Ophthalmologic Manifestations of Autoimmune Diseases
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Although the specific targets and mechanisms of systemic inflammatory diseases are multiple and variable, the eye appears to be a commonly affected organ system in a host of inflammatory conditions. As such, many inflammatory diseases have the potential to significantly threaten vision either directly through immunologic mechanisms, or indirectly either by contributing to the development of vulnerable secondary pathologic states (e.g. hypercoagulability, arteritis, and hypertension) or from side effects of treatment (e.g., steroid induced cataract or glaucoma). This chapter reviews the common ocular manifestations of autoimmune and inflammatory diseases that may present with neuro-ophthalmologic signs and symptoms.
Diseases
The immunological mechanisms underlying systemic inflammatory disorders are diverse, however many of these diseases converge at similar manifestations in the eye. Neuro-ophthalmologic manifestations of systemic inflammatory disorders may involve the optic nerve, retina, and cranial nerves. These can include, but are not limited to:
- Optic neuritis
- Ischemic optic neuropathy (AAION, NAION, PION)
- Orbital Inflammatory Syndrome
- Retinal vasculitis
- Vaso-occlusive retinopathies (BRVO, CRVO, CRAO, BRAO)
- Amaurosis fugax
OPTIC NEURITIS
Optic neuritis (ON), or optic nerve inflammation, is the most common optic neuropathy in young adults.[1] The term ON typically refers to either inflammatory or demyelinating optic neuropathy. The pathogenesis of the condition involves inflammatory demyelination, which may result in vision loss and conduction block.[1] Although remyelination can occur, visual recovery may be incomplete due to persistent demyelination and neuroaxonal loss.[1] Demyelinating ON is strongly associated with multiple sclerosis (MS) but may also be idiopathic. Antibodies that mediate ON can occur in neuromyelitis optica (NMO) or anti-myelin oligodendocytic glycoprotein (MOG) disease [1] or NMO-spectrum disorders. Demyelinating ON has also been shown to occur more frequently at higher latitudes, in those of North European ancestry, and during the spring season.[1] In contrast to demyelinating ON, inflammatory ON can be associated with a number of autoimmune disorders including systemic lupus erythematosus (SLE)[2], Sarcoidosis[3], granulomatosis with polyangiitis (GPA)[1], Inflammatory bowel disease (IBD) (i.e. Crohn’s disease and ulcerative colitis)[4], antiphospholipid antibody syndrome (APLS)[5], Sjogren syndrome[6] and Covid-19.[7]
Although controversial, some reports have also suggested that ON may also be associated with immunomodulatory therapies used to treat inflammation. For example, the biologic Infliximab (Remicade), a common immunosuppressive therapy used in the treatment of numerous autoimmune diseases, has been implicated in some cases of ON.[8]
SECONDARY ISCHEMIC OPTIC NEUROPATHY
Although typical ischemic optic neuropathy (ION) can be categorized as arteritic or non-arteritic, and anterior (optic disc involvement) or posterior (retrobulbar involvement), atypical ION can occur in autoimmune disease. In systemic inflammatory diseases, ischemia of the optic nerve can be secondary to vasculitis obstructing blood flow to the optic nerve, or occlusion due to a secondary hypercoagulable state (e.g. anti-phospholipid antibody).[4][9][10][11] Inflammatory ION has been associated with the following disorders: SLE,[5][12][13] giant cell arteritis (GCA),[12] Polyarteritis nodosa (PAN),[12] Takayasu arteritis,[13] Granulomatosis with polyangiitis (GPA),[14] Eosinophilic granulomatosis with polyangiitis (EGPA),[15] and Crohn’s disease.[4] Additionally, optic neuropathy has been reported following Covid-19 immunizations.[16]
While arteritic anterior ischemic neuropathy (AAION) is most frequently associated with GCA, rare causes of AAION can include inflammatory diseases that lead to vasculitis (i.e. SLE, PAN).[12]
Typical non-arteritic (NAION) however can occur in patients with autoimmune disease who also harbor the more common vasculopathic etiologies, such as diabetes mellitus, ischemic heart disease, hyperlipidemia, atherosclerosis, and arteriosclerosis.[12]Rare cases of NAION has also been reported following Covid-19 immunizations.[17]
In contrast to typical NAION, a retrobulbar ION (or posterior ION) is rare. It has been suggested that patients with posterior ION (PION) unassociated with recent surgery undergo evaluation for systemic vascular diseases (e.g. GCA).[13] In GCA specifically, total visual loss can result from ION secondary to vasculitis of the end arteries supplying the optic nerve head, and occlusion of the central retinal artery via ophthalmic artery involvement.[18] PION, however, has been reported in multiple systemic autoimmune disorders.[19]
ORBITAL INFLAMMATORY SYNDROME (OIS) or ORBITAL INFLAMMATORY PSEUDOTUMOR
Inflammation of the orbital contents (i.e., orbital inflammatory syndrome or OIS) including the extraocular muscles, orbital fat, or orbital optic nerve can occur in association with systemic autoimmune disorders.[20] Although most OIS is idiopathic (IOIS), OIS has been reported to occur in multiple autoimmune conditions including GPA,[20] GCA,[20] IBD,[4][20] SLE,[20] EGPA,[20] scleroderma,[20] and sarcoidosis,[20] dermatomyositis,[21] and rheumatoid arthritis.[21]
RETINAL VASCULITIS
Retinal vasculitis (RV) describes inflammation of the retinal vessels and can be due to either a primary ocular process, or secondary to an underlying autoimmune, inflammatory, infectious, or neoplastic disease.[22] RV can cause a disruption in the blood-retinal barrier which can be confirmed by fluorescein angiography (FA).[23] Although many cases of RV are isolated, up to 42.5 percent of patients presenting with RV had undiagnosed systemic disease.[24] RV can present as either retinal arteritis, phlebitis, or a mix of both.[25]
Common autoimmune associations of RV may include the following:
