capsular cataract | Opacity with ringed appearance like an onion | Opacity with dense white spots at the edge often associated with thin or absent posterior capsule | Combinations of grade I, II, III, IV with nuclear sclerosing cataract |
Daljit Singh classification of posterior polar cataract:
- Type 1: PPC associated with posterior subcapsular cataract.
- Type 2: Round or oval discoid opacity with a ringed appearance like an onion with or without grayish spots at the edge.
- Type 3: Round or oval discoid opacity sharply defined with dense white spots at the edge often associated with weak, thin, or absent posterior capsule. The white dense spots are a diagnostic sign (Daljit Singh sign) of posterior capsule rupture or extreme thinning.
- Type 4: Combination of the above 3 types with nuclear sclerotic cataract
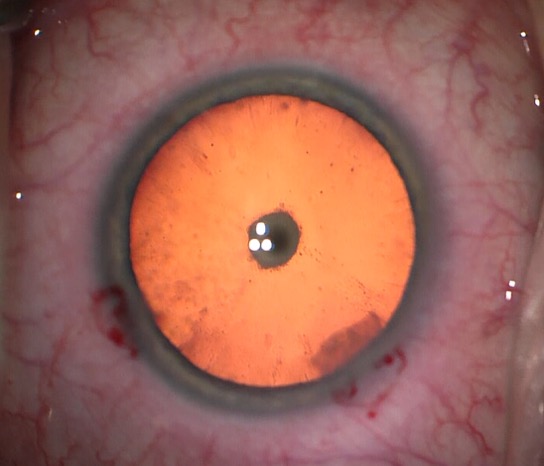
Adult patients with PPCs may present with symptoms of glare and haloes despite “normal” Snellen visual acuity. Because of their central location, even small posterior polar cataracts may be visually significant with worse symptoms under bright light or miotic conditions (Figure 1). Standard history, refraction and comprehensive examination should be performed. Screening for amblyopia will also assist with prognosis. Adjunct subjective testing may include brightness acuity testing and other variable lighting conditions to further draw out the nature of symptoms.
Figure 1: Posterior polar cataract as seen during cataract surgery
Biometric testing including biometry or ultrasound are standard for implant calculation purposes. Adjunct anterior segment imaging techniques such as optical coherence tomography (OCT), Scheimpflug imaging [20] or ultrasound as these may help to assess the potential adherence of cataract to the posterior capsule. [21] Pujari et al in their first study described the preoperative assessment of posterior capsular integrity using a posterior segment OCT with a +20 D lens, which helps to suggest a capsular deficiency in posterior polar cataracts. An intact posterior capsule shows a regularly convex contour, whereas a loss in the tracing of posterior capsule at the paracentral region and disturbance in contour with a localized protrusion of lens matter (the conical sign) depicts possible posterior capsule dehiscence and thereby aid in predicting a pre-existing deficit.[22]
In-depth patient counseling should follow accordingly. When present with other developmental pathologies, surgery can present an array of difficult challenges.
Clinical Significance
Aside from the significant visual distortion and disturbances resulting in glare and halos as described above, there are also important surgical considerations of PPCs. Because of possible adherence of PPCs to the posterior capsule, cataract extraction carries the risk of posterior capsular breaks and the potential for vitreous loss during surgery. Case reports of spontaneous rupture of the capsule have been reported as well. [23] [24] Posterior capsule rupture (PCR) has been the subject of numerous reports with its own secondary consequences and may be as high as 36 % of cases in some literature reports. [17][25] [26] [27] [28]
Surgical Technique for Treatment of Posterior Polar Cataracts
Surgical techniques [29] [30] [31] [32] [33] [34] [35] [36] [37] are driven by the characteristics of the associated nucleus density and the presence or absence of fusion of the PPC to the posterior capsule. For even rarer conditions of spontaneous rupture of the posterior capsule or associated dysgenesis, multiple sub-specialty surgery or additional adjunct techniques will be necessary. [38] [39]
Anesthesia
Anesthesia decisions should be individualized to the patient’s overall health and need. Wound construction is driven by the preferred approach and potential intraocular lens implants (IOL) that might be utilized once the cataract is extracted. There is a range of techniques reported from bimanual micro-incisional phaco [40] which carries the advantages of minimal induced corneal wound astigmatism and stable anterior chamber maintenance, to manual extracapsular cataract extraction (ECCE) for use with denser nuclei (higher rate of complications reported). [28]
Wound Construction
If considering conversion to manual ECCE, placement of the main keratome (or scleral wound) should take into consideration how easily the wound can be enlarged with minimal corneal endothelial damage. Straight clear corneal wounds may be easier to enlarge with less posterior cornea damage than long anterior multiplanar wounds. Scleral tunnels likely offer the most cornea endothelial protection and least astigmatic induction for large wounds.