Primarily retinal arteritis: SLE, PAN, EGPA, GCA[25]
Primarily retinal phlebitis: Behcet disease, multiple sclerosis, sarcoidosis, pars planitis[25]
Mix of arteritis and phlebitis: GPA, IBD[25]
VASO-OCCLUSIVE RETINOPATHIES
RV can cause secondary vascular occlusions, including central retinal vein, branch retinal vein, central retinal artery, and branch retinal artery occlusions. Multiple systemic autoimmune diseases have been associated with retinal vessel occlusion secondary to vasculitis.[26] In general, causes of these occlusions can include embolism, atherosclerotic disease, or arteritis.[27] In addition to occlusive vasculitis, secondary vasospasm may also be an underlying etiology behind vaso-occlusive retinopathy associated with autoimmune disease.[28] Retinal vascular occlusion has been associated with the following autoimmune disorders: SLE,[2][22] Sarcoidosis,[29] APLS,[5][9] Crohn’s disease[10], GPA,[26][27]EGPA[27], PAN.[26][27]Additionally, secondary vascular occlusions have been associated with Covid-19 associated coagulopathy.[30]
AMAUROSIS FUGAX
Amaurosis fugax is the symptom of temporary vision loss that occurs in one or both eyes caused by an abrupt temporary reduction in blood supply to the eye(s).[31] There are multiple causes of amaurosis fugax, and those relevant to autoimmune disease can include the development of vasculitis,[31] hypercoagulable states,[31] and vasospasm.[28] Amaurosis fugax has been associated with autoimmune diseases such as SLE[2], GCA,[28] Crohn’s disease,[32] APLS [9], and Takayasu arteritis.[33]
Pathophysiology
There are various mechanisms through which systemic autoimmune and inflammatory disorders exert their effects, and many are poorly understood. Those relevant to neuro-ophthalmology may include but are not limited to: vasculitis,[18] vasospasm,[28] immune-mediated demyelination[1], immune complex deposition[34] and hypercoagulability.[35] According to Zöller et al., in light of recent studies, autoimmune diseases may be considered as hypercoagulable disorders due to the role of inflammation in upregulating procoagulants, downregulating anticoagulants, and the development of pathogenic antibodies (i.e. anti-cardiolipin, lupus anticoagulant).[35]
Diagnosis
Symptoms
Optic Neuritis: Symptoms of optic neuritis tend to develop and may worsen over a few days, reaching maximum severity within 1-2 weeks .[36] These symptoms can include acute visual loss (usually unilateral), photopsias, dyschromatopsia, and pain with eye movement.[36]
Ischemic Optic Neuropathy: NAION: In cases of NAION, patients can often present with a sudden, unilateral painless yet severe decline in vision.[37] Symptoms may be present on awakening.[12][37]
GCA (AAION): patients can often complain of sudden unilateral or bilateral vision loss and may be associated with other symptoms (e.g. headache, jaw claudication, scalp tenderness, arthralgia and malaise).[38]
Orbital Inflammatory Syndrome: Symptoms depend on the orbital tissue involved, but typical presentation may consist of an abrupt onset of deep-rooted, boring orbital pain, diplopia, headache, and progressive vision loss.[39][40] Pain with eye rotation would be suggestive of orbital myositis.[39] Binocular simultaneous involvement could suggest the possibility of systemic vasculitis.[39]
Establishing the time course of symptoms may be helpful in differentiating OIS caused by inflammatory versus infectious causes.[20] Patients with OIS secondary to a systemic inflammatory process can generally present with symptoms of insidious onset, as opposed to the more acute course of infectious etiologies.[20]
Retinal Vasculitis: The classic symptoms of retinal vasculitis may include painless, decreased visual acuity, blurring of vision, scotoma, floaters, and flashes.[23] The symptoms of retinal vasculitis depend on the location of the affected retinal vessels.[25] Vasculitis near the macula will often cause loss of visual acuity, while vasculitis of peripheral retinal vessels may be asymptomatic.[25] Retinal vasculitis can be further classified into occlusive and nonocclusive types.[25]
Vaso-occlusive Retinopathies:
Branch retinal vein occlusion (BRVO): Can be asymptomatic and diagnosed during a routine ophthalmologic exam.[41] Patients may experience visual field deficits with blurry vision and scotoma.[41]
Central retinal vein occlusion (CRVO): Usually symptomatic. Typically, patients present with acute, painless, monocular blurry vision.[41]
Chronic Retinal Vein Occlusion: Patients may complain of decreased vision and a red, painful eye due to high intraocular pressure secondary to neovascular glaucoma.[41]
Central retinal artery occlusion (CRAO): Sudden, painless, severe loss of vision in one eye.[26]
Branch retinal artery occlusion (BRAO): Sudden loss of vision, but not as severe as CRAO. Vision field loss can be limited, and visual acuity may be impaired in less than half of patients.[26]
Amaurosis fugax: Patients typically describe the transient visual loss within a range of mild blurriness or fog to complete blackness that can last between seconds or minutes, depending on the etiology.[31] Symptoms of amaurosis fugax involving both eyes suggest a more posterior pathology involving the optic chiasm, optic tract, radiations, or occipital lobe.[31]
Signs
Optic Neuritis: Signs can include relative afferent pupillary defect (RAPD) (unless both eyes are affected symmetrically), variable decreased visual acuity, visual field defects (typically central or nerve fiber layer type), dyschromatopsia (particularly red), and disc edema in some cases, but may have normal appearing optic nerve (retrobulbar).[1][42] Optic disc edema is not required to make the diagnosis.[42]
Ischemic Optic Neuropathy: Signs can include RAPD, visual field deficits, decreased visual acuity, dyschromatopsia, and peripapillary splinter hemorrhages.[12][43] PION is characterized by a normal optic nerve at onset, but eventually optic atrophy may develop.
Funduscopic findings in ION are variable and depend on the location of the inflammation or occlusion.
Arteritic Anterior Ischemic Optic Neuropathy (AAION): Chalky pallor of optic disc, optic nerve edema, peripapillary splinter hemorrhages.[38] Upon resolution of optic disc edema, most cases of AAION may show cupping of the optic disc similar to that in glaucomatous optic neuropathy.[12]
Non-arteritic Anterior Ischemic Optic Neuropathy (NAION): hyperemic optic nerve with edema, peripapillary splinter hemorrhages.[43] Optic disc cupping is not frequently seen in NAION.[12]
Posterior Ischemic Optic Neuropathy (PION): PION would not cause disc edema as it involves the part of the optic nerve behind the optic disc. At time of onset, RAPD may be present but ophthalmoscopic exam may not show visible changes to the optic nerve head, and optic disc and fundus may be normal on ophthalmoscopy and FA.[12]
Orbital Inflammatory Syndrome (OIS): Signs include exophthalmos, conjunctival redness, proptosis, ptosis, palpable orbital mass, reduced ocular motility, eyelid erythema, periorbital edema, and chemosis.[39][40][44] Extraocular muscles will typically show enlarged insertions on orbital ultrasonography, a feature which helps differentiate OIS from thyroid ophthalmopathy.[20][45]
Retinal Vasculitis: Signs include perivascular sheathing, cotton-wool spots, frosted branch angiitis, retinal vascular occlusion, retinal hemorrhages, white sheathing or cuffing of affected vessels (segmental or continuous), and optic disc edema.[18][22][23][46] In occlusive retinal vasculitis, retinal ischemia may cause neovascularization, leading to vitreous hemorrhage.[25] Peripheral vessels are more commonly involved than central vessels.[46] The presence of signs such as vascular leakage, macular edema, areas of capillary dropout, and retinal neovascularization indicate the severity of inflammation and retinal ischemia.[22][23]
Vaso-occlusive Retinopathies:
In patients with retinal vein occlusion, macular edema is the most common cause of vision loss.[47] Vision loss can also be caused by subsequent neovascularization, leading to retinal detachment, neovascular glaucoma, or vitreous hemorrhage.[47]
Signs of retinal vein occlusion (CRVO, BRVO) may include intraretinal hemorrhage, macular edema, dilated and tortuous veins, and cotton wool spots, and neovascularization of the retina, anterior chamber angle, and iris.[48] In patients with chronic retinal vein occlusion, neovascularization of the iris or of the anterior chamber angle may lead to neovascular glaucoma, which may cause redness of the eye.[41]
Signs of retinal artery occlusion (CRAO, BRAO) on initial examination may include retinal opacity in the posterior pole, cherry-red spot, box-carring of the blood column (due to sluggish blood-flow), retinal arterial attenuation, and optic disc edema and pallor.[27] Late stage signs may include optic nerve atrophy and cilioretinal collateral vessel development.[27]
Amaurosis fugax: Signs on fundoscopic examination can provide clues as to the cause of amaurosis fugax. Depending on etiology, signs may include disc edema (suggesting an acute event involving optic nerve), optic disc pallor (may suggest a chronic process), retinal whitening (may suggest BRAO/CRAO), neovascularization and torturous veins (may suggest carotid stenosis), and Hollenhorst plaques (may suggest ipsilateral carotid artery disease).[31] Monocular vision loss that descends like a shade could suggest retinal ischemia.[31]
Clinical Diagnosis
Optic neuritis: The diagnosis of optic neuritis is typically a clinical diagnosis, made using the above signs and symptoms. Magnetic resonance imaging (MRI) of the optic nerve may assist in diagnosis.[42]
Ischemic Optic Neuropathy: Diagnosis of ION is mainly based on fundoscopic and imaging findings.
Orbital Inflammatory Syndrome (OIS): Dilated funduscopic examination (DFE) is recommended to examine the retina and optic nerve.[20] Evaluation of the optic nerve (i.e. color vision, RAPD) and visual field testing are suggested, especially due to concern for compressive optic neuropathy.[20] Orbital examination could include palpation, measurement of exophthalmos, and evaluation of extraocular movements (EOM).[20] Orbital biopsy is not always required in the evaluation of OIS but may be performed if the patient is not responsive to therapy or there is diagnostic uncertainty.[39] Imaging also aids in diagnosis.
Retinal Vasculitis: The diagnosis of retinal vasculitis is primarily clinical, made via funduscopic examination and aided by imaging such as FA and optical coherence tomography (OCT).[22][25]
Vaso-occlusive Retinopathies: Diagnosis of retinal vein occlusion is primarily clinical.[47] Both a thorough slit lamp examination with gonioscopy and DFE with ophthalmoscopy could be considered.[48] A funduscopic examination is suggested in CRAO, and diagnosis is based on the classic funduscopic findings discussed above.[27][49]
Amaurosis fugax: Due to the transient nature of the visual loss, the neurologic and ophthalmologic examination of these patients is usually normal.[31] Clinical suspicion of underlying etiology is based heavily on the patient’s description of the visual symptoms and pertinent medical and family history.[31]
Imaging
Optic Neuritis: While not required for diagnosis, in most cases, orbital MRI with gadolinium may show optic nerve enhancement.[1] Furthermore, MRI can also help determine future risk of conversion to multiple sclerosis and the presence of demyelinating white matter lesions.[1] Further tests may be performed, such as lumbar puncture, chest radiographs, and visual evoked potentials.[1] OCT can be used to detect and quantify optic atrophy.[42]
Ischemic optic neuropathy: FA is typically performed[12], and MRI is useful in differentiating between AAION and NAION.[38] Differentiating non-arteritic PION from ON can sometimes be difficult. Typically, however, retrobulbar ON produces optic nerve enhancement on the post-contrast neuroimaging (e.g., magnetic resonance imaging with gadolinium)[1] while PION may not show enhancement.[19]