Viscoelastic
Viscoelastic choice is driven by the potential of vitreous loss. Having a dispersive viscoelastic for primary use is advised and may serve multiple purposes (endothelial protection, covering posterior capsule rupture, as well as lens delineation). [41]
Capsulorhexis
Recommendations for anterior capsulorhexis size vary [42], likely in accordance with the need to counter certain lens material behaviours during extraction. Larger rhexis allows for easier access of lens disassembly at the trade-off of optic capture ability. In the event of posterior capsular rupture, a small anterior capsulorhexis may allow for better sulcus fixated IOL and less area for vitreous to prolapse. Overly small anterior capsulorhexis may inhibit smooth lens disassembly and may increase rithe sk of phimosis.
Hydrodissection and Hydrodelineation
Partial hydrodissection is possible and has been described when extracting PPCs[43] though is generally avoided with preference for hydrodelineation to avoid potential disruption of adhesions between the posterior capsule and PPC. This “inside-out” technique has been described in multiple citations. [18][19][27][44]
Phacodynamics
Intra-operative stability should be the goal of all cataract surgery and is germane to removal of PPCs. Minimizing intra-op wound leak, managing any concerns of posterior pressure and selecting appropriate phaco machine settings are essential to achieving stable intra-operative phacodynamics.
For PPCs associated with softer nuclei, lower energy parameters during each step are sufficient and may be accompanied with torsional cutting as available with newer generation platforms. Minimal rotation of lens material is advised to avoid extraneous capsule manipulation and stress. Lower than typical aspiration flow rates may be advised as rapidly flowing free fragments may not be the goal during removal.
Removal of as much lens material as possible prior to any direct manipulation of the posterior polar lens component is advantageous in the event of a possible posterior capsule rupture and potential retained lens fragments. Various alternative pre-chopping [45], as well as sculpting and chopping [46] maneuvers have been described to help delay posterior polar manipulation. [15] Nuclear material is removed first with subsequent anterior epi-nuclear and cortical material next.
When performing bimanual surgery on a soft lens with PPC, a blunt or rounded second hand instrument can be used to help “feed” anterior lens material into phaco needle or irrigation-aspiration (I/A) hand-piece. This decreases the need to “strip” capsule adjacent material under a tense fashion which may be connected to the PPC. Rapid, high vacuum assisted pulling is undesirable. Vacuum should be utilized judiciously with an intended balance of having anterior material drawn gently to the central anterior area where it can be removed while minimizing radiating posterior tension. Denser nuclei will have higher energy requirements with specific additional focus on minimizing lens "chatter” and high velocity fragments flowing in the anterior chamber.
After most of the lens material has been removed (leaving the PPC as the main remnant) judgement is made at this time whether to attempt to remove the material either 1) primarily with gentle removal with the I/A hand piece, or 2) with a vitrector or a primary posterior capsulotomy with attempt to minimize disruption of the anterior hyaloid face. Needle assisted posterior capsulotomy or vitrector assisted capsulotomy offer more control than the I/A hand piece for PPCs that are fused to the posterior capsule. Dispersive viscoelastic should be readily available at this phase as well if posterior capsular rupture occurs.
In the case of posterior capsular rupture limited anterior vitrectomy is performed and judgement is made on the appropriate location for the IOL. In posterior polar cataracts, the deficient/abnormal capsular morphology could be, pre-operatively identified using ASOCT and specifically categorized into 3 categories, conical, moth-eaten, and ectatic types as described by Pujari et al in their second study. In the conical variant, the posterior opacity–capsular junction does not show the usual convex contour, and there is a breach in the posterior capsule at the posterior pole, with the associated extension of opacity into the anterior vitreous to a varying extent. In the moth-eaten variant, the capsule shows the expected contour to the edge of the opacity, but beneath the opacity, it shows clearly deficient capsule. In addition, the posterior opacity shows a completely clear area within it resembling the morphology of the moth-eaten appearance of the edge of the leaves. In the ectatic type, the capsule appears intact with a densely adherent opacity; however, the capsule fails to show a regular contour. It possesses an uneven appearance with multiple ectatic bulges along the anterior vitreous.[47]
"Reverse flower bloom" technique for removal of posterior polar cataract. Key aspects of treatment are :
- -Removal of nuclear material first without disruption of posterior polar cataract face
- -Peeling of cortical material from outside in (similar to the reverse of a flower blooming) leaving central posterior polar component last
- -Removal of posterior polar cataract attachments to posterior capsule
Intraocular Lens Considerations
For uncomplicated cases or those with small posterior capsular ruptures that are round and central “in the bag” implantation may be possible with single piece acrylic lenses with careful observation of slow, guided unfolding into position. For any concerns of larger posterior capsular ruptures or radial expansions of capsular ruptures, a sulcus IOL with a 3-piece IOL may provide a better option. For total capsular instability, ACIOL or secondary sutured IOLs are needed.