Orbital Inflammatory Syndrome (OIS): MRI and computed tomography (CT) are frequently used in evaluating orbital inflammatory syndrome ,[45] demonstrating potential involvement of the lacrimal gland, globe, extraocular muscles, orbital fat, lacrimal gland, and presence of infiltration or mass in the orbital apex.[45] On MRI, inflammatory infiltrate may demonstrate hypointensity on T1 and T2-weighted imaging with gadolinium enhancement.[45] The lacrimal gland is the most commonly affected anatomic structure in OIS.[39][45] It typically presents as diffuse enlarged on CT with preservation of its shape.[39][45] CT imaging is useful in evaluating orbital bone, adjacent sinuses, and discovering potential abscess formation.[20] At minimal cost to the patient, orbital ultrasound may also play a role in visualizing extraocular muscle insertions and lacrimal gland enlargement.[20] Furthermore, if sarcoidosis is suspected, then a CT or chest radiograph could be considered.[20]
Retinal Vasculitis: FA is a useful imaging modality in diagnosing and managing retinal vasculitis.[23] Demonstration of whether vascular leakage is focal (i.e. sarcoidosis, multiple sclerosis) or diffuse (i.e. Behcet’s disease) informs the differential diagnosis for retinal vasculitis.[22] Indocyanine green angiography (ICG) may also be useful in detecting abnormal choroidal blood flow, a potential complication of retinal vasculitis.[23] Lastly, OCT can be used to identify the potential complications of cystoid macular edema and retinal neovascularization.[25]
Vaso-occlusive Retinopathies: Usually, the diagnosis of retinal vein occlusions is based solely on clinical examination, however imaging such as FA is often performed to evaluate macular edema and perfusion.[47] OCT can also be used to measure macular edema and treatment response.[47]
FA is helpful in differentiating between the various subtypes of CRVO and CRAO.[27][47] Acutely, FA may demonstrate delayed perfusion of the retinal arteries, with normal choroidal filling. In elderly patients, if delayed choroidal filling is observed, this may indicate an ophthalmic or carotid artery obstruction, and GCA may be considered.[26][50] After ruling out GCA, further imaging studies to evaluate the cause of occlusion could be conducted, such as carotid artery imaging and cardiac evaluation (Holter monitoring and echocardiography).[26][49]
Amaurosis fugax: In patients older than 50 years and in younger patients with vascular risk factors presenting with amaurosis fugax, carotid imaging could be considered.[31] Evaluation for GCA is also recommended in elderly patients with amaurosis fugax .[31] A cardiac evaluation (i.e. electrocardiogram (ECG), echocardiogram (EKG), Holter monitoring) may be considered after GCA and carotid disease have been excluded to search for a cardiogenic source of embolism.[31] In older patients with transient binocular vision loss and symptoms concerning for vertebrobasilar ischemia, a brain MRI can be considered.[31]
Laboratory Studies
Optic Neuritis: In typical optic neuritis cases, laboratory testing is generally low yield. The following laboratory studies may be considered in patients suspected of harboring an underlying etiology in atypical cases including non-demyelinating conditions or vasculitis: erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), syphilis serology, cerebrospinal fluid studies, complete blood count (CBC), renal function tests, liver function tests, vitamin B12, folate, serum angiotensin converting enzyme (ACE), autoantibodies (i.e. anti-double stranded DNA (anti-dsDNA), anti-neutrophil cytoplasmic antibodies (ANCA)), tuberculosis quantiferon (interferon gamma release assay) testing, and anti-aquaporin 4 antibodies (NMO) or MOG antibodies.[1]
Ischemic Optic Neuropathy: Suggested laboratory studies include CBC, ESR, and CRP.[12]
Orbital Inflammatory Syndrome (OIS): Depending on clinical suspicion, labs such as CBC, ESR, CRP, antinuclear antibody (ANA), ANCA, rheumatoid factor (RF), serum protein electrophoresis (SPEP), angiotensin converting enzyme (ACE), and thyroid function studies may be considered.[20]
Retinal Vasculitis: Suggested laboratory studies include CBC, urinalysis (UA), ESR, CRP, ANCA, ANA, human immunodeficiency virus (HIV) and syphilis serologies.[46]
Vaso-occlusive Retinopathies: An evaluation for cardiovascular risk factors could be performed.[47][49] This may include CBC, renal function tests, fasting serum glucose, hemoglobin A1c (HbA1c), and fasting lipid levels.[47] ESR and CRP are suggested in patients greater than age 50 years.[26][49] An investigation of thrombotic factors is usually considered in patients with bilateral retinal vein occlusions, previous history of thrombotic events, or patients less than age 50 years.[41][47] This includes testing for hypercoagulable states.[41][47] In young CRAO patients without cardiovascular risk factors, evaluation for vasculitis (ANA, ANCA, ACE), sickle cell disease, myeloproliferative disorders, and hypercoagulable states could be considered.[49][50]
Amaurosis fugax: Hypercoagulability testing may be warranted in younger patients, and ESR and CRP in older patients (> 50 y) to rule out GCA.[31]
Differential Diagnosis
Optic Neuritis: The differential diagnoses include, but are not limited to ION, optic nerve compression, post-infectious and infectious optic neuropathy, Leber hereditary optic neuropathy, and toxic optic neuropathy.[1]
Ischemic optic neuropathy: The differential diagnoses include, but are not limited to genetic, toxic, metabolic, infectious, inflammatory, compressive, and infiltrative optic neuropathies.[51]
Orbital Inflammatory Syndrome (OIS): The differential diagnoses include but are not limited to malignancy (primary and metastatic), congenital mass lesions (dermoid cyst, lymphangioma), infectious disease (e.g., Lyme, syphilis, tuberculosis), trauma, orbital cellulitis, thyroid eye disease,[20] carotid cavernous fistula, Tolosa-Hunt syndrome, contiguous sinusitis, and amyloidosis.
Retinal vasculitis: The differential diagnoses include, but are not limited to infection (cytomegalovirus, herpes simplex virus, HIV, varicella zoster virus, syphilis, tuberculosis, toxoplasmosis), inflammation (i.e. sarcoidosis, multiple sclerosis), and malignancy (i.e. paraneoplastic syndrome, leukemia).[22][25]
Vaso-occlusive Retinopathies: The differential diagnoses include, but are not limited to diabetic retinopathy, hypertensive retinopathy, retinopathy secondary to blood cell dyscrasias, and HIV.[48]
Amaurosis fugax: The differential diagnoses include, but are not limited to hypotension, cardiogenic embolism, coagulopathy, carotid stenosis, retinal vein occlusion, retinal vasospasm, optic neuropathy, and compressive optic neuropathy.[31]
Management
General Treatment
Optic Neuritis: Although patients with optic neuritis improve with or without treatment at final visual outcome, intravenous methylprednisolone (IVMP) in the acute treatment for ON sped the rate of visual recovery.[1][52] This approach was supported by the hallmark Optic Neuritis Treatment Trial (ONTT), which found that the use of IVMP was associated with higher rate of visual recovery, while use of oral prednisone alone was associated with increased risk of recurrent ON attacks compared to IV and placebo groups.[53] However, a recent randomized controlled trial by Morrow et. al suggests that oral steroids may be a suitable alternative.[52]
In the ONTT, acute attacks of ON were treated with either 1) 3 days of IVMP, followed by 11 days of oral prednisone, 2) oral prednisone monotherapy for 14 days, or 3) placebo.[53] However, doses of IV and oral medication were not bioequivalent.[52] Recently, Morrow et. al (2018) compared the efficacy of IVMP to a bioequivalent dose of oral prednisone and found that there was no significant difference in visual recovery between the two groups.[52] Advantages of oral steroids over IV steroids include cost-effectiveness, increased accessibility and ease of administration.[52]
For ON refractory to corticosteroids, intravenous immunoglobulin (IVIG) or plasmapheresis (PLEX) may be considered.[1] Patients with suspected NMO may require PLEX after IV steroids treatment.[1]
Ischemic Optic Neuropathy (ION): Treatment depends on the type of ION.
NAION: There are no proven effective therapies for NAION. While Hayreh and Zimmerman found that systemic corticosteroid therapy in the acute phase of NAION improved visual outcomes[12], this study was not randomized, and examiners were not blinded .[43] Studies examining aspirin as a potential therapy have been conflicting.[12] Optic nerve sheath decompression has been shown to be ineffective in randomized clinical trial for NAION.[12]
AAION: Treatment is emergent systemic high dose corticosteroid therapy due to concern for GCA[12].
PION: Treatment depends on the type of PION. For postsurgical PION, no specific treatment has been proven yet; management is mostly based on preventative measures before, during and after surgery.[12] Patients with arteritic PION are treated with emergent systemic corticosteroids due to concern for GCA.[12][19] While a limited study reports that visual outcomes of non-arteritic PION improved with early aggressive corticosteroid therapy[12], systemic corticosteroids have not been definitely proven as an effective therapy.[19]
Orbital Inflammatory Syndrome (OIS): Non-steroidal anti-inflammatory drugs have been shown to be an effective therapy.[21] Systemic corticosteroids are typically the first-line therapy for OIS.[20][21][39] Low dose radiation therapy can be considered for non-idiopathic OIS that is refractory to corticosteroids, or in patients who cannot tolerate systemic corticosteroids.[20][21] Other medical therapies may include methotrexate, cyclophosphamide, azathioprine, cyclosporine, mycophenolate mofetil, anti-tumor necrosis factor (anti-TNF) alpha biologics, and interferon (IFN) alpha.[20][21] Although rare, orbital decompression may be performed if there is progressive optic nerve compression.[39]
Retinal vasculitis: Treatment depends on the underlying etiology and severity of the vasculitis.[25] For cases of mild vasculitis affecting the peripheral retina that minimally affects vision, patients can be followed by observation alone.[25] In other cases of RV however, local or systemic corticosteroids and antimetabolites such as methotrexate[25] can be used, with corticosteroids achieving a more rapid improvement in symptoms.[25] Severe RV secondary to a systemic autoimmune disease may be treated with monoclonal antibody (anti-TNF) or IFN alpha (often done in RV secondary to Behcet’s disease).[25] Of the biologics, anti-TNFs are the preferred choice to treat RV.[25] If retinal vasculitis is occlusive (as with APLS), then treatment can include antiplatelet and/or anticoagulant medications.[25] While these therapies have been implemented in treating RV, none have been approved by the United States Food and Drug Administration as specific treatments for the condition.[25]
Vaso-occlusive Retinopathies: For retinal vein occlusion, treatment options depend on etiology of the occlusion. These options include glucocorticoid injections, anti-vascular endothelial growth factor (anti-VEGF) agent injections, or laser therapy.[47]
For acute central retinal artery occlusions, treatment strategies have been proposed to dilate retinal arteries (isosorbide dinitrate, carbogen inhalation), increase blood oxygen content (hyperbaric oxygen), dislodge emboli (eyeball massage), and increase retinal artery perfusion pressure by reducing intraocular pressure (topical antiglaucoma medications, acetazolamide and mannitol, anterior chamber paracentesis) but none of these countermeasures have been proven to be effective compared to placebo.[49]
Amaurosis fugax: Treatment varies depending on underlying etiology. Treatments may include carotid endarterectomy and antithrombotic medications.[54] If retinal vasospasm is thought to be the cause of transient vision loss, treatment may include aspirin or calcium channel blockers.[54]
In summary, autoimmune disorders may present with ophthalmic symptoms and signs, and specific treatment should be directed to the underlying etiology after appropriate work up, as suggested above.