Post-operative Management
Follow up is similar for routine post-surgical cataract cases when surgery is uncomplicated. For complicated cases with posterior capsular rupture, additional attention is required for pressure-related and associated vireo-retinal sequelae.
References
- ↑ Addison, P.K. et. al. Posterior polar cataract is the predominant consequence of a recurrent mutation in the PITX3 gene. Br J Ophthalmology. 89(2), pp138-141 (2005).
- ↑ Yamada, L. et. al. Genetically distinct autosomal dominant posterior polar cataract in a four-generation Japanese family. Am J Ophthalmol, 129 (2), pp. 159–165 (2000).
- ↑ Tulloh, C.G. Hereditary posterior polar cataract with report of a pedigree. Br J Ophthalmol, 39 (6), pp. 374–379 (1955).
- ↑ Ionides, A.C. et. al. A locus for autosomal dominant posterior polar cataract on chromosome 1p. Hum Mol Genet, 6 (1997), pp. 47–51.
- ↑ Bidinost, C. et. al. Heterozygous and homozygous mutations in PITX3 in a large Lebanese family with posterior polar cataracts and neurodevelopmental abnormalities. Invest Ophthalmol Vis Sci, 47 (4), pp. 1274–1280 (2006).
- ↑ Berry, V. et. al. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet, 69, pp. 1141–1145 (2001).
- ↑ Liu M. et. al. Wang Identification of a CRYAB mutation associated with autosomal dominant posterior polar cataract in a Chinese family. Invest Ophthalmol Vis Sci, 47, pp. 3461–3466 (2006).
- ↑ Berry V. et. al. Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4). J Med Genet, 41, p. e109 (2004).
- ↑ Burdon K.P. et. al. The PITX3 gene in posterior polar congenital cataract in. Australia Mol Vis, 12, pp. 367–371 (2006).
- ↑ Pras, E. et. al. A new locus for autosomal dominant posterior polar cataract in Moroccan Jews maps to chromosome 14q22-23. J Med Genet, 43, p. e50 (2006).
- ↑ Völcker HE et. al. Surgery of posterior polar cataract in persistent hyperplastic primary vitreous. Klin Monbl Augenheilkd. 1983 Aug;183(2):79-85. German (1983).
- ↑ Summers, K.M. et. al. Anterior segment mesenchymal dysgenesis in a large Australian family is associated with the recurrent 17 bp duplication in PITX3. Mol Vis, 14, pp. 2010–2015 (2008)
- ↑ Kalantan, H. (2012). Posterior polar cataract: A review. Saudi Journal of Ophthalmology , Volume 26 , Issue 1 , 41 – 49
- ↑ Yanoff, M. and Sassani, J.W. Lens. In Ocular Pathology, 10, 323-349.e3 (2015).
- ↑ Jump up to: 15.0 15.1 15.2 15.3 Lee, M.W. and Lee, Y. C. Phacoemulsification of posterior polar cataracts—a surgical challenge. Br J Ophthalmol. Nov; 87(11): 1426–1427 (2003).
- ↑ Schroeder, H.W. et. al. The management of posterior polar cataract: the role of patching and grading Strabismus, 13 (4), pp. 153–156 (2005).
- ↑ Jump up to: 17.0 17.1 Mistr, S.K. et. al. Preoperative considerations and outcomes of primary intraocular lens implantation in children with posterior polar and posterior lentiglobus cataract. J AAPOS, 12 (1), pp. 58–61. (2008).
- ↑ Jump up to: 18.0 18.1 18.2 Vasavada AR and Singh R.: Phacoemulsification with posterior polar cataract. J Cataract Refract Surg. 25: 238–245. (1999).
- ↑ Jump up to: 19.0 19.1 19.2 Vasavada AR, Raj SM, Vasavada V, and Shrivastav S. (2012). Surgical approaches to posterior polar cataract: a review. Eye, Vol.26(6), p.761-770 (2012).
- ↑ Arora, R. et. al. Tear-drop sign of posterior capsule dehiscence on Scheimpflug imaging. Eye 24, 737–738; published online 12 June 2009. (2010).
- ↑ Skalka, H.W. Ultrasonic diagnosis of posterior lens opacity. Ophthalmic Surg, 8 (6), pp. 72–76 (1977).
- ↑ Pujari A, Selvan H, Yadav S, Urkude J, Singh R, Mukhija R, Makwana T, Sharma N. Preoperative assessment of posterior capsular integrity using a posterior segment OCT with a +20 D lens: The 'conical sign' to suggest capsular deficiency in posterior polar cataracts. J Cataract Refract Surg. 2020 Jun;46(6):844-848.