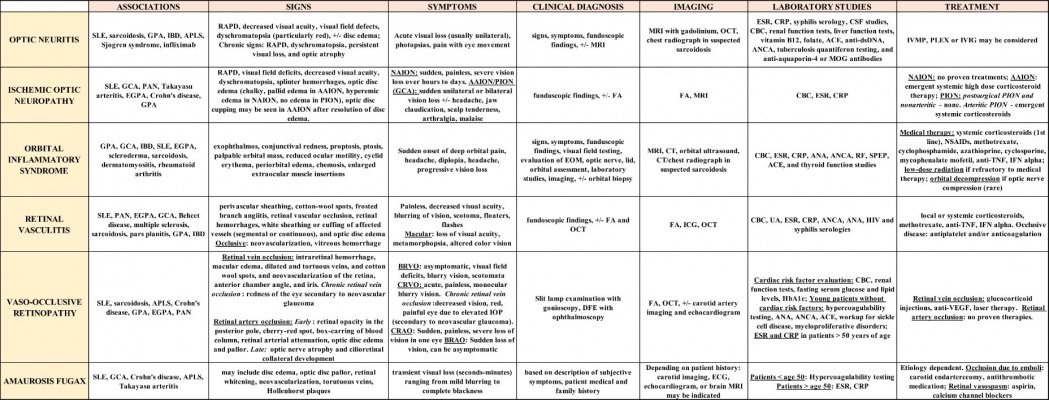
Table below depicts a summary of the signs, symptoms, diagnostic testing, and treatment of potential neuro-ophthalmologic manifestations of autoimmune diseases (References cited. [1][2][3][4][5][6][8][9][10][12][13][14][15][18][20][21][22][23][25][26][27][28][29][31][32][33][36][37][38][39][40][41][42][43][44][46][47][48][49][45][50][54]) Click on the image for a magnified view.
Abbreviations: SLE – systemic lupus erythematosus,GPA – granulomatosis with polyangiitis,IBD – inflammatory bowel disease,APLS – antiphospholipid antibody syndrome, PAN – polyarteritis nodose,EGPA – eosinophilic granulomatosis with polyangiitis,GCA – giant cell arteritis,RAPD – relative afferent pupillary defect,AAION – arteritic anterior ischemic optic neuropathy,NAION – non-arteritic anterior ischemic optic neuropathy,PION – posterior ischemic optic neuropathy,BRVO – branch retinal vein occlusion,CRVO – central retinal vein occlusion,IOP – intraocular pressure,CRAO – central retinal artery occlusion,BRAO – branch retinal artery occlusion,FA – fluorescein angiography,OCT – optical coherence tomography,DFE – dilated fundoscopic examination,EOM – extraocular movements,MRI – magnetic resonance imaging,CT – computed tomography,ICG – indocyanine green angiography,ECG – electrocardiogram,EKG – echocardiogram,ESR – erythrocyte sedimentation rate,CRP – C-reactive protein,CSF – cerebrospinal fluid,CBC – complete blood count,ACE – angiotensin converting enzyme,Anti-dsDNA – anti-double stranded DNA,ANCA – antineutrophil cytoplasmic antibody,MOG – myelin oligodendrocyte glycoprotein,ANA – Antinuclear antibody,RF – rheumatoid factor,SPEP – serum protein electrophoresis,UA – urinalysis,HIV – human immunodeficiency virus,HbA1c – hemoglobin A1c,VDRL – venereal disease research laboratory test,IVMP – intravenous methylprednisolone,PLEX – plasmapheresis,IVIG – intravenous immunoglobulin,NSAIDs – nonsteroidal anti-inflammatory drugs,Anti-TNF – anti-tumor necrosis factor,IFN – interferon,Anti-VEGF – anti-vascular endothelial growth factor.
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 Toosy, A. T., Mason, D. F., & Miller, D. H. (2014). Optic neuritis. The Lancet Neurology, 13(1), 83-99.
- ↑ 2.0 2.1 2.2 2.3 Sivaraj, R. R., Durrani, O. M., Denniston, A. K., Murray, P. I., & Gordon, C. (2007). Ocular manifestations of systemic lupus erythematosus. Rheumatology, 46(12), 1757-1762.
- ↑ 3.0 3.1 Kidd, D. P., Burton, B. J., Graham, E. M., & Plant, G. T. (2016). Optic neuropathy associated with systemic sarcoidosis. Neurology® Neuroimmunology & Neuroinflammation, 3(5), e270. http://doi.org/10.1212/NXI.0000000000000270
- ↑ 4.0 4.1 4.2 4.3 4.4 Katsanos, A., Asproudis, I., Katsanos, K. H., Dastiridou, A. I., Aspiotis, M., & Tsianos, E. V. (2013). Orbital and optic nerve complications of inflammatory bowel disease. Journal of Crohn's and Colitis, 7(9), 683-693.
- ↑ 5.0 5.1 5.2 5.3 Sanna, G., D'Cruz, D., & Cuadrado, M. J. (2006). Cerebral manifestations in the antiphospholipid (Hughes) syndrome. Rheumatic Disease Clinics, 32(3), 465-490.
- ↑ 6.0 6.1 Tang, W. Q., & Wei, S. H. (2013). Primary Sjögren's syndrome related optic neuritis. International journal of ophthalmology, 6(6), 888.
- ↑ Jossy A, Jacob N, Sarkar S, Gokhale T, Kaliaperumal S, Deb A. COVID-19-associated optic neuritis – A case series and review of literature. Indian J Ophthalmol [Internet]. 2022 Jan 1 [cited 2022 Sep 7];70(1):310. Available from: /pmc/articles/PMC8917537/
- ↑ 8.0 8.1 7 Foroozan, R., Buono, L. M., Sergott, R. C., & Savino, P. J. (2002). Retrobulbar optic neuritis associated with infliximab. Archives of Ophthalmology, 120(7), 985-987.
- ↑ 9.0 9.1 9.2 9.3 Atanassova, P. A. (2007). Antiphospholipid syndrome and vascular ischemic (occlusive) diseases: an overview. Yonsei medical journal, 48(6), 901-926.
- ↑ 10.0 10.1 10.2 Falavarjani, K. G., Parvaresh, M. M., Shahraki, K., Nekoozadeh, S., & Amirfarhangi, A. (2012). Central retinal artery occlusion in Crohn disease. Journal of American Association for Pediatric Ophthalmology and Strabismus, 16(4), 392-393.
- ↑ Crompton, J. L., Iyer, P., & Begg, M. W. (1980). Vasculitis and ischaemic optic neuropathy associated with rheumatoid arthritis. Clinical & Experimental Ophthalmology, 8(3), 219-227.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 12.17 12.18 12.19 Hayreh, S. S. (2009). Ischemic optic neuropathy. Progress in retinal and eye research, 28(1), 34-62.
- ↑ 13.0 13.1 13.2 13.3 Sadda, S. R., Nee, M., Miller, N. R., Biousse, V., Newman, N. J., & Kouzis, A. (2001). Clinical spectrum of posterior ischemic optic neuropathy. American journal of ophthalmology, 132(5), 743-750.
- ↑ 14.0 14.1 Tarabishy, A.B., Schulte, M., Papaliodis, G.N. and Hoffman, G.S., 2010. Wegener's granulomatosis: clinical manifestations, differential diagnosis, and management of ocular and systemic disease. Survey of ophthalmology, 55(5), pp.429-444.
- ↑ 15.0 15.1 Kattah, J. C., Chrousos, G. A., Katz, P. A., McCasland, B., & Kolsky, M. P. (1994). Anterior ischemic optic neuropathy in Churg‐Strauss syndrome. Neurology, 44(11), 2200-2200.
- ↑ Haseeb A, Elhusseiny AM, Chauhan MZ, Elnahry AG. Optic neuropathy after COVID-19 vaccination: Case report and systematic review. Neuroimmunol Reports [Internet]. 2022 Jan 1 [cited 2022 Sep 7];2:100121. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2667257X22000675
- ↑ Haseeb A, Elhusseiny AM, Chauhan MZ, Elnahry AG. Optic neuropathy after COVID-19 vaccination: Case report and systematic review. Neuroimmunol Reports [Internet]. 2022 Jan 1 [cited 2022 Sep 7];2:100121. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2667257X22000675
- ↑ 18.0 18.1 18.2 18.3 McCluskey, P., & Powell, R. J. (2004). The eye in systemic inflammatory diseases. The Lancet, 364(9451), 2125-2133.
- ↑ 19.0 19.1 19.2 19.3 Tamhankar, M., & Volpe, N. J., Brazis, P.W., Wilterdink, J.L. (2016) Posterior ischemic optic neuropathy. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018.)
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 20.12 20.13 20.14 20.15 20.16 20.17 20.18 20.19 20.20 20.21 20.22 Gordon, L. K. (2006). Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye, 20(10), 1196-1206.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 Espinoza, G. M. (2010). Orbital inflammatory pseudotumors: etiology, differential diagnosis, and management. Current rheumatology reports, 12(6), 443-447.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 El-Asrar, A. M. A., Herbort, C. P., & Tabbara, K. F. (2009). Differential diagnosis of retinal vasculitis. Middle East African journal of ophthalmology, 16(4), 202.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 Agarwal, A., Tripathy, Koushik. (2014). Retinal Vasculitis. [online] Eyewiki. Available at: https://eyewiki.org/Retinal_Vasculitis (Accessed on December 3 2017).
- ↑ Shulman, S., Kramer, M., Amer, R., Sorkin, N., Schaap-Fogler, M., Rosenblatt, A., & Habot-Wilner, Z. (2015). Characteristics and long-term outcome of patients with noninfectious retinal vasculitis. Retina, 35(12), 2633-2640.
- ↑ 25.00 25.01 25.02 25.03 25.04 25.05 25.06 25.07 25.08 25.09 25.10 25.11 25.12 25.13 25.14 25.15 25.16 25.17 25.18 25.19 Rosenbaum, J. T., Sibley, C. H., & Lin, P. (2016). Retinal vasculitis. Current opinion in rheumatology, 28(3), 228-235.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 23 Hedges, T.R., Brazis, P.W., Trobe, J., Wilterdink, J.L. Central and branch retinal artery occlusion. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018)
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 27.7 27.8 Hayreh, S. S. (2011). Acute retinal arterial occlusive disorders. Progress in retinal and eye research, 30(5), 359-394.
- ↑ 28.0 28.1 28.2 28.3 28.4 Flammer, J., Pache, M., & Resink, T. (2001). Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Progress in retinal and eye research, 20(3), 319-349.
- ↑ 29.0 29.1 Rothova, A. (2000). Ocular involvement in sarcoidosis. British Journal of Ophthalmology, 84(1), 110-116.
- ↑ Bapaye MM, Nair AG, Bapaye CM, Bapaye MM, Shukla JJ. Simultaneous Bilateral Central Retinal Artery Occlusion following COVID-19 Infection. https://doi.org/101080/0927394820211891262 [Internet]. 2021 [cited 2022 Nov 2];29(4):671–4. Available from: https://www.tandfonline.com/doi/abs/10.1080/09273948.2021.1891262
- ↑ 31.00 31.01 31.02 31.03 31.04 31.05 31.06 31.07 31.08 31.09 31.10 31.11 31.12 31.13 31.14 31.15 Givre, S., Van Stavern, G.P., Brazis, P.W., Trobe, J., Wilterdink, J.L. Amaurosis fugax (transient monocular or binocular visual loss). Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018.)
- ↑ 32.0 32.1 Greenfield, S. M., Teare, J. P., Whitehead, M. W., & Thompson, R. P. H. (1993). Amaurosis fugax, Crohn's disease and the anticardiolipin antibody. Lupus, 2(4), 271-273.
- ↑ 33.0 33.1 Schmidt, J., Kermani, T. A., Bacani, A. K., Crowson, C. S., Cooper, L. T., Matteson, E. L., & Warrington, K. J. (2013, August). Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. In Mayo Clinic Proceedings (Vol. 88, No. 8, pp. 822-830). Elsevier.
- ↑ Aronson, A.J., Ordonez, N.G., Diddie, K. R., & Ernest, T. (1979). Immune-complex deposition in the eye in systemic lupus erythematosus. Arch Intern Med, 139, 1312-1313.