- ↑ Ho, S.F. et. al. Spontaneous dislocation of posterior polar cataract. J Cataract Refract Surg, 33 (8), pp. 1471–1473 (2007)
- ↑ Ashraf, H. et. al. Bilateral spontaneous rupture of posterior capsule in posterior polar cataract. Clin Experiment Ophthalmol, 36 (8), pp. 798–800 (2008).
- ↑ Osher, R.H. et. al. The torn posterior capsule: its intraoperative behavior, surgical management, and long-term consequences. J Cataract Refract Surg, 16 (4), pp. 490–494 (1990).
- ↑ Osher, R.H. et. al. Posterior polar cataracts: a predisposition to intraoperative posterior capsular rupture. J Cataract Refractive Surg 16, pp. 157-162. (1990).
- ↑ Jump up to: 27.0 27.1 Hayashi, K. et. al. Outcomes of surgery for posterior polar cataract. Cataract Refract Surg,29, pp. 45–49 S. (2003).
- ↑ Jump up to: 28.0 28.1 Das, R. et. al. Surgical and visual outcomes for posterior polar cataract. Br J Ophthalmol, 92 (11), pp. 1476–1478 (2008).
- ↑ Gavris M. et. al. Phacoemulsification in posterior polar cataract. Oftalmologia. 48(4): 36–40. (2004).
- ↑ Pong, J. et. al. Managing the hard posterior polar cataract J Cat Refract Surg, 34 (2008), pp. 530–531 (2008).
- ↑ Salahuddin. Inverse horse-shoe technique for the phacoemulsification of posterior polar cataract. Can J Ophthalmol, Apr 45 (2), pp. 154–156.(2010)
- ↑ Chee, S.P. Management of the hard posterior polar cataract. J Cataract Refract Surg, 33 (9), pp. 1509–1514. (2007)
- ↑ Allen, D. et. al. Minimizing risk to the capsule during surgery for posterior polar cataract. J Cataract Refract Surg, 28, pp. 742–744. (2002).
- ↑ Nagappa, S. et. al. Modified technique for epinucleus removal in posterior polar cataract. Ophthalmic Surg Lasers Imaging, 42 (1), pp. 78–80 (2011)
- ↑ Gavriş, M. et. al. Phacoemulsification in posterior polar cataract. Oftalmologia, 48 (4), pp. 36–40 (2004).
- ↑ Siatiri, H. et. al. Posterior polar cataract: minimizing risk of posterior capsule rupture. Eye (Lond), 20 (7), pp. 814–816 Epub 2005 Oct 28 (2006).
- ↑ Kumar, S. Phacoemulsification in posterior polar cataract: does size of lens opacity affect surgical outcome? Clin Experiment Ophthalmol, 38 (9), pp. 857–861. (2010).
- ↑ Kumar, V. Posterior polar cataract surgery: a posterior segment approach. Eye (Lond), 23 (9), p. 1879 (2009)
- ↑ Vajpayee, R.B. et. al. ‘Layer by layer’ phacoemulsification in posterior polar cataract with pre-existing posterior capsular rent. Eye (Lond), 22 (8), pp. 1008–1010 (2008).
- ↑ Haripriya, A. Bimanual microphaco for posterior polar cataracts. J Cataract Refract Surg, 32 (6), pp. 914–917 (2006).
- ↑ Taskapili, M. et. al. Phacoemulsification with viscodissection in posterior polar cataract: minimizing risk of posterior capsule tear. Ann Ophthalmol (Skokie), 39 (2), pp. 145–149 (2007).
- ↑ Singh, K. et. al. Oval capsulorhexis for phacoemulsification in posterior polar cataract with preexisting posterior capsule rupture. J Cataract Refract Surg. Jul; 37(7):1183-8 (2011).
- ↑ Fine, I.H. et. al. Management of posterior polar cataract. J Cataract Refract Surg, 29, pp. 16–19 (2003)
- ↑ Masket S. Consultation section: Cataract surgical problem. J Cataract Refract Surg.; 23:819–824. (1997)
- ↑ Lim, Z. et. al. Modified epinucleus pre-chop for the dense posterior polar cataract. Ophthalmic Surg Lasers Imaging, 39 (2), pp. 171–173. (2008)
- ↑ Vasavada, A.R. et. al. Inside-out delineation. J Cataract Refract Surg, 30, pp. 1167–1169 (2004).
- ↑ Pujari A, Yadav S, Sharma N, Khokhar S, Sinha R, Agarwal T, Titiyal JS, Sharma P. Study 1: Evaluation of the signs of deficient posterior capsule in posterior polar cataracts using anterior segment optical coherence tomography. J Cataract Refract Surg. 2020 Sep;46(9):1260-1265.