- ↑ 35.0 35.1 Zöller, B., Li, X., Sundquist, J., & Sundquist, K. (2012). Autoimmune diseases and venous thromboembolism: a review of the literature. American journal of cardiovascular disease, 2(3), 171.
- ↑ 36.0 36.1 36.2 Osborne, B., Balcer, L.J., Gonzalez-Scarano, F., Brazis, P.W., Wilterdink, J.L. Optic Neuritis: Pathophysiology, clinical features, and diagnosis. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018.)
- ↑ 37.0 37.1 37.2 33 Tamhankar, M., & Volpe, N. J., Brazis, P.W., Wilterdink, J.L. (2016) Nonarteritic anterior ischemic optic neuropathy: Clinical features and diagnosis. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018.)
- ↑ 38.0 38.1 38.2 38.3 Rodriguez De Los Reyes, R.A., Swisher, J., Lee, A.G., Vickers, A., Cestari, D., (2018). Arteritic Anterior Ischemic Optic Neuropathy. [online] Eyewiki. Available at: https://eyewiki.org/Arteritic_Anterior_Ischemic_Optic_Neuropathy_(AAION) [Accessed on December 3 2017].
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 39.6 39.7 39.8 39.9 Holds, J. B., Chang, W., Dailey, R. A., Foster, J. A., Kazim, M., McCulley, T. J., Kersten, R. C. (2008). Orbit, Eyelids, and Lacrimal System (Basic and Clinical Science Course, 2008-2009). San Francisco, CA: American Academy of Ophthalmology.
- ↑ 40.0 40.1 40.2 Narla, L. D., Newman, B., Spottswood, S. S., Narla, S., & Kolli, R. (2003). Inflammatory pseudotumor. Radiographics, 23(3), 719-729.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 37 Covert, D. J., Han, D. P., Trobe, J., & Sullivan, D.J. Retinal vein occlusion: Epidemiology, clinical manifestations, and diagnosis. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018.)
- ↑ 42.0 42.1 42.2 42.3 42.4 Jirawuthiworavong, G.V., Miller, A.M. (2015). Demyelinating Optic Neuritis. [online] Eyewiki. Available at: https://eyewiki.org/Demyelinating_Optic_Neuritis (Accessed on December 3 2017).
- ↑ 43.0 43.1 43.2 43.3 Cestari, D., Miller, A.M., Lee, A.G., Kupersmith, M.J., Pineles, S.L. (2015). Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION). [online] Eyewiki. Available at: https://eyewiki.org/Non-Arteritic_Anterior_Ischemic_Optic_Neuropathy_(NAION). (Accessed on December 3 2017).
- ↑ 44.0 44.1 Chundury, R.V., Chundury, A., Espinoza, G., Lee, A.G., Burkat, C.N., Jirawuthiworavong, G.V. Wladis, E.(2017). Nonspecific Orbital Inflammation. [online] Eyewiki. Available at: https://eyewiki.org/Nonspecific_Orbital_Inflammation_(Idiopathic_orbital_inflammation,_Orbital_inflammatory_syndrome,_Orbital_pseudotumor) (Accessed on December 3 2017).
- ↑ 45.0 45.1 45.2 45.3 45.4 45.5 45.6 Weber, A. L., Romo, L. V., & Sabates, N. R. (1999). Pseudotumor of the orbit: clinical, pathologic, and radiologic evaluation. Radiologic Clinics of North America, 37(1), 151-168.
- ↑ 46.0 46.1 46.2 46.3 Tolentino, M., Dana, R., Merkel, P.A., Trobe, J., Curtis, M.R. Retinal vasculitis associated with systemic disorders and infections. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018.)
- ↑ 47.00 47.01 47.02 47.03 47.04 47.05 47.06 47.07 47.08 47.09 47.10 47.11 Wong, T. Y., & Scott, I. U. (2010). Retinal-vein occlusion. New England Journal of Medicine, 363(22), 2135-2144.
- ↑ 48.0 48.1 48.2 48.3 Comer, G.M, Lim, J.I, Marcet, M.M., Shah, V.A. (2014). Retinal Vein Occlusion. [online] Eyewiki. Available at: https://eyewiki.org/Retinal_Vein_Occlusion [Accessed on December 3 2017].
- ↑ 49.0 49.1 49.2 49.3 49.4 49.5 49.6 Varma, D. D., Cugati, S., Lee, A. W., & Chen, C. S. (2013). A review of central retinal artery occlusion: clinical presentation and management. Eye, 27(6), 688.
- ↑ 50.0 50.1 50.2 Lim, J.I, Feldman, B.H., Lee, B., Tripathy, K., Karth, P., Shah, V.A. (2015). Retinal Artery Occlusion. [online] Eyewiki. Available at: https://eyewiki.org/Retinal_Artery_Occlusion (Accessed on December 3 2017).
- ↑ Osborne, B., Balcer, L.J. Optic Neuropathies. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 21, 2018)
- ↑ 52.0 52.1 52.2 52.3 52.4 Morrow SA, Fraser JA, Day C, Bowman D, Rosehart H, Kremenchutzky M, Nicolle M. Effect of Treating Acute Optic Neuritis With Bioequivalent Oral vs Intravenous Corticosteroids: A Randomized Clinical Trial. JAMA neurology. 2018 Jun 1;75(6):690-6.
- ↑ 53.0 53.1 Beck RW, Cleary PA, Anderson Jr MM, Keltner JL, Shults WT, Kaufman DI, Buckley EG, Corbett JJ, Kupersmith MJ, Miller NR, Savino PJ. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. New England Journal of Medicine. 1992 Feb 27;326(9):581-8.
- ↑ 54.0 54.1 54.2 Pula, J. H., Kwan, K., Yuen, C. A., & Kattah, J. C. (2016). Update on the evaluation of transient vision loss. Clinical ophthalmology (Auckland, NZ), 10, 297.